Abstract
Chronic obstructive pulmonary disease (COPD) has a high prevalence and a major impact on health-related quality of life (HRQL). COPD exacerbations are an important cause of morbidity and mortality, affecting cardiovascular risk, and are associated with poorer health status. The aim of this study was to assess the association between cardiovascular risk (CVR) and HRQL, according to exacerbator or non-exacerbator phenotype. We undertook a cross-sectional, observational, descriptive study of 107 patients with COPD. Patients with two or more moderate exacerbations or one severe exacerbation in the previous year were considered as exacerbators. The CVR was calculated with the Framingham scale and SCORE (Systematic Coronary Risk Evaluation) and the HRQL was assessed with the generic questionnaire Short Form-36 Health Survey (SF-36), the St George Respiratory Questionnaire (SGRQ) and the COPD Assessment Test (CAT). Statistical analysis was done with SPSS version 26.0 for Windows. The SF-36 and the SGRQ showed lower values for the exacerbator phenotype, indicating a poorer quality of life. The CAT questionnaire showed values above 10 for the exacerbator phenotype, and lower values in the non-exacerbator group. After categorizing the sample according to their median age (65 years), we found a greater deterioration in HRQL in patients under 65 years of age according to the SF-36, the SGRQ and the CAT. We also detected differences in HRQL between non-exacerbator patients with a high CVR according to the Framingham (≥ 20%) and SCORE (≥ 5%) scales compared to those without this risk. A tendency towards worse HRQL was observed in non-exacerbator patients with a high CVR, which was statistically significant for the SGRQ impact domain on the SCORE scale. The CAT also showed a worse quality of life in non-exacerbator patients with a high CVR, which was significant in the Framingham model (Framingham high risk 8.41 vs non-high risk 6.05, p < 0.01). These differences were not observed in exacerbator patients. Our findings confirm that a high CVR influences HRQL in patients with COPD, especially in non-exacerbator patients with a high CVR, measured according to the SGRQ and the CAT.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease state characterised by the presence of persistent respiratory symptoms such as dyspnoea, cough and/or expectoration, exacerbations, and chronic airflow limitation. COPD is secondary to chronic exposure to tobacco smoke as the main causative agent, among other inhaled substances. Moreover, it is a chronic systemic inflammatory disease even in those patients who no longer smoke, leading to an increase in inflammatory cells in the airway, greater oxidative stress and, consequently, a higher prevalence of other chronic inflammatory diseases. The most prevalent comorbidities in COPD (hypertension, dyslipidaemia, diabetes mellitus) and its complications (heart failure, atrial fibrillation, stroke, retinopathy, neuropathy and ischaemic heart disease) condition quality of life1,2,3,4,5. Thus, the estimation of cardiovascular risk (CVR), which encompasses the main vascular risk factors, is an important indicator of the potential effects of these comorbidities in the development of cardiovascular disease and the best tool to establish priorities in cardiovascular prevention6.
Health-related quality of life (HRQL) is important in itself and represents one of the main aims of all health interventions7,8. Quality of life questionnaires are designed to provide standard measures of health impairment and must evaluate the gap between the current disease-related HRQL and the desirable lifestyle. There are both generic and specific quality-of-life questionnaires9. The SF-36 questionnaire has proved its validity in the evaluation of COPD patients and offers a good correlation with the baseline dyspnoea index, allowing assessment of the quality of life of COPD patients in relation to previous admissions and comorbidities10. Among the COPD-specific questionnaires, the SGRQ allows us to quantify the impact of airway diseases on the health condition and perceived well-being of respiratory patients11. It is sensitive to changes in disease progression and therapeutic response. The CAT also provides a quick, self-administered and simple way to measure the impact of COPD on HRQL12. Exacerbations in COPD and their severity affect many different factors related with the HRQL of these patients. As widely reported, patients who experience frequent exacerbations have a worse HRQL in comparison to patients who experience fewer exacerbations (< 2/year)13,14. Indeed, improving the HRQL is associated with a lower risk of exacerbations15.
Both comorbidities and exacerbations in COPD patients have a direct impact on the HRQL. In addition, they are an important cause of hospital morbidity and mortality and increase the risk of cardiovascular disease, including myocardial infarction and stroke16,17,18. A few studies have analysed the association between CVR and HRQL: a cohort of patients with hepatitis C was studied, finding a relation between patients with a high CVR and worse HRQL, though not significantly so19. Another study analysed mental disorders and CVR and their association with HRQL, where a subgroup with depressive disorders presented a higher CVR and worse HRQL results20. Very few studies in patients with COPD have found an association between CVR and HRQL, either globally or according to phenotype, associating a worsening HRQL in exacerbator patients due to the exacerbations themselves and hospital admissions rather than to other reasons, such as a high CVR.
A comparative analysis of the estimated CVR in COPD patients according to their phenotype (exacerbator or non-exacerbator) and its effect on HRQL is relevant as it may contribute to orienting different preventive strategies and, thus, a more efficient preventive intervention.
Our aim in this study was to establish whether there exists a relation between CVR and HRQL among patients with COPD according to exacerbator phenotype and whether differences exist between the two groups.
Material and methods
Study design
Observational, cross-sectional, descriptive, random study of 107 patients diagnosed with COPD and referred to the monographic COPD office at the Regional University Hospital of Malaga, Spain.
Inclusion and exclusion criteria
Inclusion: Age between 40 and 75 years, history of smoking greater > 10 packs/year, spirometry with post-bronchodilator FEV1/FVC ratio < 70%, post-bronchodilator FEV1 < 80%, clinical stability during the eight weeks prior to inclusion in the study (according to information from the patient and electronic medical history), ability to answer the questionnaires and availability to attend for the supplementary tests.
Exclusion: presence of concomitant pulmonary disease (such as pulmonary tuberculosis), malignant disease, recent cardiovascular event (within previous 6 months), presence of COPD exacerbation within 8 weeks of the study (in this case inclusion was postponed until 8 weeks had passed), patients who had previously participated in another study or clinical trial, and those who did not sign the informed consent.
The study was undertaken in accordance with the norms of good clinical practice and the ethical concepts of the Declaration of Helsinki (21). All the patients signed the informed consent and the study was accepted by the provincial Research Ethics Committee on 20 December 2013 with the code 6/2020 PI 10.
Data collection
A detailed medical record was elaborated with the following variables:
-
Demographic data: age, gender and marital status
-
Smoking habits
-
Personal history: Hypertension, dyslipidaemia, diabetes, cardiovascular disease (CVD), atrial fibrillation
-
Charlson comorbidity index22. Comorbidity was defined as any additional entity (disease, health condition) that has existed or may occur during the clinical course of a patient with a disease23
-
Treatments, including continuous home supplemental oxygen, long-acting beta2-agonist, long-acting muscarinic antagonist, or inhaled corticosteroids.
-
The Modified Medical Research Council (mMRC) dyspnoea scale, according to patient-reported data and data in the electronic medical record of the Andalusian Health Service (SAS). Based on these data, it was established whether the patient had an exacerbator or non-exacerbator phenotype. Exacerbators were considered patients with COPD who had had two or more exacerbations in the previous year, moderate (in which their usual treatment had been modified by adding antibiotics and/or corticosteroids) or severe (with hospitalisation). These exacerbations had to be at least 8 weeks apart from the conclusion of previous exacerbation treatment to avoid mistaking them for treatment failures1.
Physical examination:
-
Height and weight measured by stadiometer and scales (SECA 665, Seca), from which the body mass index (BMI) was calculated
-
Abdominal circumference, with a normal tape measure
-
Two blood pressure (BP) readings from the same arm employing a validated automatic device (OMROM M4-I, Omron Electronics, Hoofddorp, Netherlands) with an interval of 1–2 min between readings. The BP was measured with a manual sphygmomanometer with participants resting for at least five minutes. Three recordings were taken and the average of the second and third readings was used in statistical analyses25.
Analytical determinations:
Laboratory measurements were analysed in the Regional University Hospital of Malaga, obtaining a blood sample after 12-h fasting.
-
Complete blood count (haemoglobin, haematocrit, white blood cells, eosinophilic and differential blood count), coagulation, glucose, urea, creatinine, uric acid, sodium, potassium, stable glycosylated haemoglobin (HbA1c), cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides, transaminases, total proteins, serum albumin, immunoglobulin E, and alpha-1-antitrypsin.
Estimation of CVR.
CVR was estimated from the following models.
-
Framingham global CVR score, with a Framingham ≥ 20% considered a high CVR26,
-
European SCORE risk chart for low-risk countries27, following the previously published procedure and considering a SCORE ≥ 5% as a high CVR.
Respiratory function:
-
Spirometry and bronchodilator test. All the patients underwent a spirometry with a bronchodilator test following the guidelines of the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) for this technique20 with a JAEGER (OXICOM) pneumotachograph.
-
Static volumes: Measured using a JAEGER (OXICOM) plethysmograph, determining total lung capacity, residual volume and inspiratory capacity following the SEPAR Procedures Manual28,29,30.
-
A 6-min walk test (6MWT): recording metres, O2 saturation at the beginning and at the end of the test, initial and final heart rate, and the initial and final Borg Scale of dyspnoea perceived by the patient before and after exercise31. The 6MWT was carried out following the 2002 guidelines of the American Thoracic Society32 and always performed by the same nurse.
-
BODE used as a prognostic tool in COPD, which combines FEV1, distance travelled in the 6MWT, the mMRC Dyspnoea Scale value and BMI parameters33.
Quality of life questionnaires:
-
The Short Form-36 Health Survey (SF-36). This is a generic questionnaire composed of 36 items, examining eight dimensions of health status: physical functioning, social functioning, role limitations due to physical problems, role limitations due to emotional problems, mental health, vitality, pain and general health perception. The score ranges from 0 to 100 for each dimension, and does not allow an overall score to be calculated10,34.
-
The Saint George Respiratory Questionnaire (SGRQ), a specific tool to assess HRQL and quantify the impact of airway diseases on health status and perceived wellbeing of respiratory patients11,35. It is composed of 50 items, distributed in 3 dimensions: symptoms, activity and impact. The score ranges from 0 to 100, with 100 being the maximum change in HRQL; the minimum change considered clinically relevant has been set at 4 units36.
-
The Chronic Obstructive Pulmonary Disease Assessment Test (CAT), developed to assess the impact of COPD in HRQL simply and quickly, is a specific and self-administered questionnaire. Each item has 5 response options. The final score of the scale is equal to the sum of the scores for each item, with a range from 0 to 40, where a higher score indicates a greater impact of COPD on the patient quality of life12,37,38,39. We considered a score > 10 a worse quality of life. We established a time of 8 weeks between the onset of exacerbation and HRQL testing to ensure that the patient was in a stable phase.
Statistical analysis
Information was processed in a database developed specifically for this purpose. Statistical analysis was performed using SPSS software version 26.0 for Windows (IBM Corp., Armonk, NY, USA). Quantitative variables are expressed as the mean (measures of centralisation) ± standard deviation (measures of variability). In the case of qualitative variables, relative and absolute frequencies are used. Normality analysis was performed for all variables using the Kolmogorov–Smirnov test. For comparison between quantitative variables between groups, parametric (Student T-test) or non-parametric (Mann–Whitney U test) tests were used, as appropriate. Comparison between groups of qualitative variables was performed using the Chi-Square test or Fisher test, depending on whether or not the data followed a normal distribution. Univariate and multivariate logistic regression analyses were performed to assess the association between scores and the endpoint variables after adjustment for confounders. Variable associations were assessed by estimating the Pearson or Spearman correlation coefficient. Values of less than 0.05 (p < 0.05) for two tails were considered statistically significant differences.
Results
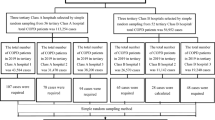
Of the 136 patients initially evaluated, 27 were excluded due to unavailability to perform the necessary examinations and another two were excluded because they did not meet the inclusion criteria. Out of the remaining 107 patients whose clinical history, laboratory tests and respiratory function tests were completed, the quality-of-life tests could not be performed in 3 patients for various reasons (see Fig. 1). Table 1 presents the general characteristics of the population by phenotype. Most patients (73.8%) were men, and the average age was 63.1 ± 6.6 years. The most frequent spirometry pattern was severe obstruction according to GOLD1, with a substantial level of air-trapping. Significant differences were found in diastolic blood pressure, which was higher in the non-exacerbator group (87.2 ± 12.4 vs 81.8 ± 11.5 mmHg; p = 0.022), and more so in the patients under 65 years of age. The average BODE Index25 was within the moderate stage, showing significant differences between the exacerbator and non-exacerbator groups (3.4 ± 1.6 vs. 2.6 ± 1.5; p = 0.018); 23.4% of the patients had CVD, 16.8% had diabetes mellitus, 37.4% had dyslipidaemia, and 40.2% had the metabolic syndrome. One in five had some comorbidity in addition to COPD and 15% had more than one comorbidity. In the Framingham and SCORE models, more than 50% were within the high or very high cardiovascular risk range18,19.
The generic SF-36 questionnaire showed lower values for the exacerbator phenotype (worse HRQL), reaching statistical significance in the scores for bodily pain (57.5 ± 35.2 vs. 73.7 ± 28.9; p = 0.033), physical functioning (41.4 ± 25.3 vs. 60.3 ± 24.7; p < 0.001), general health perception (31.4 ± 17.2 vs. 44.0 ± 20.7; p = 0.004) and vitality (44.1 ± 26.2 vs. 56.3 ± 24.2; p = 0.017). In the respiratory disease-specific questionnaires, the SGRQ also showed a worse HRQL for patients with the exacerbator phenotype, reaching statistical significance in all scores. The CAT questionnaire showed values above 10 in the exacerbator phenotype, indicating the need to change treatment or establish new therapeutic measures; these values were significantly higher than in the non-exacerbator phenotype (11.8 ± 6.1 vs. 7.4 ± 5.5; p < 0.001) (Table 2). A good correlation was found between the values of the specific questionnaires (SGRQ and CAT) for both phenotypes, as shown in Fig. 2. Table 3 shows the differences in the values of the quality-of-life questionnaires (SF-36, SGRQ and CAT) between patients who did or did not require hospital admission due to exacerbation of their COPD in the year prior to the study. A worse quality of life was found in those patients who had to be admitted, significantly so in the SGRQ scores for activity (70.7 ± 25.5 vs. 55.6 ± 24.2; p = 0.016) and symptoms (59.9 ± 23.8 vs. 45.2 ± 25.0) and in the CAT questionnaire (13.4 ± 7.8 vs. 8.6 ± 5.4; p = 0.017). When analysing the subject population according to the median age (65 years), a significant deterioration in HRQL was observed in the exacerbator phenotype in those patients under 65 years of age, where there was a greater worsening in the scores of bodily pain (56.5 ± 33.9 vs. 81.8 ± 21.1; p = 0.006), physical functioning (7.7 ± 25.9 vs. 59.8 ± 23.8; p = 0.004) and general health perception (30.2 ± 18.4 vs. 44.0 ± 23.3; p = 0.047) in the SF-36 questionnaire. In the SGRQ, the deterioration was greater in the scores for activity (69.8 ± 26.9 vs. 50.5 ± 23.0; p = 0.006) and impact (45.9 ± 24.5 vs. 30.2 ± 18.2; p = 0023) for the exacerbator subgroup under 65 years of age and in the symptoms score (56.9 ± 21.5 vs. 35.1 ± 20.0; p = 0.001) in the exacerbators over 65 years of age. The CAT questionnaire was also significantly worse in the exacerbators under 65 years of age (12.6 ± 6.0 vs. 7.1 ± 5.9; p = 0.001) (Table 4). Logistic regression analysis adjusted for confounders (Table 5) showed that the exacerbator phenotype and COPD severity were risk factors associated with worse HRQL.
When analysing differences in HRQL according to phenotype, but categorising patients according to CVR (high: Framingham scales ≥ 20% and SCORE ≥ 5%), we observed a tendency towards worse HRQL in non-exacerbator patients with a high CVR, which reached statistical significance for the SGRQ impact score on the SCORE scale (24.0 ± 17.7 vs. 32.9 ± 18.7; p = 0.039) (Fig. 3). These differences were not observed in exacerbator patients with a high CVR (Table 6). When relating this same aspect to the CAT questionnaire (Fig. 4), a worse HRQL was also observed in non-exacerbator patients with a high CVR, which was significant on the Framingham scale (6.1 ± 5.7 vs 8.41 ± 5.2; p = 0.043). These differences were not observed in exacerbator patients (Table 6).
Discussion
Our study found important differences in the CVR and HRQL between exacerbator and non-exacerbator patients, and particularly among patients younger than 65 years and those who had more hospitalisations. The patients with an exacerbator phenotype and the more severe patients experienced a greater worsening in overall HRQL than those with a non-exacerbator phenotype. In relation to the association between CVR and HRQL in our study, it was notable that the non-exacerbator patients with a high CVR were those with a worse HRQL. This finding has not previously been reported.
Our results on HRQL in patients with COPD exacerbations are largely consistent with the literature. Various studies have found an independent association of HRQL, determined by the SF-36 questionnaire, and the risk of hospitalisation and mortality in COPD patients10,40. In this study, the SF-36 variables related to physical aspects (pain, physical functioning and general health perception) were the most affected in the exacerbator phenotype. Furthermore, among those patients admitted during the previous year, there was a tendency towards worse values related to physical functioning and general health perception, further highlighting the importance of exacerbations and, therefore, the impact of lack of control of the disease on HRQL38.
The correlation between CAT and SGRQ is very good according to the literature, a circumstance confirmed in our study with a correlation in figures very similar to those described39. A study with patients with lung function characteristics very similar to ours found higher CAT values for both the exacerbator and the non-exacerbator groups and higher differences between the two phenotypes41.
This study confirms with multivariate analysis that patients with exacerbations have worse HRQL regardless of sex and age, as well as the fact that more severe patients (with worse BODE) have worse HRQL. Accordingly, the relevance of controlling the HRQL of our patients is as important as monitoring their lung function or exercise capacity and improving it is a fundamental objective of their treatment, an aspect currently considered in all clinical management guidelines in COPD1,2. However, when HRQL was analysed according to CVR, higher CVR values were related with worse HRQL in non-exacerbator patients, a finding not seen in the patients with more frequent exacerbations, whose HRQL was conditioned by their exacerbations.
A recent Canadian cohort study compared healthy people with people with pathological spirometry or in the PRISm (preserved ratio impaired spirometry) range, finding a significantly higher prevalence of CVD among those with impaired spirometry and COPD compared to those with normal spirometry. The prevalence of CVD was significantly higher in participants with PRISm results and GOLD stage II COPD, leading them to conclude that people with poor spirometry, especially those with moderate COPD and patients with PRISm, have a higher incidence of CVD compared to those with normal spirometry, such that having COPD increases the risk of developing CVD42. A previous study by our group analysed CVR in the total sample of COPD patients and found elevated values in all the scales used, both in exacerbator and non-exacerbator patients, but there were no significant differences according to clinical phenotypes43.
In our study, we found differences in HRQL between non-exacerbator patients with high CVR according to the Framingham (≥ 20%) and SCORE (≥ 5%) scales compared to those with no CVR. Although there are no studies in this regard, this could be due to the impact of CVD on HRQL44 and the finding of higher BP levels in our non-exacerbator population with high CVR. A Spanish study applied the SF-36 test to measure the quality of life and related it to CVR using the SCORE and European Society of Hypertension (ESH) tables. It was observed that in the SF-36 subscales “physical functioning” and “health change”, significantly higher values were obtained in subjects with a low-moderate risk SCORE (< 5%) compared to those with a high-risk SCORE (> 5%). In the analysis between hypertension and quality of life (SF-36) those without hypertension had higher scores on all subscales45,46. A meta-analysis of 20 observational studies concluded that hypertensive subjects have poorer levels of HRQL than non-hypertensive subjects47. In this regard, studies have linked a worsening HRQL with increasing comorbidities in COPD patients. Van Manen et al.48 showed that three or more comorbidities correlated better with HRQL scores than with FEV1 or dyspnoea measured by mMRC. Putcha et al. investigated the impact of comorbidities on HRQL, such that for each additional comorbidity, the odds of a deterioration in quality of life increased by 43%. The most prevalent comorbidities, such as heart failure, diabetes or osteoporosis, were individually associated with a significant decrease in quality of life score, adjusted for age, sex, race and other comorbidities49,50,51,52.
Currently, the analysis of cardiovascular risk factors (CVRF) has great value in defining a common strategy and preventive measures to avoid, from an early age, CV worsening in our COPD patients, so that a better HRQL is associated with a lower CVR53. It is important to recognise and treat CVD and the CVRF (like smoking, cholesterol and hypertension) as soon as possible. The adequate treatment of hypertension54, diabetes or dyslipidaemia, according to the level of estimated CVR, can contribute to improving HRQL in that it reduces the associated complications5,6,55. The GOLD strategy document1 establishes that the presence of comorbidities should not, in general, alter the treatment of COPD and that the comorbidities should be treated following usual guidelines, independently of the presence of COPD. Other specialities are aware of the importance of identifying and treating the key comorbidities. For example, the American Diabetes Association guidelines on diabetes recognise the need to go beyond just the glycaemia, highlighting the importance of efficient control of hypertension in patients with diabetes56.
One of the most notable limitations of our study concerns the fact that the sample was selected from a specific COPD clinic with the patients in advanced stages of the disease, which limits the extrapolation of the results to all COPD patients. Another concern is that the application of any CVR scale has limitations for estimating the overall CVR at the individual level, as its accuracy does not exceed 60%. However, we have used several different scales to attempt to obtain an estimate of CVR as broad and reliable as possible. The sample size may have been insufficient, as we could have obtained greater differences between the two phenotypes with a larger population. Nor did we have a control group of smokers without airway obstruction, which makes it difficult to analyse the results in aspects such as CVR and HRQL, which may be attributable either to smoking or to a direct consequence of the disease. This study is nevertheless relevant as it is one of the few studies to examine CVRF, CVR and quality of life in patients with COPD. Judging by the results of the study, it is already known that exacerbators have a worse quality of life independently of their CVR, but it was the non-exacerbators with a high CVR (poorly studied to date) who have their quality of life restricted and in whom we could undertake an early preventive intervention.
In conclusion, non-exacerbator patients, hypertense and with a high CVR, showed a worse quality of life as measured by the most used HRQL questionnaires. These findings could allow us to control CVRFs (hypertension) more strictly in non-exacerbator COPD patients, so that we can improve their quality of life with certain interventions aimed at reversing a possible worse prognosis.
Data availability
Date are available on request due to privacy restrictions. The data presented in this study are available on request from the corresponding author. In compliance with Spanish Organic Law 15/1999, the data are not publicly available.
References
Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2023 Report. GOLD Executive Summary. Disponible en: https://goldcopd.org/2023-gold-report-2/ [último acceso, 16 de abril de 2023]
López-Campos, J. L. et al. Actualización de la Guía Española de la EPOC (GesEPOC): comorbilidades, automanejo y cuidados paliativos. Arch Bronconeumol. 58, 334–344 (2022).
Jing, et al. Related factors of quality of life of type 2 diabetes patients: a systematic review and meta-analysis. Health Qual. Life Outcomes 16, 189. https://doi.org/10.1186/s12955-018-1021-9 (2018).
Redfield, M. M. & Borlaug, B. A. Heart Failure with preserved ejection fraction. A review. JAMA JAMA. 329(10), 827–838. https://doi.org/10.1001/jama.2023.2020 (2023).
Du, Y. et al. Health-related quality of life and associated factors in elderly individuals with dyslipidemia in rural Northern China. Qual. Life Res. 32, 3547–3555. https://doi.org/10.1007/s11136-023-03489-9 (2023).
Chantzaras, A. & Yfantopoulos, J. Association between medication adherence and health-related quality of life of patients with hypertension and dyslipidemia. Hormones 22, 665–676. https://doi.org/10.1007/s42000-023-00471-5 (2023).
Putcha, N. et al. Comorbidities and chronic obstructive pulmonary disease: Prevalence, influence on outcomes, and management. Semin. Respir. Crit. Care Med. 36, 575–591 (2015).
Quittner, A. L. Measurement of quality of life in cystic fibrosis. Curr. Opin. Pulm. Med. 4(6), 326–331 (1998).
Sanjuas, C. Instrumentos de medida de la calidad de vida: ¿son preferibles los genericos o los especificos?. FMC. 18(7), 452–454 (2011).
Sprenkle, M. D., Niewoehner, D. E., Nelson, D. B. & Nichol, K. L. The Veterans Short Form 36 questionnaire is predictive of mortality and health-care utilization in a population of veterans with a self-reported diagnosis of asthma or COPD. Chest. 126(1), 81–89 (2004).
Jones, P. W., Quirk, F. H., Baveystock, C. M. & Littlejohns, P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am. Rev. Respir. Dis. 145(6), 1321–1327 (1992).
Jones, P. W., Tabberer, M. & Chen, W. H. Creating scenarios of the impact of COPD and their relationship to COPD assessment test (CAT) scores. BMC Pulm. Med. 11, 42 (2011).
Seemungal, T. A. et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 157, 1418–1422 (1998).
Solem, C. T. et al. Exacerbation-related impairment of quality of life and work productivity in severe and very severe chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 8, 641–652 (2013).
Martin AL, Marvel J, Fahrbach K, Cadarette SM, Wilcox TK, Donohue JF. (2020) The association of lung function and St George's Respiratory Questionnaire with exacerbations in COPD: a systematic literature review and regression analysis. Respir JR Hurst et al European Journal of Internal Medicine 73 (1–6): 5
Miller, J. et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 107, 1376–1384 (2013).
Kunisaki, K. M. et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am. J. Respir. Crit. Care Med. 198, 51–57 (2018).
Goto, T., Shimada, Y. J., Faridi, M. K., Camargo, C. A. Jr. & Hasegawa, K. Incidence of acute cardiovascular event after acute exacerbation of COPD. J. Gen. Intern. Med. 33, 1461–1468 (2018).
McPherson, S. et al. Increased cardiovascular risk and reduced quality of life are highly prevalent among individuals with hepatitis C. BMJ Open Gastro. 7, e000470. https://doi.org/10.1136/bmjgast-2020-000470 (2020).
Quintí Foguet Boreua, Pere Roura Pocha, Anna Bullón Chiaa. Factores de riesgo cardiovascular, riesgo cardiovascular y calidad de vida en pacientes con trastorno mental severo. Aten Primaria. 2013; 45(3):141---148
World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects JAMA. 2013;310(20):2191-2194. doi:https://doi.org/10.1001/jama.2013.281053
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40(5), 373–383 (1987).
Feinstein, A. R. The pre-therapeutic classification of co-morbidity in chronic disease. J. Chronic Dis. 23(7), 455–468 (1970).
Bestall, J. C. et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 54(7), 581–586 (1999).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. Eur. Heart J. 39, 3021–3104 (2018).
D’Agostino, R. B. Sr. et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 117(6), 743–753 (2008).
Conroy, R. M. et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 24(11), 987–1003 (2003).
Garcia-Rio, F. et al. Espirometria. Normativa Sociedad Espanola de Patologia Respiratoria. (SEPAR). Arch Bronconeumol. 49(9), 388–401 (2013).
Roca, J. et al. References values for forced spirometry. Group of the European Community Respiratory. Health Survey. Eur Respir J. 11(6), 1354–1362 (1998).
Compte, L., Macian, V., Blanco, M. & Rodriguez, M. Manual SEPAR de procedimientos de evaluacion de la funcion pulmonar. Volumenes pulmonares. Luzan 5, 37–66 (2002).
Borg, G. A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 14(5), 377–381 (1982).
Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 166(1), 111–117 (2002).
Celli, B. R. et al. The body-mass index airflow obstruction dyspnea and exercise capacity index in chronic obstructive pulmonary disease. N Engl. J. Med. 350(10), 1005–1012 (2004).
Vilagut, G. et al. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: componentes físico y mental. Med Clin (Barc). 130(19), 726–735 (2008).
Ferrer, M. et al. Validity and reliability of the St George’s Respiratory Questionnaire after adaptation to a different language and culture: the Spanish example. Eur Respir J. 9(6), 1160–1166 (1996).
Ferrer, M. et al. Interpretation of quality of life scores from the St George’s Respiratory Questionnaire. Eur. Respir. J. 19(3), 405–413 (2002).
Jones, P. W. Quality of life measurement for patients with diseases of the airways. Thorax. 46(9), 676–682 (1991).
Jones, P. W. et al. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 34(3), 648–654 (2009).
Tsiligianni, I. G. et al. Assessing health status in COPD A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). BMC Pulm Med. 12, 20 (2012).
Fan, V. S., Curtis, J. R., Tu, S. P., McDonell, M. B. & Fihn, S. D. Ambulatory care quality improvement project I. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest. 122(2), 429–436 (2002).
Varol, Y., Ozacar, R., Balci, G., Usta, L. & Taymaz, Z. Assessing the effectiveness of the COPD assessment test (CAT) to evaluate COPD severity and exacerbation rates. COPD. 11(2), 221–225 (2014).
Krishnan, S., Tan, W. C. & Farias, R. Impaired spirometry and COPD Increase the risk of cardiovascular disease a canadian cohort study. Chest https://doi.org/10.1016/j.chest.2023.02.045 (2023).
Domenech, A. et al. High risk of subclinical atherosclerosis in COPD exacerbator phenotype. Respiratory Medicine 141, 165–171 (2018).
Yeo, J., Karimova, G. & Bansal, S. Co-morbidity in older patients with COPD: its impact on health service utilisation and quality of life, a community study. Age Ageing. 35(1), 33–37 (2006).
Alonso sáenz de miera MJ, et al. análisis de la calidad de vida y su relación con el riesgo cardiovascular en una población mediterránea con bajo riesgo. Clin Invest Arterioscl. 21(6), 268–272 (2009).
Arija, V., Villalobos, F. & Pedret, P. Physical activity, cardiovascular health, quality of life and blood pressure control in hypertensive subjects: randomized clinical trial. Health Qual. Life Outcomes 16, 184. https://doi.org/10.1186/s12955-018-1008-6 (2018).
Trevisol, D. J., Moreira, L. B., Kerkhoff, A., Fuchs, S. C. & Fuchs, F. D. Health-related quality of life and hypertension: a systematic review and meta-analysis of observational studies. J. Hypertens. 29, 179–188. https://doi.org/10.1097/HJH.0b013e328340d76f) (2011).
Van Manen, J. G. et al. Added value of co-morbidity in predicting health-related quality of life in COPD patients. Respir. Med. 95(6), 496–504 (2001).
Putcha, N., Puhan, M. A., Hansel, N. N., Drummond, M. B. & Boyd, C. M. Impact of co-morbidities on self-rated health in self-reported COPD: an analysis of NHANES 2001 2008. COPD. 10(3), 324–332 (2013).
Chai, C.-S. et al. Clinical phenotypes of COPD and health-related quality of life: a cross-sectional study. Int. J. Chron. Obstruct. Pulmon. Dis. 1(14), 565–573. https://doi.org/10.2147/COPD.S196109 (2019).
Smith, M. C. & Wrobel, J. P. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 9, 871–888 (2014).
Grigoryeva, N. Y., Maiorova, M. V., Korolyova, M. E. & Samolyuk, M. O. Comorbidity and polymorbidity of the patient with chronic obstructive pulmonary disease and cardiovascular diseases. Ter Arkh. 91(1), 44–47 (2019).
Lobos Bejaranoa, J. M. & Brotons, C. C. Factores de riesgo cardiovascular y atención primaria: evaluación e intervención. Aten Primaria. 43(12), 668–677 (2011).
Parikh, M. A. et al. Angiotensin-converting inhibitors and angiotensin II receptor blockers and longitudinal change in percent emphysema on computed tomography. The Multi-Ethnic Study of Atherosclerosis lung study. Ann. Am. Thorac. Soc. 14, 649–658 (2017).
Rabe, K. F., Hurst, J. R. & Suissa, S. Cardiovascular disease and COPD: dangerous liaisons?. Eur Respir Rev 27, 180057. https://doi.org/10.1183/16000617.0057-2018] (2018).
Standards of medical care in diabetes – 2014. Diabetes Care 2014; 37: Suppl. 1, S14–S80.
Funding
This project was funded with NEUMOSUR grant 9/2013.
Author information
Authors and Affiliations
Contributions
AM, AD, PVF, MASCH, and CO contributed to the design of the study. PVF and MASCH assessed cardiovascular risk, while CO focused on evaluating quality of life according to phenotypes. AM and AD also participated in recruitment, patient data collection, and drafting the main manuscript. P.R-E conducted statistical analysis and prepared tables and figures. All authors reviewed manuscript versions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montiel, A.M., Ruiz-Esteban, P., Del Río, A.D. et al. Differences in cardiovascular risk and health-related quality of life in COPD patients according to clinical phenotype. Sci Rep 14, 9687 (2024). https://doi.org/10.1038/s41598-024-60406-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60406-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.