Abstract
Cyanobacteria inhabiting extreme environments constitute a promising source for natural products with biotechnological applications. However, they have not been studied in-depth for this purpose due to the difficulties in their isolation and mass culturing. The Atacama Desert suffers one of the highest solar irradiances that limits the presence of life on its hyperarid core to endolithic microbial communities supported by cyanobacteria as primary producers. Some of these cyanobacteria are known to produce scytonemin, a UV-screening liposoluble pigment with varied biotechnological applications in cosmetics and other industries. In this work we carried out a strain selection based on growth performance among 8 endolithic cyanobacteria of the genera Chroococcidiopsis, Gloeocapsa and Gloeocapsopsis isolated from non-saline rocks of the Atacama Desert. Then we investigated the influence of NaCl exposure on scytonemin production yield. Results in the selected strain (Chroococcidiopsis sp. UAM571) showed that rising concentrations of NaCl lead to a growth decrease while triggering a remarkable increase in the scytonemin content, reaching maximum values at 20 g L−1 of NaCl over 50-fold higher scytonemin contents than those obtained without NaCl. Altogether, these findings point out to cyanobacteria from the Atacama Desert as potentially suitable candidates for pilot-scale cultivation with biotechnological purposes, particularly to obtain scytonemin.
Similar content being viewed by others
Introduction
Microorganisms inhabiting extreme environments, both extremophile and extremotolerant strains, have become a promising source of new bioactive compounds of interest1. The capacity of producing these interesting products is explained by their survival strategies to deal with harsh environmental conditions and to achieve competitive advantages in those extreme environments where essential resources are also scarce or difficult to uptake2. Thus, the genetic adaptation of microorganisms inhabiting extreme conditions could be expected to allow the synthesis of novel metabolites with unique structures and specific biological activity3, helping in their colonization4.
Among environmental factors acting in extreme habitats, it is well known that UVR exerts lethal effects on biological systems since it is absorbed by biomolecules5. Thus, some cyanobacteria are able to produce scytonemin, the most widespread sunscreen pigment exclusively produced by these organisms6,7. It is a yellow–brown lipid-soluble dimeric compound composed of indolic and phenolic subunits8 that occurs in both oxidized (MW 544 Da) and reduced (MW 546 Da) forms which in vivo absorption maximum is at 370 nm and purified at 386 nm, in the UVA region. This pigment is located in the exopolysaccharidic (EPS) sheath of certain terrestrial cyanobacterial species and is highly stable under different abiotic stresses being able to reduce about 90% of the radiation that reaches the inside of cells9. Scytonemin production is not constitutive, occurring in response to adverse environmental conditions, such as UV radiation, oxidative stress10, nutrient deficits11, and desiccation and salt stress10,11,12, whose physiological effects and responses are considered to be related13. Its biosynthetic pathway operon has been poorly studied, first described in 2007 by Soule et al.14 in Nostoc punctiforme ATCC 29133 and subsequently in genera Anabaena, Rivularia, Scytonema, Calothrix, Nodularia, Chlorogloeopsis and Lyngbya, the cluster comprises 18 genes, including genes coding for scytonemin precursors, scytonemin assembly (scyA, scyB, scyC, scyD, scyE and scyF) and regulators. This cluster presents a mosaic structure, differing in gene content, organization, and direction among species15. Among those genes, scyC gene has been the most studied and used for phylogenetic studies16. ScyC is essential for scytonemin formationcatalysing the cyclization and decarboxylation of a β-ketoacid to form a ketone in the early stages of scytonemin biosynthesisfor its17. Furthermore, a prospective study using Nostoc punctiforme genes pointed out to scyC along with scyA and scyB as the only three genes required for heterologous production of scytonemin in E. coli18.
Scytonemin has become of particular interest to the cosmetic industry due to its UV-absorbing and antioxidant capacity. Moreover, its hydrophobic nature could facilitate penetration into the skin, thus increasing protection against harmful sun radiation18. It also has antitumor potential, being capable of inducing apoptosis in tumor cells by inhibiting the PLK1 (polo-like kinase 1) enzyme, which is involved in the development of several types of cancer, such as osteosarcoma and myeloma19,20.
The presence of cyanobacteria able to produce scytonemin has been reported in the endolithic microbial communities of some deserts21,22. Within those, the Atacama Desert (North Chile) is perhaps the most challenging polyextreme environment on Earth holding the highest surface ultraviolet radiation (UVR), photosynthetic active radiation (PAR) and annual mean surface solar radiation23. Under these extreme conditions in this desert, life is often limited to endolithic microbial communities (inside the rocks) supported by primary producers, mainly cyanobacteria, some of which are highly resistant to desiccation, light overexposure, and ionizing radiation24. However, the capacity of cyanobacterial strains from Atacama to produce this UV-screening compound when exposed to increasing NaCl concentrations remains unexplored. Besides its role in osmotic stress, NaCl addition to the culture medium is often used to reduce microbial contamination in open cultures for biotechnological purposes.
Taking all these aspects into account, this work addresses (i) a cyanobacterial strain selection among 8 endolithic strains of the genera Chroococcidiopsis, Gloeocapsa and Gloeocapsopsis, which have been isolated from the Atacama Desert, characterizing their growth rate and scytonemin synthesis capacity, along with (ii) the effect of NaCl exposure in scytonemin synthesis.
Results
Strain selection
Growth rate varied between endolithic cyanobacterial strains (Table 1, Fig. S1). Thus, Chroococcidiopsis sp. UAM570 and UAM571 together with Gloeocapsa sp. UAM572 reached the highest growth rate (0.24 day−1), while the lowest was measured for Chroococcidiopsis sp. UAM580 (0.17 day−1). The only strain belonging to the genus Gloeocapsopsis, UAM573, showed an average growth rate (0.22 day−1).
Since there was more than one strain that exhibited the highest growth rate, growth experiments exposing Chroococcidiopsis sp. UAM571 and Gloeocapsa sp. UAM572 to NaCl were performed (Table S1, Fig. S2). Chroococcidiopsis sp. UAM571 exhibited a higher growth rate for all NaCl concentrations and was then selected to perform the scytonemin production experiment.
Scytonemin production by Chroococcidiopsis sp. UAM571 exposed to NaCl
To broadly understand the scytonemin production of Chroococcidiopsis sp. UAM571 three different features were studied: (i) content per biomass DW (mg scytonemin g DW−1), (ii) concentration per volume of culture (g scytonemin·L−1), and (iii) productivity, which means related to culture volume and time (µg scytonemin L−1 day−1).
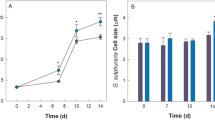
The study of the scytonemin content of Chroococcidiopsis sp. UAM571 revealed that its value increased through time and higher NaCl concentrations (Fig. 1A, Table 2). Thus, the highest scytonemin content (3.17 mg g DW−1) was obtained when it was exposed to 20 g L−1 NaCl-supplemented BG11 for 14 days, which represented more than 0.3% of biomass DW. This scytonemin content value was significantly higher compared to the control culture, reaching a drastic 53-fold difference, and to its exposure to 10 g L−1 NaCl, reaching a threefold difference (< 0.05; ANOVA, Bonferroni). The exposure to 10 g L−1 NaCl supplemented media also showed an increase in scytonemin content, up to 17 times higher than the one obtained in control conditions (Table 2). However, no significant differences were found between scytonemin content values under this NaCl concentration and control conditions. On the other hand, even though a difference of threefold and almost twofold was observed when comparing the scytonemin content between exposure times, this factor resulted non-significant for any of the NaCl concentrations (p > 0.05; ANOVA, Bonferroni) (Table 2, Fig. 1A).
(A) Scytonemin content in Chroococcidiopsis sp. UAM571 grown in BG11 with 0 g L−1 (green), 10 g L−1 (blue) and 20 g L−1 (orange) NaCl on days 7 (plain bars) and 14 (dotted bars) of experiment. (B) Evolution of total scytonemin concentration in the culture of Chroococcidiopsis UAM571 in BG11 with 0 g L−1, 10 g L−1 and 20 g L−1 NaCl. The content is expressed in mg scytonemin g−1 of DW (A) and mg scytonemin L−1 culture (B). Error bars represent SD (n = 3). The same letter indicates the absence of significant differences (p > 0.05; ANOVA, Bonferroni).
In contrast to scytonemin content results, the scytonemin concentration observed at both exposure times (7 and 14 days) of the highest NaCl concentration tested showed significant differences between them and with the rest of culture conditions studied (p < 0.05; ANOVA, Bonferroni) (Fig. 1B). The highest scytonemin concentration (0.80 mg L−1) was observed for 20 g L−1 NaCl-supplemented culture after 14 days.
When analysing the scytonemin productivity, it increased with NaCl concentration, reaching its maximum when the culture was grown under 20 g L−1 NaCl-supplemented BG11 (57.4 µg L−1 day−1) (Table 2) and 3 times higher than the one observed at 10 g L−1 NaCl. The statistical analysis revealed significant differences in scytonemin productivity between all NaCl concentrations tested (< 0.05; ANOVA, Bonferroni).
The increasing scytonemin production with time and NaCl concentration was also observed in the microscopy images as an increasing intensity of brown-yellow color in the EPSs layer of the cells (Fig. 2). These changes in color were also accompanied by a higher EPSs layer thickness when cells were exposed to increasing NaCl concentrations (Fig. 2d–g). were scytonemin was accumulated (Fig. 2e–g). To confirm this apparent increase in EPS we performed an extraction and spectrophotometric determination in samples exposed to 20 g NaCl L−1 and 0 g NaCl L−1. The quantification supported microscopic findings since UAM571 showed an average EPS content of 35.4 mg EPSs g−1 DW under 20 g NaCl L−1 compared to a 1.4-fold reduced production without NaCl (25.2 mg EPSs g−1 DW on average).
Bright field microscopy images of Chroococcidiopsis sp. UAM571 cells during scytonemin production experiment. (a) Stock culture; (b,c) cells exposed to control conditions (0 g L−1 NaCl) after 7 and 14 days; (d,e) cells exposed to 10 g L−1 NaCl supplemented BG11 culture medium after 7 and 14 days; (f,g) cells exposed to 20 g L−1 NaCl supplemented BG11 culture medium after 7 and 14 days. Scale bar indicates 20 µm. Black arrowheads point to EPSs, arrows point to scytonemin associated to EPSs capsules, black circle indicates green cells in 20 g L−1 NaCl supplemented BG11 culture.
Phylogenetic study of scyC gene
The comparison of scy C gene sequence from Chroococcidiopsis sp. UAM571 (515 bp) using BLAST tool from NCBI showed the highest similarity with Chroococcidiopsis sp. CCMEE 029 strain (CP083761) with a percent of identity of 90.70%. This result was consistent with the phylogenetic study of this gene (Fig. 3) where UAM571 scyC sequence was grouped with the one belonging to CCMEE 029 strain and others from the same genus, Chroococcidiopsis. The subcluster I (cluster A) where the scyC sequence of this study was located showed an intra-group similarity range of 64–98%. Cluster A is mostly composed of cyanobacteria isolated from terrestrial environments (Anabaenopsis circularis NIES-21, Nostoc linckia NIES-25, Cyanothece sp. PCC7822, Chroococcidiopsis sp. CCMEE29), while cyanobacteria conforming Cluster B belong originally to both aquatic Rivularia sp. PCC 7116, Nodularia spumigena CCYA9414 and Nostoc edaphicum CCNP1411) and terrestrial environments (Cylindrospermum sp. NIES-4074, Nostoc sp. NIES-3756).
Phylogenetic tree according to the Maximum Likelihood principle based on the sequences of 48 cyanobacterial strains for the scyC gene. Sequences conforming cluster A (containing subclusters I and II) are highlighted in green. Sequences conforming cluster B (containing subclusters III and IV) are highlighted in yellow. The sequence of this study is highlighted in bright green. Sequence similarity range of each cluster and subcluster is indicated in brackets. Bootstrap values above 60% are indicated by orange sized dots at each branch.
Discussion
Cyanobacteria are known to produce a large variety of bioactive compounds and those inhabiting such an extreme habitat as the Atacama Desert, especially in terms of solar radiation, are expected to produce compounds to deal with that extreme UVR as scytonemin, a UV screening compound synthesized exclusively by certain members of this phylum. However, the challenge of isolating, characterizing, and growing these organisms under laboratory conditions results in scarce information about the genes involved in the biosynthetic pathway of this compound, and the influence of different abiotic factors on their production10,11,12. Thus, our study aims to evaluate growth and scytonemin production capacity of cyanobacterial strains from the Atacama Desert under different NaCl concentrations, and to find their position within the evolutionary context based on the scyC gene.
Eight endolithic cyanobacterial strains belonging to the genera Chroococcidiopsis, Gloeocapsa and Gloeocapsopsis previously isolated from gypcrete and calcite from the Atacama Desert25,26 were used in this study. After a preliminary screening based on their growth rate in control conditions and under different NaCl concentrations, Chroococcidiopsis sp. UAM571 was selected to specifically study its growth and scytonemin production capacity when exposed to NaCl. Growth rates of endolithic cyanobacteria at their original habitat remain undetermined, although Davila et al.27 described that, in halite, these microbial communities count on conditions for photosynthesis only 10 days per year. Despite the expected low growth rate for Chroococcidiopsis sp. UAM571 in its original habitat, the observed growth rates in this study without NaCl (0.24 day−1) were similar to other strains from the same genus under the same conditions, 0.11–0.4 day−110,28. However, this growth rate showed a 1.5 to tenfold difference with others from cyanobacteria isolated from aquatic environments such as Microcystis aeruginosa (0.29–0.62 day−1), Cylindrospermopsis raciborskii (0.67 day−1) and Anabaena flos-aquae (1.41 day−1)29,30.
Salinity is a crucial parameter in the screening for commercial production of cyanobacteria and microalgae since it can be utilized to cope with cross-contamination of the culture system by non-target microalgae/cyanobacteria and other microorganisms like bacteria, viruses, fungi and zooplankton (e.g., ciliates, copepods, rotifers)31. Despite salinity has been described as a limiting factor for growth in cyanobacteria isolated from non-hypersaline environments as the freshwater Microcystis aeruginosa, or Cylindrospermopsis sp. and Anabaena sp. from brackish environments, whose survival rates decreased at 6–15 g L−1 NaCl32, Chroococcidiopsis sp. UAM571 showed a broad tolerance to NaCl, up to 20 g L−1. Moreover, the observed growth rate at 20 g L−1 was only 1.7 times lower when compared to the control, similar to the one observed in Chroococcidiopsis sp. M88-VD-G for the same conditions (1.6 times)10, although its growth rate when exposed to NaCl was not as high as the one from the endolithic Leptolyngbya sp. ISTCY10 (0.25 day−1 at 25 g L−1 NaCl)33. Besides the effect in growth, microscopic observations and spectrophotometric quantification indicated higher production of EPSs in UAM571 related to NaCl exposure, which is coherent with previous findings in other cyanobacteria34,35. That higher EPSs production in Chroococcidiopsis sp. UAM571 exposed to NaCl presents another advantage for its commercial production since the EPSs make easier the self-flocculation and, therefore, the concentration of biomass in a smaller volume, decreasing the expenses derived from biomass recovery.
The influence of abiotic factors in scytonemin production is often studied by combining them with UVR exposure6,10,11,25, while only a few studies worked on osmotic stress as a single factor to induce scytonemin synthesis10,12. In this context, this study improves the knowledge in NaCl as a single stressor for scytonemin production, which, in addition to allowing mass cultivation as previously mentioned, will drop the production costs. The increasing NaCl concentration effect on scytonemin production by UAM571 was previously observed in M88-VD-G strain from the same genus10 and Lyngbya aestuarii12, that reached maximum scytonemin concentrations at 20 and 56 g L−1 NaCl respectively. The high tolerance and productivity observed in the latter cyanobacterial strain compared to Chroococcidiopsis sp. UAM571 could be explained by the isolation source of this Lyngbya aestuarii strain, a salty lagoon, thus this organism probably counts on adaptation strategies to grow and produce scytonemin exposed to NaCl. Considering the positive effect in scytonemin production by NaCl exposure, leading to a 53-fold increase in Chroococcidiopsis (this study10), it could be additionally multiplied by a factor of 10 when combined with UVR in this genus10. This points out to the combination of both factors (NaCl and UVR) as an interesting option to test in future studies in UAM571. However, any future experimental approach targeting biotechnological purposes should include detailed techno-economic analyses to evaluate whether the increased production with UVR counteracts the potentially higher costs of energy and specific materials (UVR light sources and transmitting materials for cyanobacterial growth) when simulating indoor large-scale cultivations.
The overall study of biomass and scytonemin productivity related to DW on Chroococcidiopsis sp. UAM571 suggests that, as a result of the increasing NaCl concentration, the relative quantity of EPS per cell would be higher35 providing a larger space for scytonemin to be deposited36. Besides this indirect effect of NaCl on scytonemin content via EPSs-synthesis triggering, it remains to be determined whether NaCl has a direct effect on scytonemin biosynthesis itself. One possibility may be to perform transcriptomics studies (e.g., with qPCR or RNA-Seq) selecting the most suitable scy genes once the scytonemin synthesis cluster is fully described in the genus Chroococcidiopsis.
Considering the growth and scytonemin production capacity of Chroococcidiopsis sp. UAM571 and the increasing biotechnological interest of scytonemin7,18,37 not yet commercially available, it seems crucial to estimate its potential mass productivity. With this purpose, we performed a simplified simulation of biomass (g DW m−2 day−1) and scytonemin productivity (mg m−2 day−1) based on our experimental data extrapolated to standard mass culturing devices including both a single system and two different multi-system arrangements (Table S2). Briefly, the single system would consist in a bubble column of 400 L and 0.3 m238, while each multi-system column arrangement would propose a different spatial distribution of those systems on an extension of 1 hm239,40. Therefore, single system growth of Chroococcidiopsis sp. UAM571 would produce a total biomass of up to 22 g DW m−2 day−1, which is similar to the biomass productivity found in commercially used cyanobacteria as Arthrospira spp. (8.2–27 g DW m−2 day−1) (rev.41) Anabaena sp. (9.4–23.5 g DW m−2 day−1)42. Regarding the pigments’ productivity, the highest scytonemin productivity would be 0.08 g·scytonemin m−2·day−1, about threefold lower than other cyanobacterial pigments with biotechnological interest as phycoerythrin and phycocyanin (2.6–2.8 g m−2 day−1)38 yet this might be counteracted by the higher potential market value of scytonemin compared to phycobiliproteins43. When simulating the multi-system arrangements displayed over 1 hm2, a biomass of 37–55 kg and up to 200 g of scytonemin could be produced by Chroococcidiopsis sp. UAM571 per day, even considering that productivity per m2 is reduced between 4 and 6 times depending on the arrangement (Table S2). In light of these promising estimates, future studies with UAM571 strain from Atacama may focus on performing indoor and outdoor cultivations to validate the actual profitability of mass producing scytonemin with this platform.
The biosynthetic pathway of scytonemin has not been studied deeply and annotated sequences of the genes involved in its production in NCBI GenBank are still scarce. However, the presence of the scyC gene has been published for at least 3 cyanobacterial genera, among them, Nostoc44,45,46, Chlorogloeopsis47 and Nodularia48,49 and could be found in whole-genome sequences of cyanobacterial strains belonging to other genera such as Chroococcidiopsis, Cyanothece, Anabaena, Calothrix and Scytonema. The use of specific primers for scyC amplification in the chosen cyanobacterial strain allowed to obtain a 515 bp sequence. The low sequence identity in 3 clusters of the phylogenetic tree (≤ 70%) (Fig. 3) point to scyC as a poorly conserved gene.Within the genus Chrooccocidiopsis, UAM571 is the only Atacama strain being the other strains isolated from Negev Desert (Israel) and from soils in Germany and China (Fig. 3), according to the information available for those sequences in the NCBI Genbank. It remains to be solved. Obtaining further scyC sequences from endolithic Atacama strains would help top disentangle whether they form a separate subgroup given the isolation and extreme characteristics of this Desert. Altogether thephylogenetic trends suggested by Fig. 3 highlight the need to further study the genes involved in scytonemin biosynthesis in different genera to better understand not just the evolutionary history of scytonemin synthesis but also the regulation and the influence of abiotic factors on its production.
In summary, according to the results achieved and the estimates, the production of scytonemin by the Atacama Desert endolithic cyanobacteria Chroococcidiopsis sp. UAM571 would be optimal at 20 g L−1 NaCl since it is between 3 and 5 times higher than when exposed to 10 g L−1 NaCl, while presenting a moderate biomass productivity. The obtained values of biomass and scytonemin productivity in this work allow its consideration for mass cultivation with biotechnological purposes following outdoor open cultivation approaches. This study also provides new prospects in phylogenomics of scytonemin biosynthetic pathway in the genus Chroococcidiopsis. Future studies elucidating its scy cluster infull might allow transcriptomics determination (e.g., via qPCR or RNA-Seq) of salinity effects, alone or in combination with UV exposure, on the production of this biotechnologically and ecologically relevant pigment while also providing new gene alternatives for its heterologous production in E. coli18.
Methods
Organisms and culture conditions
Growth experiments were conducted in 8 endolithic cyanobacterial strains isolated from samples taken in the Atacama Desert (Northern Chile) in 201525,26 belonging to 3 different genera (Chroococcidiopsis, Gloeocapsa and Gloeocapsopsis), different microhabitats (cryptoendolithic, chasmoendolithic and hypoendolithic) and substrates (gypcrete and calcite) (Table 3). Their identification at genus level and phylogenetic relationships based on 16S rRNA can be found in previous publications26 and the sequences are available in the National Center for Biotechnology Information GenBank under accession numbers MW544037–MW544043. All cyanobacterial strains are maintained in BG11 liquid culture media50, 25 °C and 7 μmol photons·m−2·s−1 as part of the Universidad Autónoma de Madrid culture collection.
Growth studies for strain selection
Ten-day growth curves were performed for the 8 study strains in 50 mL BG11 culture medium, under orbital shaking (135 rpm), at a temperature of 25 °C and constant light of 38.9 μmol photons m−2 s−1. An optical density at 750nm (OD750nm) of 0.1 was used as a starting point for all cultures performing biological triplicates for each strain. Growth was determined by measuring OD750nm with a UV–Vis spectrophotometer (Hitachi U-2000 Spectrophotometer) on days 3, 7 and 10 of the experiment.
Experiment on Chroococcidiopsis sp. UAM571
Effect of NaCl on growth
Fourteen-day growth curves were performed Chroococcidiopsis sp. UAM571 in 150 mL BG11 culture medium with 0, 10 and 20 g L−1 NaCl concentrations, performing biological triplicates. Conditions of starting point OD750nm, light, temperature and agitation were maintained as indicated in “Growth studies for strain selection” section.
Scytonemin content determination
Scytonemin content was analysed for each culture condition at days 7 and 14 of the experiment. The extraction and quantification were conducted as in Ref.25, with minor modifications. Briefly, cyanobacterial biomass of 5 mL of each experimental condition and replicate was collected through filtration on 0.2 µm pore-size polycarbonate filters (Cyclopore TM, Whatman). Filters were then suspended in 1:1 (v/v) methanol: ethyl acetate, agitated through vortex for 1 min and subjected to sonication bath for 10 min. Then the filters were removed, and the extracts incubated overnight at 4 °C in darkness to facilitate the extraction of scytonemin6. Finally, the extracts were filtered through 0.2 μm pore-sized sterilized syringe-driven filter (Symta, Madrid, Spain).
Scytonemin content was determined by measuring the scytonemin absorbance maximum at 384 nm, pooled carotenoids (490 nm), and that of chlorophyll a at (663 nm) and following previous equations from Ref.9. Extinction coefficient (ε) for scytonemin was 112.6 L g−1 cm−151. Absorbance values were corrected for residual scatter by subtracting the absorbance at 750 nm. All absorbance measurements were made on Genesys 150 UV–Visible Spectrophotometer (Thermo scientific). All scytonemin measurements were normalized to dry weight (DW) obtained as described in Ref.52.
Finally, light microscopy observations of the cells were also performed to follow the production of scytonemin via their change in colour over time (from blue-green to yellow–brown as more scytonemin accumulates) using a OLYMPUS BX63 microscope with a equipped with a Leica DC 300 F digital camera (Leica Microsystems, Germany). All images were obtained with OLYMPUS cellSens Dimension software.
Exopolysaccharides (EPSs) quantification
Exopolysaccharides were quantified in cultures exposed to 0 and 20 g NaCl L−1 for 14 days. 10-mL culture aliquots of each condition and replicate were centrifuged (10,000×g; 30 min) and the biomass pellet stored at − 20 °C until processed. Extraction and quantification of EPSs followed the methods by Singh et al.53 including spectrophotometric determination54. The concentration of EPS (mg L−1) was standardized to biomass (dry weight) and expressed as content in mg EPS g−1 DW to allow comparisons between conditions.
DNA extraction and, scyC gene PCR, sequencing, and phylogenetic analysis
Genomic DNA from the selected cyanobacterial strain was isolated with UltraClean Microbial DNA Isolation Kit (MO BIO Laboratories) following the manufacturer instructions. To increase cell lysis, 3 cycles of freeze-defreeze were performed using liquid nitrogen and a 60 °C thermal bath. PCR of the scyC gene from the scytonemin cluster was performed by using PfuTurbo Cx Hotstart DNA Polymerase (Agilent) and the primers specifically designed in this study scyChF (GTCTACTTCCATTGG) and scyChR (CCGCTTGATATTCAT) with a target region of 570 bp, applying 1 cycle of denaturalization at 95 °C for 1 min, followed by 35 cycles of 95 °C for 30 s, annealing step at 43 °C for 1 min and elongation at 72 °C for 1 min, finalizing with an elongation step at 72 °C for 5 min. Sequencing of PCR product was performed by using the kit Big DyeTM Terminator v3.1 Cycle Sequencing (Applied Biosystems) in a ABI Prism 3730 Genetic Analyzer (Applied Biosystems) at the Spanish National Center of Oncology Research (CNIO, Madrid).
For the phylogenetic analysis, obtained sequence of the scyC gene was aligned with sequences obtained from the NCBI GenBank (NCBI MegaBlast tool http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed 18.07.23) using the Clustal W 1.4 software55. The final alignment length was 515 bp. A phylogenetic tree was constructed in MEGA11 software 11.0.13 version56 using the Maximum Likelihood (ML) method. The best-fitting evolutionary model, chosen following the BIC (Bayesian Inference Criterion), was Tamura-3 model with gamma distribution57. 1000 bootstrap replicates were performed for the tree.
Statistical analysis
Statistical comparisons were performed using one- and two-factor ANOVA tests and the Bonferroni post-hoc test. A significance level of p = 0.05 was used in all cases. All statistical analyses were carried out using GraphPad Prism 9.1.0.221.
Data availability
The DNA sequence of Chroococcidiopsis sp. UAM571 scyC gene generated and analysed during the current study is available in the GenBank repository, under the Accession Number PP194459.
References
Neifar, M., Maktouf, S., Ghorbel, R. E., Jaouani, A. & Cherif, A. Extremophiles as source of novel bioactive compounds with industrial potential. In Biotechnology of Bioactive Compounds: Sources and Applications (eds Gupta, V. K. et al.) 245–267 (Wiley Blackwell, 2015).
Núñez-Montero, K. & Barrientos, L. Advances in antarctic research for antimicrobial discovery: A comprehensive narrative review of bacteria from antarctic environments as potential sources of novel antibiotic compounds against human pathogens and microorganisms of industrial importance. Antibiotics 7, 90. https://doi.org/10.3390/antibiotics7040090 (2018).
Okoro, C. K. et al. Diversity of culturable actinomycetes in hyper-arid soils of the Atacama Desert, Chile. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 95, 121–133 (2009).
Bratchkova, A. & Ivanova, V. Bioactive metabolites produced by microorganisms collected in Antarctica and the Arctic. Biotechnol. Biotechnol. Equip. 25, 1–7. https://doi.org/10.5504/bbeq.2011.0116 (2011).
Diffey, B. L. Solar ultraviolet radiation effects on biological systems. Phys. Med. Biol. 36, 299 (1991).
Rastogi, R. P. & Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 87, 244–256 (2014).
Rastogi, R. P., Madamwar, D. & Incharoensakdi, A. Sun-screening bioactive compounds mycosporine-like amino acids in naturally occurring cyanobacterial biofilms: Role in photoprotection. J. Appl. Microbiol. 119, 753–762 (2015).
Proteau, P. J., Gerwick, W. H., Garcia-Pichel, F. & Castenholz, R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 49, 825 (1993).
Garcia-Pichel, F. & Castenholz, R. W. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J. Phycol. 27, 395–409 (1991).
Dillon, J. G., Tatsumi, C. M., Tandingan, P. G. & Castenholz, R. W. Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.). Arch. Microbiol. 177, 322–331 (2002).
Fleming, E. D. & Castenholz, R. W. Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ. Microbiol. 9, 1448–1455 (2007).
Rath, J., Mandal, S. & Adhikary, S. P. Salinity induced synthesis of UV-screening compound scytonemin in the cyanobacterium Lyngbya aestuarii. J. Photochem. Photobiol. B 115, 5–8 (2012).
Singh, H. Desiccation and radiation stress tolerance in cyanobacteria. J. Basic Microbiol. 58, 813–826. https://doi.org/10.1002/jobm.201800216 (2018).
Soule, T., Stout, V., Swingley, W. D., Meeks, J. C. & Garcia-Pichel, F. Molecular genetics and genomic analysis of scytonemin biosynthesis in Nostoc punctiforme ATCC 29133. J. Bacteriol. 189, 4465–4472 (2007).
Klicki, K. et al. The widely conserved ebo cluster is involved in precursor transport to the periplasm during scytonemin synthesis in Nostoc punctiforme. Mbio 9(10), 2018. https://doi.org/10.1128/mBio (2018).
D’Agostino, P. M. et al. Bioinformatic, phylogenetic and chemical analysis of the UV-absorbing compounds scytonemin and mycosporine-like amino acids from the microbial mat communities of Shark Bay, Australia. Environ. Microbiol. 21, 702–715 (2019).
Balskus, E. P. & Walsh, C. T. An enzymatic cyclopentyl[b]indole formation involved in scytonemin biosynthesis. J. Am. Chem. Soc. 131, 14648–14649 (2009).
Pathak, J. et al. Cyanobacterial secondary metabolite scytonemin: A potential photoprotective and pharmaceutical compound. Proc. Natl Acad. Sci. India Sect. B Biol. Sci. 90, 467–481. https://doi.org/10.1007/s40011-019-01134-5 (2020).
Duan, Z. et al. Lentiviral shRNA screen of human kinases identifies PLK1 as a potential therapeutic target for osteosarcoma. Cancer Lett. 293, 220–229 (2010).
Zhang, G., Zhang, Z. & Liu, Z. Scytonemin inhibits cell proliferation and arrests cell cycle through downregulating Plk1 activity in multiple myeloma cells. Tumor Biol. 34, 2241–2247 (2013).
Wierzchos, J. et al. Adaptation strategies of endolithic chlorophototrophs to survive the hyperarid and extreme solar radiation environment of the Atacama Desert. Front. Microbiol. 6, 934 (2015).
Vítek, P. et al. Distribution of scytonemin in endolithic microbial communities from halite crusts in the hyperarid zone of the Atacama Desert, Chile. FEMS Microbiol. Ecol. 90, 351–366 (2014).
Cordero, R. R. et al. Ultraviolet radiation in the Atacama Desert. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 111, 1301–1313 (2018).
Wierzchos, J., Casero, M. C., Artieda, O. & Ascaso, C. Endolithic microbial habitats as refuges for life in polyextreme environment of the Atacama Desert. Curr. Opin. Microbiol. 43, 124–131. https://doi.org/10.1016/j.mib.2018.01.003 (2018).
Casero, M. C., Ascaso, C., Quesada, A., Mazur-Marzec, H. & Wierzchos, J. Response of endolithic chroococcidiopsis strains from the polyextreme Atacama Desert to light radiation. Front. Microbiol. 11, 614875 (2021).
Casero, M. C. et al. The composition of endolithic communities in gypcrete is determined by the specific microhabitat architecture. Biogeosciences 18, 993–1007 (2021).
Davila, A. F. et al. In situ metabolism in halite endolithic microbial communities of the hyperarid Atacama Desert. Front. Microbiol. 6, 153663 (2015).
Olsson-Francis, K. & Cockell, C. S. Use of cyanobacteria for in-situ resource use in space applications. Planet Space Sci. 58, 1279–1285 (2010).
Melero-Jiménez, I. J., Martín-Clemente, E., García-Sánchez, M. J., Bañares-España, E. & Flores-Moya, A. The limit of resistance to salinity in the freshwater cyanobacterium Microcystis aeruginosa is modulated by the rate of salinity increase. Ecol. Evol. 10, 5045–5055 (2020).
Thomas, M. K. & Litchman, E. Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia 763, 357–369 (2016).
von Alvensleben, N., Stookey, K., Magnusson, M. & Heimann, K. Salinity tolerance of Picochlorum atomus and the use of salinity for contamination control by the freshwater cyanobacterium Pseudanabaena limnetica. PLoS ONE 8, e63569 (2013).
Moisander, P. H., McClinton, E. & Paerl, H. W. Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb. Ecol. 43, 432–442 (2002).
Singh, J., Tripathi, R. & Thakur, I. S. Characterization of endolithic cyanobacterial strain, Leptolyngbya sp. ISTCY101, for prospective recycling of CO2 and biodiesel production. Bioresour. Technol. 166, 345–352 (2014).
Ozturk, S. & Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. 17, 595–602 (2010).
Bemal, S. & Anil, A. C. Effects of salinity on cellular growth and exopolysaccharide production of freshwater Synechococcus strain CCAP1405. J. Plank. Res. 40, 46–58 (2018).
Gao, X. Scytonemin plays a potential role in stabilizing the exopolysaccharidic matrix in terrestrial cyanobacteria. Microb. Ecol. 73, 255–258 (2017).
Singh, S. P., Kumari, S., Rastogi, R. P., Singh, K. L. & Sinha, R. P. Photoprotective and biotechnological potentials of cyanobacterial sheath pigment, scytonemin. Afr. J. Biotechnol. 9, 580–588 (2010).
Velu, C., Cirés, S., Alvarez-Roa, C. & Heimann, K. First outdoor cultivation of the N2-fixing cyanobacterium Tolypothrix sp. in low-cost suspension and biofilm systems in tropical Australia. J. Appl. Phycol. 27, 1743–1753 (2015).
Mirón, A. S., Gómez, A. C., Camacho, F. G., Grima, E. M. & Chisti, Y. Comparative evaluation of compact photobioreactors for large-scale monoculture of microalgae. In Progress in Industrial Microbiology Vol. 35 (eds Osinga, R. et al.) 249–270 (Elsevier, 1999).
Chini Zittelli, G., Rodolfi, L., Biondi, N. & Tredici, M. R. Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 261, 932–943 (2006).
Borowitzka, M. A. & Moheimani, N. R. Open pond culture systems. In Algae for Biofuels and Energy (eds Borowitzka, M. A. & Moheimani, N. R.) 133–152 (Springer, 2013).
Moreno, J., Vargas, M. Á., Rodríguez, H., Rivas, J. & Guerrero, M. G. Outdoor cultivation of a nitrogen-fixing marine cyanobacterium, Anabaena sp. ATCC 33047. Biomol. Eng. 20, 191–197 (2003).
Chini Zittelli, G., Lauceri, R., Faraloni, C., Silva Benavides, A. M. & Torzillo, G. Valuable pigments from microalgae: Phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. https://doi.org/10.1007/s43630-023-00407-3 (2023).
Chen, Z. et al. Genomic and transcriptomic insights into the habitat adaptation of the diazotrophic paddy-field cyanobacterium Nostoc sphaeroides. Environ. Microbiol. 23, 5802–5822 (2021).
Tippelt, A., Busche, T., Rückert, C. & Nett, M. Complete genome sequence of the cryptophycin-producing cyanobacterium Nostoc sp. strain ATCC 53789. Microbiol. Resour. Announc. 9, 10 (2020).
Fidor, A. et al. Nostoc edaphicum CCNP1411 from the Baltic Sea—A new producer of nostocyclopeptides. Mar. Drugs 18, 442 (2020).
Soule, T. et al. A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria. BMC Genom. 10, 1–10 (2009).
Teikari, J. E., Hou, S., Wahlsten, M., Hess, W. R. & Sivonen, K. Comparative genomics of the Baltic Sea toxic cyanobacteria Nodularia spumigena UHCC 0039 and its response to varying salinity. Front. Microbiol. 9, 299361 (2018).
Voß, B. et al. Insights into the physiology and ecology of the brackish-water-adapted cyanobacterium Nodularia spumigena CCY9414 based on a genome-transcriptome analysis. PLoS ONE 8, e60224 (2013).
Rippka, E., Deruelles, J. & Waterbury, N. B. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111, 1 (1979).
Garcia-Pichel, F., Sherry, N. D. & Castenholz, R. W. Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chiorogloeopsis sp.. Photochem. Photobiol. 56, 17–23 (1992).
Cirés, S., Wörmer, L., Timón, J., Wiedner, C. & Quesada, A. Cylindrospermopsin production and release by the potentially invasive cyanobacterium Aphanizomenon ovalisporum under temperature and light gradients. Harmful Algae 10, 668–675 (2011).
Singh, S., Verma, E., Niveshika, T. B. & Mishra, A. K. Exopolysaccharide production in Anabaena sp. PCC 7120 under different CaCl2 regimes. Physiol. Mol. Biol. Plants 22(4), 557–566 (2016).
Dubois, M., Gilles, K. A., Hamilton, J. K., Roberts, P. A. & Smith, F. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Tamura, K. Estimation of the Number of Nucleotide Substitutions When There are Strong Transition-Transversion and G+C-Content Biases. https://academic.oup.com/mbe/article/9/4/678/1254082 (1992).
Acknowledgements
This work has been co-funded by Comunidad de Madrid and European Regional Development Fund (FEDER) and the company Microalgae Solutions S.L. via the “Cheque Innovación” programme awarded to the project CYANOACTIVE; and by Comunidad de Madrid and Universidad Autonoma de Madrid via LIPEPSTREM project (SI3/PJI/2021-00461). M. C. Casero is recipient of a Juan de la Cierva-Formación postdoctoral grant FJC2020-044126-I funded by MCIN/AEI/10.13039/501100011033 and by European Union NextGenerationEU/PRTR. Our acknowledgement to Dr. Benito Gómez Silva (Universidad de Antofagasta, Chile) and his team for all their assistance in 2015 sampling campaign in Atacama Desert during their participation as research team members in project MINECO. REF. CGL2013-42509-P (Spanish Ministry of Economy Competitiveness, 2013-2016). We are grateful to Luis García Aguirre for his assistance with EPSs extraction in strain UAM571. We would also like to thank two anonymous reviewers for their constructive comments that allowed to improve a previous version of this manuscript. This manuscript has been released as a pre-print at Research Square (https://doi.org/10.21203/rs.3.rs-3899919/v1).
Funding
The funding was provided by Comunidad de Madrid (LIPEPSTREM project (SI3/PJI/2021-00461), 49/067123.9/20), Universidad Autonoma de Madrid (LIPEPSTREM project (SI3/PJI/2021-00461)), Ministerio de Ciencia e Innovación (Juan de la Cierva-Formación postdoctoral Grant (FJC2020-044126-I)), Agencia Estatal de Investigación (Juan de la Cierva-Formación postdoctoral grant FJC2020-044126-I), European Union NextGenerationEU/PRTR (Juan de la Cierva-Formación postdoctoral Grant (FJC2020-044126-I)), European Regional Development Fund (49/067123.9/20).
Author information
Authors and Affiliations
Contributions
M.C.C and M.A.H.: experiment design, lab work, data production, data interpretation, writing first draft of manuscript; J.P.R., D.V. and A.Q.: data interpretation, manuscript edit; S.C.: project design, experiment design, data interpretation, manuscript final edit. All authors reviewed and confirmed the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Casero, M.C., Herrero, M.Á., De la Roche, J.P. et al. Effect of salinity on scytonemin yield in endolithic cyanobacteria from the Atacama Desert. Sci Rep 14, 9731 (2024). https://doi.org/10.1038/s41598-024-60499-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60499-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.