Abstract
We aimed to analyze the risk factors and construct a new nomogram to predict non-sentinel lymph node (NSLN) metastasis for cT1-2 breast cancer patients with positivity after sentinel lymph node biopsy (SLNB). A total of 830 breast cancer patients who underwent surgery between 2016 and 2021 at multi-center were included in the retrospective analysis. Patients were divided into training (n = 410), internal validation (n = 298), and external validation cohorts (n = 122) based on periods and centers. A nomogram-based prediction model for the risk of NSLN metastasis was constructed by incorporating independent predictors of NSLN metastasis identified through univariate and multivariate logistic regression analyses in the training cohort and then validated by validation cohorts. The multivariate logistic regression analysis revealed that the number of positive sentinel lymph nodes (SLNs) (P < 0.001), the proportion of positive SLNs (P = 0.029), lymph-vascular invasion (P = 0.029), perineural invasion (P = 0.023), and estrogen receptor (ER) status (P = 0.034) were independent risk factors for NSLN metastasis. The area under the receiver operating characteristics curve (AUC) value of this model was 0.730 (95% CI 0.676–0.785) for the training, 0.701 (95% CI 0.630–0.773) for internal validation, and 0.813 (95% CI 0.734–0.891) for external validation cohorts. Decision curve analysis also showed that the model could be effectively applied in clinical practice. The proposed nomogram estimated the likelihood of positive NSLNs and assisted the surgeon in deciding whether to perform further axillary lymph node dissection (ALND) and avoid non-essential ALND as well as postoperative complications.
Similar content being viewed by others
Introduction
Breast cancer is the most common malignancy in women worldwide and seriously threatens women's physical and mental health1,2. Axillary lymph node (ALN) metastasis is the earliest and most common metastatic pathway of breast cancer, and ALN status is one of the most important prognostic factors in breast cancer3,4,5. In recent years, sentinel lymph node biopsy (SLNB) has become a common method of assessing the axilla's status to achieve minimal trauma and the most efficient treatment6. SLNB can be a safe alternative to axillary lymph node dissection (ALND) when the sentinel lymph node (SLN) is negative7,8, and the management of the axilla when the SLN is positive is currently a hot topic of research9. According to the ACOSOG Z0011 trial, no axillary surgical treatment is recommended for patients with 1–2 positive SLNs who undergo breast-conserving surgery combined with postoperative radiotherapy. AMAROS and IBCSG23-01 offer evidence in favor of axillary management for patients with SLN micrometastases. However, the management of the axilla in patients undergoing mastectomy with 1–2 positive SLNs remains controversial.
SLN-positive patients inevitably undergo ALND, despite postoperative complications that can seriously affect the quality of life, such as arm/shoulder mobility restriction, lymphedema, numbness, and paresthesia10. Unfortunately, some studies have shown that 20–60% of SLN-positive patients have negative non-sentinel lymph nodes (NSLN) after ALND11,12,13. The feasibility of omitting ALND in patients with 1–2 SLN-positive has also been illustrated by the fact that some studies have shown no differences in disease-free survival (DFS) and overall survival (OS) between patients who undergo ALND and those who do not14,15,16. Therefore, it is urgent to develop a predictive model to assess the risk of NSLN metastasis and identify patients at low risk. However, how do surgeons determine if a patient with positive SLNs needs further axillary surgery?
Some conventional models could assess the NSLN metastasis in breast cancer patients who are SLN-positive, but the majority of these predictive models were developed using data from Western populations17,18. The effects of biomarkers, heredity, lifestyle, and socio-economic status on breast cancer vary among different races19,20,21,22. Additionally, there are fewer NSLN metastasis prediction models based on breast cancer in the Chinese population23,24. Several studies have assessed the predictive capacity of established models in predicting ALN metastasis in Chinese women breast cancer patients, but they found inconsistencies when compared to the original studies7,25,26. Xu Guo et al27,28,29. developed a prediction model that combined axillary ultrasonography (AUS) with deep learning radionics (DLR). The model could be conveniently applied in primary hospitals. However, its predictive capacity was low when imaging was negative due to inherent ultrasound defects. Ke Xiang et al14. developed a model to predict the risk of NSLN metastasis, but they used a limited number of cases and lacked external validation.
Therefore, predictors and prediction models for NSLN metastasis in cT1-2 breast cancer patients need further research. In this study, we developed a new nomogram to assess the risk of NSLN metastasis, enabling surgeons to avoid unnecessary ALND by screening patients with a low risk of NSLN metastasis.
Results
Clinical and pathological characteristics
A total of 1,765 cT1-2 breast cancer patients with SLN metastases during the review period and 935 of these cases were excluded according to the exclusion criteria (48 cases for received neoadjuvant therapy, 584 for not receiving ALND, 289 for non-invasive breast cancer, and 14 for missing clinical data). Finally, 830 cT1-2 breast cancer patients with positive SLNs were enrolled and were assigned to the training cohort (n = 410), internal validation cohort (n = 298), and external validation cohort (n = 122) according to the periods and centers. Of the 830 patients enrolled, 525 patients (63.3%) had one positive SLN, 206 (24.8%) had two positive SLNs, and 99 (11.9%) had three or more positive SLNs. The average age in the training, internal validation, and external validation cohorts was 51.0 ± 11.0, 50.7 ± 10.8, and 52.1 ± 9.9 years, respectively. The mean tumor size was 1.85 ± 0.95, 2.00 ± 0.87, and 1.97 ± 0.95 cm in the corresponding cohorts. The number of positive SLNs was 1.56 ± 0.89, 1.52 ± 0.82, and 1.44 ± 0.75, and the positive rate of NSLN metastasis was 26.8%, 24.2%, and 38.5% in the training, internal validation, and external validation cohorts. Descriptive characteristics of the population are presented in Table 1 and Fig. 1.
Independent predictors for NSLN metastasis
The univariate analysis based on clinicopathologic information was conducted to explore the potential predictors of NSLN metastasis in the training cohort. As shown in Table 2, the number of positive SLNs (P < 0.001), the proportion of positive SLNs (P < 0.001), lymph-vascular invasion (P = 0.001), perineural invasion (P = 0.003), and ER status (P = 0.029), were detected to be significantly associated with NSLN metastasis. These five variables were further incorporated into multivariate logistic regression analyses. The results showed that the number of positive SLNs (P < 0.001; OR: 1.801; 95% CI: 1.295–2.506), the proportion of positive SLNs (P = 0.029; odds ratio (OR): 3.671; 95% confidence intervals (CI): 1.139–11.832), lymph-vascular invasion (P = 0.029; OR: 1.790; 95% CI: 1.063–3.016), perineural invasion (P = 0.023; OR: 1.984; 95% CI: 1.098–3.583), and estrogen receptor (ER) status (P = 0.034; OR: 3.164; 95% CI: 1.092–9.165) were identified as independent risk factors for NSLN metastasis. Therefore, factors such as the positive number of SLNs > 2, the proportion of positive SLNs > 50%, lymph-vascular invasion, perineural invasion, and ER-positive were considered risk factors for NSLN metastasis.
Nomogram construction
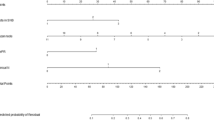
Based on the five risk factors obtained from the multivariate analysis in the training cohort, the model was presented as a visible nomogram. As shown in Fig. 2, the number of positive SLNs had the greatest effect on NSLN metastasis with a maximum score of 100 points. Next in influence was the proportion of positive SLNs with a maximum score of 50 points. The effect of ER status has a maximum score of 38 points. The effects of perineural invasion and lymph-vascular invasion were not negligible with 29 and 12 points, respectively.
Nomogram predicting the probability of NSLN metastasis. The five variables were located in rows 2–6 of the nomogram. The corresponding scores were obtained by inputting each of the patient's characteristics into the nomogram and drawing an upward line to row 1 (Points). The scores were then summed and a line was drawn downwards to row 7 (Total points) to obtain the total score. Put the total score in a downward line to row 8 (Risk of NSLN metastasis) to determine the risk of NSLN metastasis.
Model validation
To evaluate the predictive capacity of the nomogram model for the NSLN metastasis risk, we used the receiver operating characteristic (ROC) curve. As shown in Fig. 3a,d,g, the area under the curve (AUC) was 0.730 (95%CI: 0.676–0.785), 0.701 (95%CI: 0.630–0.773), and 0.813 (95% CI: 0.734–0.891) in the training, internal validation, and external validation cohorts. The calibration curve and the decision curve analysis (DCA) were plotted to assess the nomogram's effectiveness. The calibration curves (Fig. 3b,e,h) illustrated a strong agreement between the predicted and observed values across all three cohorts. The DCA demonstrated more benefits within the probability range of 10%-70%, as shown in Fig. 3c,f,i. Overall, the nomogram shows a good ability to discriminate and calibrate.
Nomogram verification for training cohort (a–c), internal validation cohort (d–f), and external validation cohort (g-i). a, d, g: ROC curves. b, e, h: calibration curves. The "ideal" line as the reference standard. The "apparent" line shows the agreement between the observed and predicted probabilities. The "bias-corrected" line shows the agreement between the corrected predicted and the observed probabilities. A closer proximity of the apparent or bias-corrected line to the ideal line indicates better consistency between predicted values and actual values. c, f, i: DCA curves. The "treat all" line represents the assumption that all non-sentinel lymph nodes of the patients were positive. The "treat none" line represents the assumption that none non-sentinel lymph nodes of the patients were positive. The "model" line represents the nomogram. The DCA curve lies above the "none" and "all" baselines in the threshold probability range of 0.1 to 0.7, indicating acceptable model performance in this range. ROC: receiver operating characteristics; AUC: areas under the ROC curve; CI: confidence interval; DCA: decision curve analysis.
Among the training cohort, at a cutoff of 14% for the risk of NSLN metastasis (with total points of 50), there were 106 cases reported as "negative", of which 98 cases were true negatives based on the results of ALND and 8 cases that were false negatives, and 304 reported as positive (102 true positives and 202 false positives). The Youden Index ranges from − 1 to 1, and a value closer to 1 indicates a better discriminatory ability of the test. The cutoff value of 26% is determined based on the maximum Youden index. The sensitivity, specificity, false-negative rate, false-positive rate, and negative predictive value of different cutoffs are shown in Table 3. The false-negative rate below 10% is generally considered acceptable. Considering the clinical application, we set the cut-off value for the nomogram at 14%, which means that if the risk of NSLN metastasis is less than 14% (50 points in total), it is recommended to omit ALND.
Discussion
The concept of breast cancer treatment is gradually shifting towards precision and individualized treatment, and how to maximize the benefits of treatment while minimizing the trauma is a goal that surgeons have been pursuing30. The Z0011 trial results have influenced treatment decisions in breast cancer by allowing SLN-positive patients who underwent breast-conserving surgery combined with postoperative radiotherapy to omit ALND31. In our study, approximately 14.8% (123/830) of patients received breast-conserving surgery. Although our study focused on patients who may not be fully matched to the Z0011 trial, it provided a more representative perspective on breast cancer in the Chinese population. Notably, nearly 60% of breast cancer patients with positive SLNs in some studies did not show NSLN metastases in further ALND. Within our study population, 72.4% had no detectable NSLN metastases. To identify SLN-positive and NSLN-negative patients, we analyzed the clinical and pathological characteristics of SLN-positive but NSLN-negative patients, incorporated meaningful factors for further analysis, and constructed a visual nomogram.
Through univariate analysis and multivariate logistic regression analysis in the training cohort, we have identified five parameters that are associated with NSLN metastasis: the number of positive SLNs, the proportion of positive SLNs, lymph-vascular invasion, perineural invasion, and ER status. Consistent with previous studies, a high number of positive SLNs and a high proportion of positive SLNs were associated with NSLN metastases7,15,32. An increased number of SLN metastases usually indicates a higher likelihood of lymph node involvement, which predicts an increased risk of NSLN metastases. However, the number of SLNs detected during surgery is influenced by the experience of the surgeon, the usage of the tracer, and anatomical differences in the patient. A higher proportion of positive SLNs may lead to an overestimation of the risk of NSLN metastasis if too few SLNs are detected during surgery. To reduce the bias resulting from the reasons mentioned above, the majority (797, 96.0%) of SLNs detected during surgery in our study were two or more. To further explore the occurrence of NSLN metastasis in patients with 1–2 SLN metastases, we divided the training cohort of patients with 1 or 2 SLN metastases into the 1-SLN-positive group and the 2-SLN-positive group. We found that 2-SLN-positive patients had a higher NSLN metastasis rate (31.7% vs. 17.7%, P = 0.004). Positive lymph-vascular invasion and perineural invasion indicate a more aggressive tumor with greater metastatic potential. Consistent with previous research33,34,35, our multivariable analyses showed that lymph-vascular invasion and perineural invasion were independent risks of NSLN metastasis. Currently, the association between ER status and NSLN metastasis is contentious36. Several studies have shown a negative association between ER status and NSLN metastasis14,35,37,38. In our investigation, ER positivity was a risk factor for NSLN metastasis, which is consistent with the findings of the study conducted by Alsumai et al34,39,40.
Furthermore, there was no significant connection between HER-2 status and NSLN metastasis found in our study (P = 0.644), which differs from the findings of Zhao et al35,38,40,41. An increased Ki-67 index is usually accompanied by a highly metastatic state42. However, our study did not find a relationship between Ki-67 and NSLN metastasis, which differs from the findings of the study conducted by Augusto Pereira et al3,43. Inconsistent conclusions may be attributed to ethnic differences and require further research.
Several models have been proposed to predict the presence of NSLN metastasis in breast cancer patients. The MSKCC nomogram contains eight predictors with an AUC of 0.711–0.761 in the Chinese population7,10,13,25. Including fewer parameters in the nomogram than in the MSKCC nomogram would not only reduce the interactions caused by the inclusion of too many factors but also provide greater practicality for real-world applications. Our nomogram was composed of five variables which included the number of positive SLNs, the proportion of positive SLNs, lymph-vascular invasion, perineural invasion, and ER status. The established nomogram had an excellent performance in external validation, suggesting its wide applicability. For patients with a low risk of NSLN metastasis, omission of ALND may be considered. Moreover, a false-negative rate of less than 10% is acceptable clinically25. When the predicted cutoff value in our nomogram was 14%, the sensitivity, specificity, false-negative rate, and false-positive rate were 92.7%, 32.7%, 7.3%, and 67.3%. Therefore, considering the clinical application and false-negative rate, the cutoff value of the nomogram is finally set to 14%, which means that if the NSLN metastatic rate was less than 14% according to our prediction nomogram, the exemption of ALND would be recommended. For example, a 65-year-old breast cancer patient who is ER-positive with negative lymph-vascular invasion and perineural invasion. In this case, five SLNs were detected, with one SLN positivity, the overall score was 44, corresponding to a 13% probability of NSLN metastasis. This patient falls into the low-risk group and may be a candidate for omitting ALND. The studies by Gao et al. found that the survival benefit from ALND was not significant in mastectomy patients with 1–2 positive SLN. These studies also provide evidence supporting the omission of ALND16,44,45.
The present study still has some limitations. First, this study is retrospective, and larger prospective studies are needed to validate the model. Second, the external cohort sample is limited and requires a multi-center prospective study to increase the number of cases to improve the accuracy and representativeness of the prediction model.
Conclusions
In conclusion, we found that the number of positive SLNs, the proportion of positive SLNs, lymph-vascular invasion, perineural invasion, and ER status were independent risk factors for NSLN metastasis and utilized these factors to develop a nomogram. The nomogram showed good predictive performance. To maximize patient benefit while minimizing damage to the organism, surgeons can use this nomogram to identify patients with a low risk of NSLN metastasis and omit further ALND. Our nomogram has clinical applicability.
Materials and methods
Patients
The clinical data of patients with cT1-2 breast cancer who underwent surgery from January 2016 to December 2021 at multi-center was retrospectively analyzed. All patients were classified as cN0 (negative axillary ultrasound or clinical examination). Inclusion criteria: (1) 18 years of age or older; (2) Paraffin pathology diagnosis of invasive breast cancer (3) One or more SLN macrometastases; (4) Successful SLNB and ALND; (5) The preoperative clinical diagnosis was T1-2, N0 according to the 8th American Joint Committee on Cancer (AJCC). Exclusion criteria: (1) Presence of other malignant tumors; (2) Metastatic breast cancer; (3) Having undergone only SLNB without ALND; (4) Treated with neoadjuvant chemotherapy or radiotherapy; (5) Missing clinical data. Finally, a total of 830 patients were included for further analysis.
Training cohort: 410 patients treated at The Fourth Hospital of Hebei Medical University between 2016 and 2019.
Internal validation cohort: 298 patients treated at The Fourth Hospital of Hebei Medical University between 2020 and 2021.
External validation cohort: 122 patients treated at Xingtai People's Hospital (n = 94) and the Affiliated Hospital of Hebei Engineering University (n = 28) between 2020 and 2021.
Ethics approval and consent to participate
This retrospective study was approved by The Ethics Committee of The Fourth Hospital of Hebei Medical University (2022KY054). According to the requirements of the ethics committee, local legislation, and institutional requirements, informed consent was waived by The Ethics Committee of The Fourth Hospital of Hebei Medical University because of the retrospective nature of our study. The study was performed in compliance with the Declaration.
Data collection
Data was collected from eligible patients’ records, including age, type of surgery, tumor size, the number of positive SLNs, proportion of positive SLNs, number of positive NSLNs, lymph-vascular invasion, perineural invasion, histological grade, the status of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor-2 (HER-2), and Ki-67.
Diagnostic criteria
The status of ER, PR, and HER-2 was identified through immunohistochemistry (IHC). According to the 2020 American Society of Clinical Oncology/College of American Pathologists Guideline (2020 ASCO/CAP), tumor nuclei staining of ≥ 1% was defined as positive for ER or PR, and nuclei staining of < 1% was defined as negative46. HER-2 status was defined according to the results of IHC: 0 or 1 + were considered HER-2 negative, while 3 + was considered HER-2 positive. In cases of 2 + , additional fluorescence in situ hybridization (FISH) was required to determine whether the HER-2 gene was amplified or not. HER-2 status was considered negative unless the HER-2 gene was amplified; otherwise, it was considered positive47,48.
SLNB and ALND procedures
Patients who tested SLN-positive would undergo further ALND. In the cases where the intraoperative frozen section (FS) or touch imprint cytology (TIC) showed a negative SLN but the postoperative paraffin pathology revealed a positive SLN, a secondary ALND was performed25.
Statistical analysis
Statistical analysis was conducted using SPSS 22.0. Continuous variables were presented as mean ± standard deviation, while categorical variables were presented as proportions. The chi-squared test or Fisher exact test was used to compare categorical variables between groups. To identify risk factors affecting NSLN metastasis, a univariate analysis was performed in the training cohort. Factors found to be statistically significant underwent multivariate logistic regression analysis. The results were then presented as OR along with their corresponding 95% CI. All tests were two-sided, and P < 0.05 indicated a statistically significant difference.
A predictive model for the risk of NSLN metastasis was constructed using R software (version 4.2.2) along with MSTATA software (www.mstata.com). A nomogram model was established according to the screened independent risk factors, and validated by the internal and external validation cohorts. The ROC was plotted to calculate the AUC and evaluate the predictive power of the nomogram model. The AUC over 0.7 indicates that the nomogram provides a reasonable estimation. A calibration curve and DCA were used to evaluate the performance of the model.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Harbeck, N. & Gnant, M. Breast cancer. The Lancet 389, 1134–1150 (2017).
Wang, X. Y. et al. Risk factors and a predictive nomogram for non-sentinel lymph node metastases in chinese breast cancer patients with one or two sentinel lymph node macrometastases and mastectomy. Curr. Oncol. 26, 210–215 (2019).
Pereira, A., Siegrist, J., Lizarraga, S. & Pérez-Medina, T. Clustering molecular subtypes in breast cancer, immunohistochemical parameters and risk of axillary nodal involvement. JPM 12, 1404 (2022).
Chen, Y., Dai, Y., Chen, Y., Xu, Z. & Ding, J. Development and validation of an ultrasonography and clinicopathological features-based nomogram for non-sentinel lymph node metastasis. Gland. Surg. 12, 402–414 (2023).
Gruber, I. et al. Prediction of non-sentinel lymph node metastases after positive sentinel lymph nodes using nomograms. Anticancer Res. 38, 4047–4056 (2018).
Cheng, M. et al. A nomogram to predict non–sentinel lymph node metastasis in patients with initial cN+ breast cancer that downstages to cN0 after neoadjuvant chemotherapy. J. Surg. Oncol. 122, 373–381 (2020).
Lei, H., Yuan, P., Guo, C. & Ying, J. Development and validation of nomograms for predicting axillary non-SLN metastases in breast cancer patients: A retrospective analysis. Front. Oncol. 13, 1096589 (2023).
Rubio, I. T. et al. Nomogram including the total tumoral load in the sentinel nodes assessed by one-step nucleic acid amplification as a new factor for predicting nonsentinel lymph node metastasis in breast cancer patients. Breast Cancer Res. Treat. 147, 371–380 (2014).
Liu, Y. et al. Axillary management for early invasive breast cancer patients: Who will truly benefit?. Front. Oncol. 12, 989975 (2022).
Chen, J. et al. Predicting sentinel lymph node metastasis in a Chinese breast cancer population: assessment of an existing nomogram and a new predictive nomogram. Breast. Cancer Res. Treat. 135, 839–848 (2012).
Qiao, E. et al. A prospective validation cohort study of a prediction model on non-sentinel lymph node involvement in early breast cancer. Ann. Surg. Oncol. 27, 1653–1658 (2020).
Di Filippo, F. et al. Elaboration of a nomogram to predict nonsentinel node status in breast cancer patients with positive sentinel node, intraoperatively assessed with one step nucleic amplification: Retrospective and validation phase. J. Exp. Clin. Cancer Res. 35, 193 (2016).
Mittendorf, E. A. et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann. Surg. 255, 109–115 (2012).
Xiang, K. et al. A multi-dimensional nomogram to predict non-sentinel lymph node metastases in T1–2HR+ breast cancer. Front. Endocrinol. 14, 1121394 (2023).
Huang, Z. et al. Risk factors of non-sentinel lymph node metastasis in breast cancer with 1–2 sentinel lymph node macrometastases underwent total mastectomy: A case-control study. World J. Surg. Onc. 21, 125 (2023).
Gao, W., Lu, S., Zeng, Y., Chen, X. & Shen, K. Axilla lymph node dissection can be safely omitted in patients with 1–2 positive sentinel nodes receiving mastectomy: A large multi-institutional study and a systemic meta-analysis. Breast Cancer Res. Treat. 196, 129–141 (2022).
Department of Radiation Oncology, Kayseri Training and Research Hospital, Kayseri, Turkey & Cihan, Y. B. Could Nomograms Used to Identify Non-Sentinel Lymph Node Metastases May Be Valuable in Radiotherapy Planning? Eur J Breast Health 15, 69–70 (2019).
Fang, Z. et al. A novel predictive nomogram including serum lipoprotein a level for nonsentinel lymph node metastases in Chinese breast cancer patients with positive sentinel lymph node metastases. Dis. Mark. 2021, 1–10 (2021).
Charan, M. et al. Molecular and cellular factors associated with racial disparity in breast cancer. IJMS 21, 5936 (2020).
Zhou, J. et al. Population-based recurrence rates among older women with HR-positive, HER2-negative early breast cancer: Clinical risk factors, frailty status, and differences by race. The Breast 59, 367–375 (2021).
Prakash, O. et al. Racial disparities in triple negative breast cancer: A review of the role of biologic and non-biologic factors. Front. Public Health 8, 576964 (2020).
Sarink, D. et al. Racial/ethnic differences in postmenopausal breast cancer risk by hormone receptor status: The multiethnic cohort study. Int. J. Cancer 150, 221–231 (2022).
Duan, H., Zhang, J., Zhang, G., Zhu, X. & Wang, W. An improved nomogram including elastography for the prediction of non-sentinel lymph node metastasis in breast cancer patients with 1 or 2 sentinel lymph node metastases. Front. Oncol. 13, 1196592 (2023).
Liu, L. et al. A novel nomogram for decision-making assistance on exemption of axillary lymph node dissection in T1–2 breast cancer with only one sentinel lymph node metastasis. Front. Oncol. 12, 924298 (2022).
Wang, N. et al. A mathematical prediction model incorporating molecular subtype for risk of non-sentinel lymph node metastasis in sentinel lymph node-positive breast cancer patients: A retrospective analysis and nomogram development. Breast Cancer 25, 629–638 (2018).
Wu, P. et al. Validation of breast cancer models for predicting the nonsentinel lymph node metastasis after a positive sentinel lymph node biopsy in a Chinese population. Technol. Cancer Res. Treat. 17, 153303381878503 (2018).
Guo, X. et al. Deep learning radiomics of ultrasonography: Identifying the risk of axillary non-sentinel lymph node involvement in primary breast cancer. EBioMedicine 60, 103018 (2020).
Sa-nguanraksa, D. et al. Nomogram to predict non-sentinel lymph node status using total tumor load determined by one-step nucleic acid amplification: first report from Thailand. Breast Cancer 26, 471–477 (2019).
Liu, D. et al. Models for Predicting Sentinel and Non-sentinel Lymph Nodes Based on Pre-operative Ultrasonic Breast Imaging to Optimize Axillary Strategies. Ultrasound in Medicine & Biology 47, 3101–3110 (2021).
García-Mejido, J. A., Sanchez-Sevilla, M., García-Jimenez, R., Fernández-Palacín, A. & Antonio-Sainz, J. Intraoperative predictive model for the detection of metastasis in non-sentinel axillary lymph nodes. Clin. Exp. Obstet. Gynecol. 49, 86 (2022).
Clinic of Breast and Endocrine Surgery, Ankara City Hospital, Ankara, Turkey et al. Associated Features with Non-Sentinel Lymph Node Involvement in Early Stage Breast Cancer Patients who Have Positive Macrometastatic Sentinel Lymph Node. Eur J Breast Health 16, 192–197 (2020).
Wang, X. et al. Sentinel lymph node positive rate predicts non-sentinel lymph node metastasis in breast cancer. J. Surg. Res. 271, 59–66 (2022).
Chen, H. et al. Correlation analysis of pathological features and axillary lymph node metastasis in patients with invasive breast cancer. J. Immunol. Res. 2022, 1–7 (2022).
Alsumai, T. S., Alhazzaa, N., Alshamrani, A., Assiri, S. & Alhefdhi, A. Factors predicting positive sentinel lymph node biopsy in clinically node-negative breast cancer. BCTT 14, 323–334 (2022).
Zhao, Y.-X., Liu, Y.-R., Xie, S., Jiang, Y.-Z. & Shao, Z.-M. A Nomogram predicting lymph node metastasis in T1 breast cancer based on the surveillance, epidemiology, and end results program. J. Cancer 10, 2443–2449 (2019).
Hermansyah, D., Indra, W., Paramita, D. & Siregar, E. Role of hormonal receptor in predicting sentinel lymph node metastasis in early breast cancer. Med Arch 76, 34 (2022).
Sohail, S. K., Sarfraz, R., Imran, M., Kamran, M. & Qamar, S. Estrogen and progesterone receptor expression in breast carcinoma and its association with clinicopathological variables among the Pakistani population. Cureus 12, e975 (2020).
Farley, C. et al. To dissect or not to dissect: Can we predict the presence of four or more axillary lymph node metastases in postmenopausal women with clinically node-negative breast cancer?. Ann. Surg. Oncol. 30, 8327–8334 (2023).
Jung, E. J. et al. Positive estrogen receptor status is a poor prognostic factor in node-negative breast cancer: An observational study in Asian patients. Medicine 100, e25000 (2021).
Si, J. et al. Axillary evaluation is not warranted in patients preoperatively diagnosed with ductal carcinoma in situ by core needle biopsy. Cancer Med. 8, 7586–7593 (2019).
Sujarittanakarn, S., Himakhun, W., Worasawate, W. & Prasert, W. The case to case comparison of hormone receptors and HER2 status between primary breast cancer and synchronous axillary lymph node metastasis. Asian Pac. J. Cancer Prev. 21, 1559–1565 (2020).
Zhang, H., Sui, X., Zhou, S., Hu, L. & Huang, X. Correlation of conventional ultrasound characteristics of breast tumors with axillary lymph node metastasis and Ki-67 expression in patients with breast cancer. J. Ultrasound Med. 38, 1833–1840 (2019).
Holm-Rasmussen, E. V., Jensen, M.-B., Balslev, E., Kroman, N. & Tvedskov, T. F. Sentinel and non-sentinel lymph node metastases in patients with microinvasive breast cancer: A nationwide study. Breast Cancer Res. Treat. 175, 713–719 (2019).
Kim, B. K. et al. Omission of axillary lymph node dissection in patients who underwent total mastectomy with 1 or 2 metastatic lymph nodes. Ann. Surg. Treat Res. 98, 283–290 (2020).
FitzSullivan, E. et al. Outcomes of sentinel lymph node-positive breast cancer patients treated with mastectomy without axillary therapy. Ann. Surg. Oncol. 24, 652–659 (2017).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. JCO 38, 1346–1366 (2020).
Zheng, L., Liu, F., Zhang, S., Zhao, Y. & Liu, Y. Nomograms for predicting the likelihood of non-sentinel lymph node metastases in breast cancer patients with a positive sentinel node biopsy. Medicine 98, e18522 (2019).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. JCO 36, 2105–2122 (2018).
Acknowledgements
We thank Xingtai People's Hospital and the Affiliated Hospital of Hebei Engineering University for providing data for external verification.
Funding
Funding was supported by Hebei Natural Science Foundation (H2020206365 and H2021206071) and S&T Program of Hebei (236Z7719G).
Author information
Authors and Affiliations
Contributions
Z.C.S. and L.Y. designed the study. X.Y.Z. and L.Y. analyzed the data, wrote the manuscript, and prepared Figures and tables. L.X.Y. and Y.C. extracted the data. C.B.C., X.L.L., and Q.L.W. reviewed and revised the manuscript. L.Y. and X.Y.Z. contributed equally to this work and should be considered co-first authors. All authors reviewed the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, L., Zhao, X., Yang, L. et al. A new prediction nomogram of non-sentinel lymph node metastasis in cT1-2 breast cancer patients with positive sentinel lymph nodes. Sci Rep 14, 9596 (2024). https://doi.org/10.1038/s41598-024-60198-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60198-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.