Abstract
Moult and migration are energetically demanding and require adequate nutrition. In some species, individuals may interrupt their fall migration to moult at discrete stopover locations outside of their breeding grounds (i.e., moult-migration) leading to competing nutritional demands for moult and migration. Here, we use DNA barcoding of fecal samples to compare the diet of moulting and actively migrating (post-moult) Swainson’s Thrushes (Catharus ustulatus) and Tennessee Warblers (Leiothlypis peregrina) during their fall migration stopover at a large urban greenspace in Montreal, Canada. Diet differed according to moult status, species, and seasonality. Swainson’s Thrushes had a broad diet with frequent detections of both insects and berry-producing shrubs; while detections in Tennessee Warblers’ diets were mainly arthropods. For both species, more actively migrating individuals consumed fleshy-fruiting plants than moulting individuals. A higher proportion of moulting birds consumed arthropods compared to active migrants, due to either arthropod availability or a dietary preference for proteinaceous foods to grow feathers. Both species and moult classes consumed more native plants than non-native plants later in the season. We show the importance of managing urban greenspaces with native plants and diverse food sources that can provide for the different dietary needs of migratory birds.
Similar content being viewed by others
Introduction
The annual life cycle of a vast number of birds include migration between their breeding and wintering grounds1, which is an energetically demanding period, increasing a songbird’s energy expenditure by up to 30%2. Most north-temperate adult passerines undergo a complete prebasic moult—the loss and regrowth of all feathers—following the breeding season to prepare for their migration3. However, some individuals of certain migratory bird species will start their migration and halt at specific stopover sites outside of their breeding grounds to moult their worn-out feathers; they are called moult-migrants3,4,5,6,7. The various sequential stages of bird’s life cycle (breeding, moult, migration, etc.) have separate nutritional demands, with migrating bird needing to store fat reserves (fuel) and rebuild muscle mass (power) to power and fuel their long-distance flights between their breeding and wintering grounds8,9. Alternatively, birds undergoing post-breeding moult could face higher protein demands when replacing body and flight feathers because moult requires protein for feather growth3,4,10. Moulting birds may have a lower physiological capacity for building fat stores at that time11, which is why stopover sites have a critical role in providing migrants areas with key resources to replenish their fat and muscle mass before carrying on with their migration5,11,12.
Stopover sites are especially important for birds in active moult, as they have exceptionally high energetic demands during this period11. Individuals going though their flight feather moult are likely to have an impaired flight efficiency, which could suggest the need for specific habitat requirements at stopover sites, differing to the ones at their breeding and wintering sites4. For example, Tennessee Warblers (Leiothlypis peregrina) moult migrants in our study area have been observed to have large overlapping stopover home ranges (∼15 ha) and were dependent on high abundance of forest and forest edge13. Food nutritional quality and abundance are crucial for efficient refueling and can influence the time spent at stopover sites, since those affect the rate at which birds replace their energy stores14,15. Buler et al12. show that densities of insectivorous migrants are positively related to arthropod abundance while frugivorous migrants are positively related to the amount of fleshy-fruited plants during autumn12.
Many insectivorous bird species breeding in North America switch to a partially frugivorous diet during their fall migration8,15. This dietary plasticity allows birds to exploit resources that are more abundant during autumn15. Dietary flexibility may have evolved due to the spatial and temporal uncertainty in insect availability during autumn stopover8,16. Migrants eating fruits use less energy-expensive foraging techniques, encounter more food items per unit of time, and execute fewer search movements than when feeding on insects8. Therefore, the use of temporally and spatially stable fruit resources decreases searching and handling time, which in turn decreases energy expenditure during foraging and results in a positive net energy budget for migrants2,8,16. Due to seasonal frugivory, many species select different habitats than those preferred at other times of the year to optimize their energy gain17. Highly frugivorous migratory species can refuel at a faster rate when fruit diversity, quality, and availability are high15.
Evidence also suggests that migratory passerines tend to prefer native over non-native plant species for feeding. Oguchi et al. (2017) showed that fall migrating Swainson's Thrushes (Catharus ustulatus) and Gray Catbirds (Dumetella carolinensis) selected foraging habitat and occurred at higher densities in predominantly native shrublands rather than in exotic-dominated shrubland habitats18. Smith et al14. demonstrated that certain native fruiting shrub species are of higher nutritional quality to songbirds during fall migration compared to invasive species due to high lipid content. Indeed, native fruits tend to have greater fat and energy density, whereas exotic fruits tend to contain more sugar and water18.
These energetic differences between native and non-native fruits can have implications for the foraging efficiency for migratory species. For example, it is estimated that birds need to consume up to three times the wet mass of non-native exotic Elaeagnus umbellata fruits compared to the ones of native Ilex verticillata to obtain the same amount of energy18. Thus, fall migrants should generally prefer lipid- and energy-rich fruits18. Unfortunately, anthropogenic alterations of stopover habitats such as introduction and spread of non-native plants have been identified as a major conservation concern18. Invasive plant species can drastically change habitat quality due to their relatively high growth rate and competitive ability compared to native plants14. This can result in substantial environmental costs, particularly if the non-native plants compete with the native ones that provides important food resources for migrating birds. Additionally, non-native plants negatively impact insect availability and abundance since they are poor at supporting the insect communities that are critical food resource for birds, notably communities with high lipid and protein like caterpillars and spiders19,20. The protein requirements of many migratory birds can be met by eating higher-protein insects, which is why shrubland habitat can be important migratory stopover sites since they contain a variety of preferred fruit-bearing shrubs and an adequate abundance of insects17.
Although the current understanding of moult and plumage cycles is limited, moult-migration seems to be more common than currently recognized for passerines in North America4,7, so documenting it should be a priority. Additionally, existing conservation plans may lack information to fully maintain and improve stopover sites, as Neotropical migrants' habitat and food requirements during their migratory period remain insufficiently studied compared to the breeding and wintering seasons21. Data on the food requirements of Neotropical migrants throughout their complete annual cycle would benefit species conservation. Indeed, moult-stopovers are critical locations to conserve because moulting birds are highly vulnerable at this time4,11 and they stop for long periods at these sites (i.e. approximately 1–1.5 months, representing 13% of the annual cycle for Swainson’s Thrushes)5. Existence of diet differentiation among the migratory songbirds, specifically regarding the ecology of the moult migrants, would provide an important new insight into identifying critical moulting stopover requirements.
To better understand food items used by birds during stopover, we describe and compare the diet of two neotropical migrants and boreal forest breeders (Swainson’s Thrush and Tennessee Warbler) during their post-moult and moult migration in a peri-urban greenspaces of Montreal, Quebec, Canada. These songbird species are known to have differing diet preferences between seasons, and the two species feed at different foraging heights. Breeding and spring migrating populations of Swainson’s Thrush tend to be insectivorous whereas fall migrating and wintering populations are more frugivorous16,22,23,24. Swainson’s Thrushes are predominantly near-ground foragers (< 2 m high), whereas Tennessee Warblers are arboreal and insectivorous, but are opportunistic fruit eaters and nectar robbers during migration25,26,27. Despite the general knowledge of shifting diets based on species and seasonality, researchers have not yet identified specific diet requirements during the vulnerable period of moult migration and how this might compare to actively migrating individuals. We used fecal DNA metabarcoding to examine the composition of Swainson’s Thrushes and Tennessee Warblers' diets and to determine if diets changed according to moulting status or species. First, we predict more Swainson’s Thrush fecal samples to contain plant DNAs and more Tennesee Warbler samples to contain arthropod DNAs, as described in previous diet studies. As birds undergoing moult migration have various competing nutritional demands (i.e. protein to replace feathers and sugars to fuel for migration), we hypothesise dietary differences between moulting and post-moult migrants8,16. We predict moult-migrants to have a primarily insectivorous diet and post-moult migrants to have a berry-rich, frugivorous diet.
Methods
Study site and species
We conducted our fieldwork at the McGill Bird Observatory (MBO) near the Macdonald Campus of McGill University, on the west side of the island of Montreal (45.43°N, 73.94°W;5,6. The migration monitoring station is part of the “Montreal Gap”, a 600 km section of land in between Lake Ontario and the Gulf of the St Lawrence, through which migrating birds pass and avoid bodies of water when carrying out their continental migration28. The MBO is within the Grand Parc de l’Ouest nature reserve, which extends over more than 3000 ha2.
We surveyed the common fruit bearing species available at the MBO for fruit availability. We sampled the present shrubs—woody plants between 0.5 and 3 m tall—within 11 random 2 m-radius circular quadrats inside the study site. Quadrats were evenly distributed across the study site by randomly selecting a distance from either close (0–50 m) or far (50 m–125 m) from the banders’ cabin (i.e. where we would release the bird) and an angle (from 0 to 360 degrees) which would be the center of the quadrat. All random selections were done using a random number generator cellular application. Surveys were performed in 2021 (July to September) and 2022 in (June–July). The most common native fleshy-fruiting shrubs found were hackberry (Celtis occidentalis), purple-flowering raspberry (Rubus odoratus), dogwood (Cornus sp.), hawthorn (Crataegus sp.), sumac (Rhus sp.), and gooseberries (Ribes sp.). Other native fruiting plant species, like riverbank grapevine (Vitis riparia), raspberry (Rubus idaeus), and butternut tree (Juglans cinerea), were found. The main introduced fruiting shrubs and vines were common buckthorn (Rhamnus cathartica), glossy buckthorn (Rhamnus frangula), and Virginia creeper (Parthenocissus quinquefolia). The MBO is a small site (22 hectares), where the bird release locations (i.e., the banders’ cabin) is representative of the bird’s foraging habitat throughout the study site and the netting area.
The MBO and its surroundings consists of wetlands, secondary forest and shrublands at the forest-agriculture border which are valuable areas for moult-migrants arriving from the boreal forest6,28. Swainson’s Thrushes and Tennessee Warblers are common fall moult-migrants at the MBO, and thus ideal focal species for this research. Regarding the Tennessee Warblers’ typical migratory window at the MBO, previous studies have shown that moult migrants arrive in early August while post-moult migrants arrive more than a month later, in September13. Moult-migrants spend around 46 days at their stopover site compared to post-moult migrants which only occupy the site an average of 8 days13. This stopover duration is very similar to Swainson’s Thrushes that stay for an average of 47 (moult migrant) versus 7 (post-moult migrants) days at the same stopover site5.
Sample collection and determination of fecal sample contents
From August 1 to October 15 2021, we captured after-hatch year Swainson’s Thrushes (N = 83) and Tennessee Warblers (N = 21) using 30 mm mist nets (full moult record sample population, hereafter thrushes and warblers). Each of those species were separated into two categories: moult-migrants (i.e., individuals currently moulting, n = 39 for thrushes and n = 14 for warblers), and post-moult migrants (i.e., birds that already completed their moult prior to arriving to the site and were actively migrating, n = 44 thrushes and n = 7 warblers). Several species of adult warblers and thrushes undergo a definitive prebasic moult of flight feathers at these stopover sites during moult migration6,29. We did not compare immature birds as they do not undergo a full (definitive) prebasic moult. We assume that birds arrived on site at the start of their moult or at least at a not very advanced moulting stage (moulting first primaries). If the bird was moulting, each flight feathers (primaries, secondaries and tertials) was scored according to a method adapted from Newton in 196630. Each feather was scored as old (0), absent (0.1) or as a percentage of growth in 0.1 increments from 0.2 to 1; one meaning fully grown2. The captured birds were all banded, aged, and weighed. A subsample of the birds caught were sexed; 56 Swainson’s Thrushes (32 females and 20 males) and 18 Tennessee warblers (8 females and 10 males). Given the lack of sexual dimorphism in both species, we sexed the birds by obtaining 100ul blood samples and performing DNA sexing following methods in Griffiths et al. (311998). All experimental protocols were approved under animal use protocol 2007–5446 from McGill University, and federal banding permits 10743AE and 10743 T issued by the Canadian Wildlife Service. Furthermore, all methods are reported in accordance with ARRIVE guidelines.
We were able to assess the diets on a subsample of Swainson’s Thrushes (n = 38) and Tennessee Warblers (n = 10) using fecal samples collected from the capture of each individual at the MBO (dietary subsample population). To do so, the birds were left for 10–30 min in a brown paper bag before their release. Fecal samples were collected from the bags and put in 15 ml tubes with ethanol. Samples were stored at − 20 °C until analysis. The biological samples were also collected under animal use protocol 2007–5446 and approved by the McGill University Animal Care Committee. We then sent the samples to the Canadian Center for DNA Barcoding (CCDB). They analyzed the fecal samples for arthropods and plants detection using DNA barcoding as a method of species identification32. A total of 54 fecal sample was sent to the CCDB laboratory (42 thrushes, with 2 recaps (n = 40) and 12 warblers with 1 recap (n = 11). There were no detections recovered by the CCBD in the diet of 2 thrushes and 1 warbler, resulting in our dietary subsample population of 48 birds (38 Swainson’s Thrushes and 10 Tennessee Warblers).
Laboratory scientists at the CCDB amplified the DNA from the fecal samples with arthropod-specific primers ZBJ-ArtF1c_t1/ZBJ-ArtR2_t1 and plant-specific primers ITS-S2F_t1/ITS4_t132. Each DNA extract was amplified twice to achieve two independent PCR and sequencing replicates. The resulting sequence reads were filtered to remove low quality reads, trimmed to remove primer and adapter sequences, and then filtered for a minimum size of 100 bp. The processed reads were then compared to a comprehensive Barcode of Life Data (BOLD) reference library33 and assigned an identity using the Basic Local Alignment Search Tool (BLAST) algorithm32. The results from BLAST searches were sorted into unique taxonomic identification per sample. See Supplementary Materials for the full DNA Testing Laboratory NGS Report with detailed methods by the CCDB. Furthermore, no plant specimens were collected for this study. We assume the plant collection and use, to create the BOLD reference library, was in accordance with all the relevant guidelines. It is important to note that while this novel technology allows scientists to track the presence of food items in an individual’s diet, there are potential shortcomings to this tool to quantify proportions of food use, and to differentiate between direct and indirect consumption when multiple trophic levels are pooled together. Metabarcoding of fecal material can also yield simple barcoding errors due to DNA degradation or deficiencies in reference libraries.
Classification of probable and improbable plant and arthropod entries
We classified if the detected arthropods and plant species found in the fecal samples of the birds were probable (i.e. present in southern Quebec) or improbable (i.e. absent from southern Quebec). Plants were assessed to species level and arthropods to the genus level, due to uncertainty about the distribution of many arthropods at the species level. We kept all detections that were deemed probable to be found in the bird’s diet at the study site. At times, some data entries provided by the lab were not as taxonomically precise as the others; some plant DNA was only identifiable at the genus-level rather than the species-level, and some arthropod entries were only identifiable at the family-rather than the genus-level. In this case, when the higher taxon was deemed probable, we accepted this entry. This resulted in keeping 53.3% of the known plant detections (32 probable plant species, which included 2 detections stopping at the genus-level). Regarding the arthropods, we deemed probable 74.3% of the arthropod detections (113 probable plant genera, which included 12 detections stopping at the family-level). To distinguish the probable versus improbable entries for the plants, we used the Canadensys Database of Vascular Plants of Canada (VASCAN), which is a comprehensive list of all vascular plants reported in Canada34. We also noted if the probable plants were native or introduced34. For the arthropods, we went through peer-reviewed articles from the journal of Zoology ‘ZooKeys’, which reported the known families of arthropods in Canada35. We also used iNaturalist to look at probable entries for plants and arthropods, only considering entries of “research-grade level”36. We eliminated species with distributions which did not overlap with the southern parts of Quebec. A table listing the probable and improbable entries of arthropods and plants can be found in the Supplementary Materials as Table S1 and S2.
Statistical analysis of fecal contents
We used generalized linear mixed models (GLMMs) to describe how moult status, species, and seasonality might influence diet, using the ‘glmm’ function in the R package ‘rms’. For the diet, we separated the two main categories of ‘Plants’ and ‘Arthropods’ in 10 key groups to assess our hypotheses. It is important to note that the direct diet of the birds, as well as the indirect diet of the arthropods ingested by the birds or other arthropods, are amplified when conducting fecal metabarcoding. With this method, it is not currently possible to separate actual direct plant consumption versus indirect consumption via phytophagous insects. Thus, we assume plant detections are coming from a mix of directly and indirectly consumed plant material because the genetic material from some of the plants detected in the analysis is coming from the guts of consumed invertebrates. We also assume this bias is similar across our different sampling groups. Plant genetic materials were separated into either "fleshy-fruiting plant" or "dry-fruiting plant", as fleshy-fruits are more likely to be directly consumed by birds9 and plants without fleshy-fruit are presumably a by-product of arthropod consumption. Plant genetic materials were also separated into native versus invasive plant categories. Arthropod items were separated into their respective orders: Araneae, Coleoptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Neuroptera, and Orthoptera. We built 12 models, each including moulting status, species and seasonality as explanatory variables and the response variable as different diet items (i.e. the four plant fruit types and eight main arthropod orders mentioned above). Interactions between predictors were not considered because none of the response variables were explained by multiple explanatory variables at the same time. Thus, since none of the individual predictors had an effect, an interaction between predictors would not either. All GLMMs were fitted with a binomial distribution since the response variable included only zeros and ones: either the diet item was detected (1) or absent (0) in the individual sample. Individual bird identification was also included as a random effect in each model to account for variation within repeated samples from the same individual. The models performed similarly with and without this random effect, which we confirmed by comparing the marginal and conditional models (see Results). Hereafter, we report statistics derived from our GLMM model. We are using an alpha level of 0.05 to assess significance and report statistical differences among groups or covariates. However, when p-values occurred between 0.1 and 0.05, we also report differences given our low sample size; such that these variables provide 'marginal' support.
Additionally, we carried out two permutational multivariate analysis of variance (PERMANOVAs) using the ‘adonis’ function in the R package ‘vegan’. Each PERMANOVA compared moult status, species, and sex to the presence of either plant or arthropod items in fecal samples, namely, (1) 17 plant orders and (2) 10 arthropod orders. We used orders to reduce the dimensionality (i.e. the number of diet-related variables) in the PERMANOVA analyses, since DNA identification to the species and genus level is less reliable. Before analyses, we removed samples where the subject’s sex was unknown and where there were no arthropod and plant items detected, which left us with a sample size 32 individuals with arthropod detected in their fecal sample and 22 individuals with plant DNA detected in their fecal sample. Note that in this analysis, individuals were double counted since the same bird could have a plant and an arthropod in its fecal sample. All analyses were performed using R Statistical Software (v4.2.1; R Core Team 2022).
Results
After removing the improbable entries, arthropod detections were found in 47 out of 48 individuals (dietary subsample population). We detected 101 arthropod genera belonging to 71 families and 10 orders. Plant detections were found in 38 out of 48 individuals (dietary subsample population). Approximately 56% of the plant sequences recovered from all replicates showed no significant similarity to any of the publicly available records on BOLD and were assigned an identity of “unknown”32. Identified plant items represented approximately 30 species belonging to 23 genera, 18 families, and 17 orders. Note that this study does not represent the entire direct diet of moult migrating Swainson’s Thrushes and Tennessee Warblers. In essence, with the methods used here, it is challenging to definitively identify the plant or arthropod taxon that the birds are not eating; our understanding is limited to just a glimpse of their diet. When referring to 'eating' in this context, indirect consumption is considered, as the detected plants and arthropods could be by-products of the arthropods the birds are eating (e.g., caterpillars eating host plants or spiders eating prey).
Species’ moult status results
Almost half of adult Swainson’s Thrushes (47%) captured (n = 39 out of 83) were undergoing flight-feather moult. Moulting was slightly more frequent among females (62.5%) (n = 20 out of 32) than males (45.8%) (n = 11 out of 24). Most adult Tennessee Warblers (67%, n = 14 out of 21) caught were undergoing moult and moulting was more frequent in females (87.5%) (n = 7 out of 8) than in males (50%) (n = 5 out of 10).
Diet description of Swainson’s Thrushes
Plant genetic materials were detected in the fecal sample of 30 out of 38 thrushes (Table 1). However, approximately 56% of the plant entries were assigned an identity of “unknown”. Plant genetic materials from the Rubus, Juglans, and Ambrosia genera were found to be most present in the digestive tract of the birds (present in 13% of individuals for all 3 genera). Regarding the diet according to their moulting status, there were detections of plant genetic material from Ambrosia, Boehmeria, and Frangula genera for moulting birds and from Rubus as well as Juglans genera for post-moulting individuals.
Arthropod items were detected in the fecal sample of 37 out of 38 thrushes (Table 2). The dominant order (present in 78.4% of individuals) was Lepidoptera. Regarding the birds’ diet according to their moulting status, species from the Lepidoptera order were the most common dietary item for both moulting and post-moulting birds. Moulting individuals also ingested Diptera, Hemiptera, Araneae, and Coleoptera, in order of frequency. Post-moulting individuals ingested Diptera, Araneae, Coleoptera, and Hemiptera.
Diet description of Tennessee Warblers
Plant genetic materials were detected in the fecal sample of 8 out of 10 warblers. When omitting the 56% of unknown plant entries, plant genetic materials were detected in the fecal sample of 4 out of 10 warblers (Table 1). Of those birds, 3 out of 4 had DNA of Solidago genus in their feces. As Solidago is not a fleshy-fruited plant, warblers may have been feeding on Solidago nectar or feeding on arthropods that had Solidago material in their digestive tracts.
Arthropod items were detected in the fecal sample of all 10 warblers captured (Table 2). Arthropod genetic materials from Lepidoptera, Diptera, Araneae, and Hemiptera orders were found to be most present in the digestive tract of the birds (present in 50% of individuals for all 4 orders). Regarding the diet according to their moulting status, species from Lepidoptera and Araneae were common dietary items for both moulting and post-moulting birds. There were more detections of Dipterans for moulting birds and Hemipterans for post-moulting birds.
Influence of moult status, species, and seasonality on diet
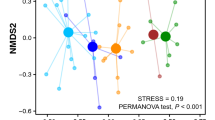
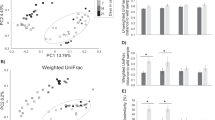
According to our general linearized mixed models, species and moult status were not statistically significant explanatory variables for most of the four fruit types and eight arthropod orders (Table 3). For fleshy fruit presence, however, moulting status of a bird (from both species pooled together) was found to be a significant explanatory variable (P = 0.04, Table 3a), such that, moulting individuals were less likely to have fleshy-fruiting plant detected in their diet than post-moult birds. More precisely, a negative trend signifies an association with post-moult migrants and a positive trend with moulting migrants. There was no variation among individuals as a comparison of the marginal and conditional models (including and excluding individual as a random effect) performed equally well or poor in every case. Finally, calendar date was positively related to the proportion of native fruit in both species’ fecal samples (P = 0.02) and marginally negatively related to the proportion of introduced fruit in the species’ fecal samples (P = 0.08, see Table 3c,d and Fig. 1). The plant species detected along with their status (native or introduced) and fruiting season can be found in Supplementary Table S3.
Introduced (in blue) and native (in red) plant genetic material detected (1) or not (0) in the individual’s diet according to seasonality for both Swainson’s Thrushes and Tennessee Warblers. Introduced plants tended to decrease in detection as time passed (P = 0.081) and native plants increased in detection as time passed (P = 0.021) (CI = 95%).
For our PERMANOVA analyses, no difference were detected in plant or arthropod presence in the birds’ fecal samples in relation to species, moult status, or sex (See Supplementary Table S4). The only statistical difference was the interaction between species and sex on arthropod detections (n = 34, p = 0.05). See the variation in individual fecal samples in Supplementary Table S5.
Discussion
This study effectively employed fecal DNA metabarcoding to discern species- and migration-specific dietary patterns. After excluding improbable detections, the proportion of Tennessee Warblers and Swainson’s Thrush with plant (8/10 and 30/38, respectively) and arthropod (10/10 and 37/38, respectively) detections were remarkably similar, allowing us to reject the hypothesis that warblers would have far more arthropod diet and thrushes a more plant-based diet. Instead, both species had diets composed of arthropods and fruit, however thrushes had a higher probability of consuming Lepidoptera than warblers. Post-moult migrating birds had more carbohydrate-rich foods (fleshy-fruit) than moulting birds (Table 3). Moreover, as the migration season progressed, birds reduced their consumption of introduced plant species and consumed more fruit- and energy-rich native shrubs than earlier in the season (Fig. 1).
Diet description of study species
Our results showed that the diet of Swainson’s Thrushes was taxonomically more varied than the Tennessee Warber’s, especially for plants. Similarly, studies carried out in Illinois and Rhode Island show that 60 to 80% of Swainson’s Thrushes’ diet during their fall migration were made of wild fruits16,24. However, our results could also be influenced by the extremely small sample size of Tennessee Warblers with plants of known genera detected in their diet (n = 4), suggesting a primarily insectivorous diet. Additionally, fewer arthropod orders were found in the diet of warblers compared to thrushes. This could be explained again by the small sample size of warblers (n = 10) or by the fact that warblers might be more specialized and thrushes more generalist regarding arthropods in their diet. Alternatively, it could also reflect differences in arthropod diversity present in arboreal foliage compared to leaf litter. In either case, these results suggest that at our study site, the feeding niche of thrushes is broader for both plants and arthropods than the warblers’ diet.
Importance of Lepidopteran species in diet
Our findings underscore the crucial role of Lepidopteran species in shaping the dietary habits of thrushes and warblers during their fall migration. Indeed, birds, particularly North American species, rely and prey extensively on caterpillars, since they are a high-energy, high-biomass, and nutritious food resource37,38,39,40,41 In fall, arboreal warblers and ground foragers benefit from the availability of Lepidopteran larvae, because those feed on tree leaves as well as pupate and over winter in soil and leaf litter42. Butler & Strazanac (2000) showed that larval composition, richness, and abundance are higher in May and August, suggesting multiple peaks in lepidopteran larvae in both spring and late summer/early fall when birds are migrating43. Our results are consistent with the synchronous availability and nutritious value of this food item since both thrushes and warblers showed that the highest proportion of individuals consumed species form the Lepidopteran order during their fall migration. Indeed, 78.4% of thrushes and 50% of warblers, as well as 66.7% of moulting individuals and 78.3% of post-moult individuals, had Lepidopterans detected in their diet, further suggesting that moths and butterflies are important food resource for the migrating birds in urban areas. Like mentioned previously, not being able to account for availability of prey in the area is a caveat of DNA barcoding, which means we cannot differentiate selection versus availability with certainty. However, even if Lepidoptera are occasionally detected as indirect food items, our results highlight the importance of Lepidoptera for supporting food webs by being consumed by both insectivorous birds and the predatory arthropods (e.g., spiders) that birds feed on.
Regarding our results from the fecal sample analysis, most of the plant sequences recovered from all replicates in the DNA barcoding analysis were unknown. This high proportion (56%) of unknown plant species could result from degraded DNA from being digested twice. Plant DNA may be highly fragmented by going through the gut of an herbivorous arthropod as well as the gut of an insectivorous bird, which means our inference on the importance of herbivorous arthropods to birds may be conservative. It is possible that herbivorous insects such as Lepidopteran and Symphyta larvae (Sawflies) would be the main prey insects from our unknown detections because most larval Lepidoptera are folivores44, highly abundant prior to pupation, and nutrient rich45,46. Additionally, the bird’s diet might consist of arachnids that prey on phytophagous insects. These uncertainties could be alleviated in future studies by combining methods (e.g. metabarcoding, isotopes and/or foraging data).
Our results also call attention to the importance of native plants for migrating birds as the season progresses (Fig. 1). Native plant communities support a higher diversity of caterpillar species, leading to greater bird abundance and diversity20,47. This relationship is supported by empirical evidence demonstrating that insect herbivores rely on plant lineages with which they have shared an evolutionary history for successful reproduction20,47,48. Consequently, landscapes dominated by non-native plants are less likely to support the same diversity and biomass of insect herbivores48, indicating that urban greenspaces with non-native plants are less supportive of breeding insectivorous birds20. Further studies that focus on stopover habitat quality, requirements, and communities would be beneficial since increasing native plant species in urban areas would positively impact both Lepidopteran and avian populations, contributing to the mitigation of urban biodiversity losses47. As well as supporting herbivorous insect communities and the birds dependent on them, prioritizing native flora in human-dominated landscapes is crucial for promoting sustainable and local food webs20.
Diet community of the fall migrants
Largely, we found no statistical difference between the plant and arthropod orders present in the fecal samples of thrushes and warblers, though our small sample size may have lacked the statistical power to determine differences. Moreover, this is partly a limitation of the DNA technique which allows us to estimate detections but not quantity of prey. Not being able to identify all items eaten with the same precision with the DNA barcoding method was another limitation; some detections were unidentifiable, and others were only identifiable to genus or family level. We also could not account for availability of prey and food items in the area, or abundance of food items in diet, although we confirm what plants were available (see methods). Species and moult status had no significant effect although there was some evidence that sex (within species) impacted arthropod-related diet (p = 0.05). Sexual segregation of micro-foraging habitat has been observed in several songbirds while on their wintering grounds49,50 and breeding grounds51,52,53. Differences in foraging height, rates, and substrate contribute to these distinct niches49,51. Since insect families shift significantly from ground level to canopy and between plant species in temperate forests54, a similar distribution in foraging strategy may reflect this difference in diets between the sexes. Likewise, the difference we found in the birds’ diet based on date might also be explained by the difference in migration timing between sexes instead of habitat-usage differences per se55.
Arthropod community
We detected a wide distribution of arthropod diversity in the thrushes and warblers’ fecal samples. Of these, Lepidopterans and Arachnids are detected at a higher frequency in bird fecal samples and are a high-quality prey item with high caloric content39,40,41. The species in these orders also have a high quantity of crude fat, crude protein, and moisture56. Individuals also had lower-quality prey in their diets, such as small-bodied Hemipterans (i.e. detections of Elasmucha, Gyponana, Scaphoideus, Lygus, Phytocoris, Hoplistoscelis, Lasiomerus; which range in size from 6 to 10 mm (average 7.3 mm)), that contain less energy due to their small size, but are often abundant57. This could suggest that some diet selection for foraging birds could be based on preference and some on abundance. However, our results might be potentially recovering the diet of the predatory arthropods eaten by the bird (e.g., spiders preying on Hemipterans) and not the diet of the individual, making it difficult to assess preference.
Plant community
Many plant orders detected in the thrushes and warblers’ fecal samples seem to be secondary consumption products. Particularly in the warblers’ feces, many detected plants produce dry seeds that are not generally consumed by non-granivorous birds: birches (Betula) from the order Fagales; rapeseed (Brassica) from the order Brassicales; goldenrods (Solidago) from the order Asterales; herbaceous plants like lambsquarters and goosefoot (Chenopodium) from the order Caryophyllales; and grasses from the order Poales. These orders, however, are known to be host plants for a large diversity of herbivorous Lepidoptera, Symphyta, Hemiptera and Coleoptera species as well as predators and pollinators58. Thus, arthropod community visiting these non-fleshy fruited plants are likely an important source of arthropod food for songbirds. The Brassicales plant order consist of various trap crops and insectary plants which hosts parasitoids, as well as insects from Lepidoptera, Coleoptera, Hemiptera, and Hymenoptera59. Tennessee Warblers were frequently spotted foraging in fields containing those herbaceous plant species and foraging in detected tree species higher in the canopy when being tracked for an affiliated study (personal observation). Thus, we suggest that many of the plants detected in the warblers’ diet were initially eaten by arthropods, which were then eaten by the birds, since Tennessee Warblers are primarily insectivorous25,26. Whether the plant found in the bird’s fecal samples was directly or indirectly eaten still shows that the plant is important for the bird's diet as these plants are the foundation of local food webs. Similarly, dry plant material from various plant orders detected in the thrushes’ fecal sample are presumably a by-product of their arthropod consumption. For example, plants from the Juglans genus do not represent an expected dietary item for Swainson’s Thrush; it is much more likely the birds were eating herbivorous arthropods that were foraging on Juglans leaves. We can infer that that species from the Juglans genus may be important contributors to thrush diet at this stopover, even if the actual dietary items are arthropods that feed on Juglans. Moreover, the thrushes’ diet contained more detections of fleshy-fruiting plant genera compared to warblers, such as hackberry (Celtis), hawthorn (Crataegus), buckthorn (Rhamnus), Siberian crab-apple (Malus baccata), apple (Malus), bitter cherry (Prunus emarginata), raspberries (Rubus), etc. from the Rosales order as well as shrubby plants like sumac (Rhus) from the Sapindales order. These results are consistent with the literature, given that Swainson’s Thrushes are near-ground foragers,omnivorous, and frugivorous,especially during the fall16,22,23,24.
Seasonality of native and non-native plant species
Seasonality influenced the detection of native and introduced plants in an individual’s diet (Fig. 1). At the start of the field season (fecal sample collection started on August 4th), there was a higher proportion of introduced plants detected in the birds’ diet (mainly buckthorn species) while, at the end of the fall migration season (last fecal sample was collected on October 6th), native plants were dominant and primarily consisted of Rubus species. This decrease in the detection of introduced plant species and increase of native plant species detected over time could be explained by the different fruiting times of these plant groups. Indeed, many introduced species at this site fruit in August and September: Ambrosia artemisiifolia, Chenopodium album, Frangula alnus, Glycine max, Rubus phoenicolasius, and Rumex acetosa. Some native species are found to fruit starting September: Solidago gigantea, Solidago rugosa, Impatiens capensis, and Lysimachia ciliate. Another plausible explanation could be that native plants are more adapted to the fall temperatures and are more robust, meaning that they can prosper later in the season whereas introduced plant species are not as adapted to the colder temperatures60. This was the case for plants on Marion Island, where invasive plants exhibited lower leaf toughness and frost tolerance than native plants60. This could explain why the migrating birds of our study primarily consumed the fruits of native species throughout the autumn season. Further research is needed regarding plant species invasion, fruit quality, and its impact on bird-fruit interactions.
Finally, since songbirds require abundant food resources over the length of their migratory routes, the diversity of native fruit and host plants in shrublands, forests and other green spaces, need to be maintained to provide suitable stopover sites. Unfortunately, anthropogenic alterations to the North American landscape have degraded habitat quality, introduced non-native species, and reduced the amount of greenspaces available for stopover sites used by long-distance Nearctic–Neotropical migrants17,61. The migration routes of many avian species follow the Atlantic or Pacific coasts where urban areas are one of the main land cover types due to the major population centers located near those coasts. Thus, migrants that encounter metropolitan areas are limited to woodlots and forests fragments within city parks as their stopover habitats61. Moult-migration is common in North America7 and moult-migrants are known to stop for long periods at these sites5, therefore, highlighting their diet amidst these circumstances is crucial. Further studies are needed to allow land managers in metropolitan areas to make science-based decisions regarding the survival and long-term conservation of migrating bird populations, which greatly depends on high quality stopover sites17,61.
Conclusion
This paper reports novel information about the rather unknown life history period of songbird species: moult-migration. We documented the diet of Swainson’s Thrushes and Tennessee Warblers at a peri-urban stopover site during their fall migration. Many neotropical migrants spend almost as much time in large greenspaces within an urban matrix in southern Canada as they are in the boreal forest for breeding13, which is not currently considered thoroughly in conservation planning. Therefore, since our results suggests that diet differs according to moulting status, species and even seasonality, the management of urban areas with a variety of fruit and arthropod-rich species that can provide for the different dietary needs of the fall migrants is crucial. We advise municipality managers to plant diverse native species that can host an abundance of native species of arthropods, so that migrants can benefit from an adequate and optimal environment at their stopover site. Indeed, native landscaping positively influences songbird abundance and may reduce biodiversity losses in human-dominated landscapes20,47. The large-scale loss in migratory bird species have serious conservation implications that could be mitigated with changes in landscape practices, especially by the management of urban stopover sites.
Data availability
The data and the codes from this research have not yet been archived, but archiving can be done and shared upon request to the corresponding author.
References
Domer, A., Ovadia, O. & Shochat, E. Energy for the road: Influence of carbohydrate and water availability on fueling processes in autumn-migrating passerines. Auk 135, 534–546. https://doi.org/10.1642/AUK-17-228.1 (2018).
Morales Bucheli, A. C. Stopover ecology of moult migrant Swainson's thrushes «Catharus ustulatus» Master of Science thesis, McGill University, (2019).
Cyr, N. E., Wikelski, M. & Romero, L. M. Increased energy expenditure but decreased stress responsiveness during molt. Physiol. Biochem. Zool. 81, 452–462. https://doi.org/10.1086/589547 (2008).
Leu, M. & Thompson, C. W. The potential importance of migratory stopover sites as flight feather molt staging areas: A review for neotropical migrants. Biol. Conserv. 106, 45–56. https://doi.org/10.1016/S0006-3207(01)00228-2 (2002).
Morales, A., Frei, B., Mitchell, G. W., Bégin-Marchand, C. & Elliott, K. H. Reduced diurnal activity and increased stopover duration by molting Swainson’s Thrushes. Ornithology https://doi.org/10.1093/ornithology/ukab083 (2022).
Junda, J. H., Duval, S. & Gahbauer, M. A. Use of discrete molting grounds by migrant passerines undergoing prebasic molt in southern Quebec. Wilson J. Ornithol. 132(72–82), 11. https://doi.org/10.1676/1559-4491-132.1.72 (2020).
Pyle, P., Saracco, J. F. & DeSante, D. F. Evidence of widespread movements from breeding to molting grounds by North American landbirds. Auk 135, 506–520. https://doi.org/10.1642/AUK-17-201.1 (2018).
Parrish, J. D. Behavioral energetic and conservation implications of foraging plasticity during migration. Stud. Avian Biol. 20, 53–70 (2000).
Oguchi, Y., Smith, R. J. & Owen, J. C. Fruits and migrant health: Consequences of stopping over in exotic- vs. native-dominated shrublands on immune and antioxidant status of Swainson’s Thrushes and Gray Catbirds. Condor 119, 800–816. https://doi.org/10.1650/condor-17-28.1 (2017).
Bauchinger, U. & Biebach, H. Transition between moult and migration in a long-distance migratory passerine: Organ flexibility in the African wintering area. J. Ornithol. 147, 266–273. https://doi.org/10.1007/s10336-006-0059-3 (2006).
Morris, S. R. et al. An 18-year study of migration and stopover ecology of Tennessee warblers in Kalamazoo county Michigan. Wilson J. Ornithol. 125, 70–78. https://doi.org/10.1676/08-131.1 (2013).
Buler, J. J., Moore, F. R. & Woltmann, S. A multi-scale examination of stopover habitat use by birds. Ecology 88, 1789–1802. https://doi.org/10.1890/06-1871.1 (2007).
Poirier, V., Frei, B., Lefvert, M., Morales, A. & Elliott, K. H. Moult migrant Tennessee Warblers undergo extensive stopover in peri-urban forests of southern Quebec. Can. J. Zool. First https://doi.org/10.1139/cjz-2023-0109 (2023).
Smith, S. B., DeSando, S. A. & Pagano, T. The value of native and invasive fruit-bearing shrubs for migrating songbirds. Northeast. Nat. 20(171–184), 114. https://doi.org/10.1656/045.020.0114 (2013).
Smith, S. B. & McWilliams, S. R. Patterns of fuel use and storage in migrating passerines in relation to fruit resources at autumn stopover sites. Auk 126, 108–118. https://doi.org/10.1525/auk.2009.09139 (2010).
Parrish, J. D. Patterns of Frugivory and energetic condition in Nearctic Landbirds during autumn migration. The Condor 99, 681–697. https://doi.org/10.2307/1370480 (1997).
Smith, S. B. et al. Fruit quality and consumption by songbirds during autumn migration. Wilson J. Ornithol. 119(419–428), 410. https://doi.org/10.1676/06-073.1 (2007).
Oguchi, Y., Pohlen, Z., Smith, R. J. & Owen, J. C. Exotic- and native-dominated shrubland habitat use by fall migrating Swainson’s Thrushes and Gray Catbirds in Michigan, USA. Condor 120, 81–93. https://doi.org/10.1650/CONDOR-17-27.1 (2017).
Tallamy, D. W., Narango, D. L. & Mitchell, A. B. Do non-native plants contribute to insect declines?. Ecol. Entomol. 46, 729–742. https://doi.org/10.1111/een.12973 (2021).
Narango, D. L., Tallamy, D. W. & Marra, P. P. Nonnative plants reduce population growth of an insectivorous bird. PNAS 115, 11549–11554. https://doi.org/10.1073/pnas.1809259115 (2018).
Marra, P. P., Cohen, E. B., Loss, S. R., Rutter, J. E. & Tonra, C. M. A call for full annual cycle research in animal ecology. Biol. Lett. 11, 20150552. https://doi.org/10.1098/rsbl.2015.0552 (2015).
Mack, D. E. & Yong, W. Swainson's Thrush Catharus ustulatus, <https://birdsoftheworld.org/bow/species/swathr/cur/introduction> (2000).
Hejl, Sallie J.; Hutto, Richard L.; Preston, Charles R.; Finch, Deborah M. 1995. Effects of silvicultural treatments in the Rocky Mountains. In: Martin, Thomas E.; Finch, Deborah M. Ecology and management of neotropical migratory birds. New York, NY: Oxford University Press. p. 220-244.
Graber, R. R., Graber, J. W. & Kirk, E. L. Illinois Birds: Turdidae. Report No. 075, (Prairie Research Institute, University of Illinois at Urbana-Champaign, 1971).
Rimmer, C. C. & McFarland, K. P. Tennessee Warbler Leiothlypis peregrina, <https://birdsoftheworld.org/bow/species/tenwar/cur/introduction> (2012).
Mcmartin, B. Impact of insecticide applications on the foraging behaviour and diet of three boreal forest species Master's Thesis thesis, University of Toronto, (1996).
Cumming, E. E. Habitat segregation among songbirds in old-growth boreal Mixedwood forest. Can. Field Nat. 118, 45–55. https://doi.org/10.22621/cfn.v118i1.881 (2004).
Gahbauer, M. A., Duval, S. & Davey, D. McGill Bird Observatory Ten-Year Report: 2005–2014. 326 (Migration Research Foundation, Ste-Anne-de-Bellevue QC., 2016).
Tonra, C. M. & Reudink, M. W. Expanding the traditional definition of molt-migration. The Auk. 135, 1123–1132. https://doi.org/10.1642/AUK-17-187.1 (2018).
Newton, I. The Moult of the Bullfinch Pyrrhula Pyrrhula. Ibis 108, 41–67. https://doi.org/10.1111/j.1474-919X.1966.tb07251.x (1966).
Griffiths, R., Double, M. C., Orr, K., & Dawson, R. J. A DNA test to sex most birds. Molecular ecology 7(8), 1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x (1998).
Nguyen, T. X. DNA Testing Laboratory NGS Report. (Canadian Center for DNA Barcoding Montreal, 2022).
Systems, B. Barecode of life data systems, <http://www.boldsystems.org> (2022).
Acadia University, U. d. M. B. C., University of Toronto Mississauga, University of British Columbia. Database of Vascular Plants of Canada (VASCAN), <https://data.canadensys.net/vascan/search> (2023).
Langor, D. W. The diversity of terrestrial arthropods in Canada. ZooKeys 819, 9–40. https://doi.org/10.3897/zookeys.819.31947 (2019).
iNaturalist. <https://www.inaturalist.org/> (2023).
Krištín, A. Birds as predators of Lepidoptera: Selected examples. Biologia 52, 319–326 (1997).
Poelen, J., Simons, J. & Mungall, C. Global biotic interactions: An open infrastructure to share and analyze species-interaction datasets. Ecol. Inform. 24, 148–159. https://doi.org/10.1016/j.ecoinf.2014.08.005 (2014).
Wilkin, T. A., King, L. E. & Sheldon, B. C. Habitat quality, nestling diet, and provisioning behaviour in great tits Parus major. J. Avian Biol. 40, 135–145. https://doi.org/10.1111/j.1600-048X.2009.04362.x (2009).
García-Navas, V. & Sanz, J. J. The importance of a main dish: nestling diet and foraging behaviour in Mediterranean blue tits in relation to prey phenology. Oecologia 165, 639–649. https://doi.org/10.1007/s00442-010-1858-z (2011).
McMartin, B., Bellocq, M. & Smith, S. Patterns of consumption and diet differentiation for three breeding warbler species during a spruce budworm outbreak. The Auk 119, 216–220. https://doi.org/10.1093/auk/119.1.216 (2009).
Schmitt, L. & Burghardt, K. T. Urbanization as a disrupter and facilitator of insect herbivore behaviors and life cycles. Curr. Opin. Insect Sci. 45, 97–105. https://doi.org/10.1016/j.cois.2021.02.016 (2021).
Butler, L. & Strazanac, J. Occurrence of Lepidoptera on selected host trees in two central Appalachian national forests. Ann. Entomol. Soc. Am. 93, 500–511. https://doi.org/10.1603/0013-8746(2000)093[0500:OOLOSH]2.0.CO;2 (2000).
Schroeder, L. A. Changes in tree leaf quality and growth performance of lepidopteran larvae. Ecology 67, 1628–1636. https://doi.org/10.2307/1939094 (1986).
Razeng, E. & Watson, D. M. Nutritional composition of the preferred prey of insectivorous birds: popularity reflects quality. J. Avian Biol. 46, 89–96. https://doi.org/10.1111/jav.00475 (2015).
Kennedy, A. C. Examining Breeding Bird Diets to Improve Avian Conservation Efforts, University of Delaware, (2019).
Burghardt, K. T., Tallamy, D. W. & Shriver, W. G. Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conserv. Biol. 23, 219–224. https://doi.org/10.1111/j.1523-1739.2008.01076.x (2009).
Zuefle, M. E., Brown, W. P. & Tallamy, D. W. Effects of non-native plants on the native insect community of Delaware. Biol. Invasions 10, 1159–1169. https://doi.org/10.1007/s10530-007-9193-y (2008).
Grubb, T. C. On sex-specific foraging behavior in the white-breasted Nuthatch. J. Field Ornithol. 53, 305–314 (1982).
Cooper, N. W., Thomas, M. A. & Marra, P. P. Vertical sexual habitat segregation in a wintering migratory songbird. Ornithology 138, ukaa080. https://doi.org/10.1093/ornithology/ukaa080 (2021).
Fogg, A. M., George, T. L. & Purcell, K. L. Intersexual variation in the foraging ecology of sexually monochromatic Western Wood-Pewees. J. Field Ornithol. 84, 40–48. https://doi.org/10.1111/jofo.12004 (2013).
Morse, D. H. A quantitative study of foraging of male and female spruce-woods warblers. Ecology 49, 779–784. https://doi.org/10.2307/1935549 (1968).
Petit, L. J., Petit, D. R., Petit, K. E. & Fleming, W. J. Intersexual and temporal variation in foraging ecology of prothonotary warblers during the breeding season. The Auk. 107, 133–145. https://doi.org/10.1093/auk/107.1.133 (1990).
Preisser, E., Smith, D. C. & Lowman, M. D. Canopy and ground level insect distribution in a temperate forest. Selbyana 12, 141–146 (1998).
Briedis, M. et al. A full annual perspective on sex-biased migration timing in long-distance migratory birds. Proc. Biol. Sci. 286, 20182821. https://doi.org/10.1098/rspb.2018.2821 (2019).
Razeng, E. & Watson, D. M. Nutritional composition of the preferred prey of insectivorous birds: popularity reflects quality. J. Avian Biol. 46, 89–96. https://doi.org/10.1111/jav.00475 (2014).
Nell, C. S. et al. Consequences of arthropod community structure for an at-risk insectivorous bird. PLOS ONE 18, e0281081. https://doi.org/10.1371/journal.pone.0281081 (2023).
Ustinova, E. N. & Lysenkov, S. N. Comparative study of the insect community visiting flowers of invasive goldenrods (Solidago canadensis and S. gigantea). Arthropod Plant Interact. 14, 825–837. https://doi.org/10.1007/s11829-020-09780-7 (2020).
Badenes-Pérez, F. R. Trap crops and insectary plants in the order brassicales. Ann. Entomol. Soc. Am. 112, 318–329. https://doi.org/10.1093/aesa/say043 (2018).
Mathakutha, R. et al. Invasive species differ in key functional traits from native and non-invasive alien plant species. J. Veg. Sci. 30, 994–1006. https://doi.org/10.1111/jvs.12772 (2019).
Seewagena, C. L., Slayton, E. J. & Guglielmo, C. G. Passerine migrant stopover duration and spatial behaviour at an urban stopover site. Acta Oecol. 36, 484–492. https://doi.org/10.1016/j.actao.2010.06.005 (2010).
Acknowledgements
We thank the banders and volunteers at the McGill Bird Observatory who helped collect data on the Swainson’s Thrushes and Tennessee Warblers migrating in 2021 and 2022. This project was funded by the Environmental Damages Fund (EDF), Kenneth M Molson Foundation, Environment and Climate Change Canada, the Canadian Wildlife Service, a Natural Sciences and Engineering Research Council of Canada—Undergraduate Student Research Award (NSERC USRA, reference #522431), a NSERC Alexander Graham Bell Canada Graduate Scholarship-Master’s (CGS M) award, a Fonds de recherche du Québec—Nature et technologies (FRQNT, reference #320544) grant, and Bird Protection Québec who is an important funder of the McGill Bird Observatory.
Author information
Authors and Affiliations
Contributions
A.B.B.: conceptualization, data curation, formal analysis, methodology and writing (original draft, review, and editing). V.P.: conceptualization, data curation, formal analysis, methodology and writing (review and editing). K.H.E.: supervision of the entire project, funding acquisition, conceptualization formal analysis, investigation, project administration, writing (review and editing). B.F.: supervision of the project, funding acquisition, conceptualization, formal analysis, investigation, writing (review and editing). D.N.: writing (review and editing).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Blanc-Benigeri, A., Poirier, V., Narango, D. et al. Diet of moulting Swainson's Thrushes (Catharus ustulatus) and Tennessee Warblers (Leiothlypis peregrina) at a stopover site during fall migration measured with fecal DNA metabarcoding. Sci Rep 14, 9913 (2024). https://doi.org/10.1038/s41598-024-59462-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59462-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.