Abstract
Chronic kidney disease (CKD) is often a common comorbidity in critically ill patients with type 2 diabetes mellitus (T2DM). This study explored the relationship between blood urea nitrogen to serum albumin ratio (BAR) and mortality in T2DM patients with CKD in intensive care unit (ICU). Patients were recruited from the Medical Information Mart database, retrospectively. The primary and secondary outcomes were 90-day mortality, the length of ICU stay, hospital mortality and 30-day mortality, respectively. Cox regression model and Kaplan–Meier survival curve were performed to explore the association between BAR and 90-day mortality. Subgroup analyses were performed to determine the consistency of this association. A total of 1920 patients were enrolled and divided into the three groups (BAR < 9.2, 9.2 ≤ BAR ≤ 21.3 and BAR > 21.3). The length of ICU stay, 30-day mortality, and 90-day mortality in the BAR > 21.3 group were significantly higher than other groups. In Cox regression analysis showed that high BAR level was significantly associated with increased greater risk of 90-day mortality. The adjusted HR (95%CIs) for the model 1, model 2, and model 3 were 1.768 (1.409–2.218), 1.934, (1.489–2.511), and 1.864, (1.399–2.487), respectively. Subgroup analysis also showed the consistency of results. The Kaplan–Meier survival curve analysis revealed similar results as well that BAR > 21.3 had lower 90-day survival rate. High BAR was significantly associated with increased risk of 90-day mortality. BAR could be a simple and useful prognostic tool in T2DM patients with CKD in ICU.
Similar content being viewed by others
Introduction
The international diabetes federation predicted that the global population of diabetes will reach to 454 million in 2030; furthermore, the number will increase to 783 million in 20451. Type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD) are both chronic diseases. A study showed that the prevalence of T2DM patients complicated with CKD reached to 64–81.6/10,000, and it will be further increased2. CKD is one of the most debilitating and expensive complication of T2DM3. T2DM complicated with CKD will reduce life expectancy by 16 years than without CKD, which will increase higher medical cost burden and mortality4,5. T2DM and CKD patients often need to restrict their diet during their daily treatment, and thus are prone to disease-related malnutrition, which further leads to poor prognosis before admission to ICU6,7.
T2DM patients with CKD are accompanied by protein-energy malnutrition and inflammation before admission to ICU. Blood urea nitrogen (BUN) not only reflects the status of kidney function, but also as an important indicator of protein nutrient metabolism level and inflammation8. A study found that high BUN levels were positively associated with increased adverse renal outcomes9. Albumin is the main component of plasma proteins, and as a commonly used biomarker in clinical practice, its slight changes can affect the prognosis of patients in various diseases. A study showed that low serum albumin levels accelerated the decline of kidney function10. Studies have reported that BAR can be used as a useful biomarker for poor prognosis of many diseases, such as gastrointestinal bleeding, sepsis, COVID-19, and acute kidney injury11,12,13.
However, since the impact of BAR among T2DM patients with CKD has not yet been investigated, in the study, we evaluated the relationship between BAR and the prognosis of T2DM patients with CKD in ICU.
Materials and methods
Data source
This was a retrospective cohort study that involved Medical Information Mart for Intensive Care III (MIMIC-III) (https://physionet.org/content/mimiciii/1.4/) database. MIMIC-III database contains clinical information on over 50,000 ICU patients at the Beth Israel Deaconess Medical Center from 2001 to 201214. After completing the online training course of the National Institutes of Health, the author (S.L.) obtained access to this database (certification number: 42883491). Accessed to database was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). We extracted patient demographics, laboratory findings, length of hospital stay, 30- and 90-day mortality, and other clinical variables by PostgreSQL V.10.0. Our study was performed in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion and exclusion criteria
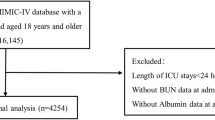
Patients admitted to the ICU for the first time were included in this study. Patients were excluded according to: (1) Less than 18 years old; (2) ICD code is not T2DM. (3) Without CKD. According to the KDIGO clinical practice guidelines, CKD was diagnosed that glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 for 3 months or more15. (4) Missing BUN or serum albumin values; (5) Less than 48 h in ICU; (6) Missing data for more than 5% of patients. Finally, a total of 1920 patients were included in this study (Fig. 1). We had listed the top five diagnosed diseases and top five diseases that were first diagnosed in this population at admission to ICU (Supplementary Table 1, 2).
Data extraction
We extracted the variables as follows: age, gender, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), coronary artery disease (CAD), chronic heart failure (CHF), hypertension, CKD stage, sequential organ failure assessment (SOFA), hemoglobin (HGB), white blood cell (WBC) count , platelet count, potassium, sodium, phosphate, creatinine, blood urea nitrogen (BUN), albumin (ALB), alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), glucose, lactate level, arterial oxygen partial pressure (PaO2), partial thromboplastin time (PTT), prognostic nutritional index (PNI) and estimate-GFR (eGFR) by structured query language in PostgreSQL. We had listed some diseases that might influence the prognosis and clinical data itself (Supplementary Table 3). BAR was calculated by dividing the BUN by the albumin.
Statistical analysis
Patients were divided into three groups: BAR < 9.2, 9.2 ≤ BAR ≤ 21.3 and BAR > 21.3, according to the interquartile ranges (IQRs) of BAR value. Continuous variables were presented as mean ± standard deviations or IQRs, and classification variables were presented as totals and percentage (%). The chi-square test was used for classified variables between groups. The Wilcoxon rank-sum test was used for non-normally distributed continuous variables, and student t-test was used for normally distributed continuous variables. Univariate and multivariate cox regression were used to assess the independent factors associated with 90-days mortality, which presented as the hazard ratio (HR) and 95% confidence interval (CI). In order to reduce the impact of confounding factors, we constructed three Cox regression models to identify the potential clinical usefulness of BAR by including covariates with p values < 0.05 in the univariate Cox analyses or for importance of clinical concern. Kaplan–Meier survival analysis was used to determine the difference in 90-day mortality between the three groups. Stratification analyses was used to assess the association of BAR with 90-day mortality. P < 0.05 was considered statistically significant. The statistical analyses were performed using the Stata software version 16.0 (Stata Corp. LLC, TX, US).
Ethical approval and consent to participate
The informed consent was waived by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The patient's information has been standardized and the project did not affect clinical care, so requirement for individual patient consent was waived.
Results
Baseline characteristics
A total of 1920 patients were enrolled and were divided into the three groups: low-BAR group (BAR < 9.2, n = 486), mid-BAR group (9.2 ≤ BAR ≤ 21.3, n = 954), and high-BAR group (BAR > 21.3, n = 480). The number of man was significantly higher in the high-BAR group with lower proportion of hypertension. A higher proportion of CHF, CKD 4 stage and CKD 5 stage, along with higher levels of SOFA score, WBC, potassium, phosphate, creatinine, ALP, lactate and PTT in the high-BAR group; Patients with higher BAR had lower age, SBP, DBP, HGB, sodium, PaO2, PNI, and eGFR (all p < 0.05) (Table 1).
BAR levels and outcome
Compared with low-BAR group (BAR < 9.2), patients with higher BAR had significantly higher the length of stay, in-hospital mortality, 30-day mortality and 90-day mortality (p < 0.05) (Table 2).
Association between the BAR and 90-day mortality
Univariate and multivariate Cox regression analyses were utilized to evaluate the significance of BAR in predicting 90-day mortality. Univariate regression analysis showed that age, weight, SBP, DBP, CAD, CKD stage, SOFA score, WBC, phosphate, lactate, PTT, eGFR, BAR, mid-BAR and high-BAR were significantly associated with 90-day mortality (p < 0.05) (Table 3). Unadjusted in model 1, high BAR level was significantly associated with higher risk of 90-day mortality (BAR as continuous variable, HR 1.018, 95% CI 1.011–1.027; mid-BAR, HR 1.261, 95% CI 1.021–1.557; high-BAR, HR 1.768, 95% CI 1.409–2.218). Adjust for age, gender, weight, SBP, DBP, CAD, CHF, hypertension, CKD stage and SOFA score in model 2, high BAR level was also significantly associated with higher risk of 90-day mortality (BAR as continuous variable, HR 1.021, 95% CI 1.012–1.030; mid-BAR, HR 1.304, 95% CI 1.043–1.631; high-BAR, HR 1.934, 95% CI 1.489–2.511). Furthermore, Adjust for model 2 plus HGB, WBC, platelet, potassium, sodium, phosphate, ALP, ALT, AST, glucose, PaO2, PTT and eGFR in model 3, high BAR level remained a greater risk of 90-day mortality (BAR as continuous variable, HR 1.018, 95% CI 1.009–1.028; mid-BAR, HR 1.277, 95% CI 1.011–1.618; high-BAR, HR 1.864, 95% CI 1. 399–2.487) (all p < 0.05) (Table 4).
Prediction of 90-day mortality
The receiver operating characteristic (ROC) curve generated using the indicator variables (BAR, ALB, and BUN) were shown in Fig. 2. The AUC value of BAR was 0.708, which showed significantly higher AUC value than the ALB and BUN (p < 0.05). Similarly, the c-statistic of BUN, ALB, and BAR showed that BAR had the highest c-statistic (Supplementary Table 4).
Subgroup analyses
Subgroup analyses were used to determine the consistency of association between BAR and risk of 90-day mortality (Table 5). Subgroup analyses showed that patients with age > 75(HR 1.024, 95% CI 1.013–1.034), man (HR 1.022, 95% CI 1.012–1.032), hypertension (HR 1.023, 95% CI 1.010–1.037), CHF (HR 1.019, 95% CI 1.008–1.030), CAD (HR 1.022, 95% CI 1.007–1.038), SOFA score ≥ 5 (HR 1.014, 95% CI 1.005–1.023), weight ≥ 77 (HR 1.027, 95% CI 1.016–1.038), CKD 3 stage (HR 1.023, 95% CI 1.011–1.035), phosphate < 4 (HR 1.019, 95% CI 1.005–1.033), WBC ≥ 11.3 (HR 1.019, 95% CI 1.010–1.029), lactate ≥ 2.4 (HR 1.024, 95% CI 1.013–1.035), ALP ≥ 103 (HR 1.022, 95% CI 1.012–1.032) and PaO2 < 155 (HR 1.019, 95% CI 1.009–1.029) had a significantly higher risk of 90-day mortality with high BAR level. The results also showed that high BAR was significantly associated with increased 90-day mortality in these patients with CKD stage 5 (Supplementary Table 5).
Kaplan–Meier analysis
The Kaplan–Meier survival curve analysis showed that BAR > 21.3 had worst prognosis. Patients in higher BAR group had significantly higher risk of 90-day mortality than low and mid-BAR groups (BAR > 21.3 vs 9.2 ≤ BAR ≤ 21.3 vs BAR < 9.2; 40.2% vs 30.6% vs 25.1%, respectively; log-rank test p value < 0.001) (Fig. 3).
Discussion
With the increasing prevalence of T2DM and CKD in worldwide, timely identification of prognostic risk factor is particularly important in clinical work. In our study, we found that higher BAR on admission to ICU was significantly associated with an increased risk of 90-day mortality in T2DM patients with CKD. And BAR could serve as an independent predictive factor of 90-day mortality. Further, the K-M curve also presented that the high BAR group had a worse prognosis. Our study was the first largest study to explored the relationship between BAR and prognosis in T2DM patients with CKD in the ICU.
BUN is a nitrogen-containing compound that it is influenced by renal function, neurohormone, and sympathetic nervous activity. BUN is mainly filtered through the glomeruli and excreted through urine. When glomerular filtration function decreases, BUN concentration will increase. BUN can not only be used to estimate glomerular filtration function, but also to assess the body's nutritional status, low blood volume, protein metabolism and others8. Many studies have found that BUN was a powerful predictor of prognosis in patients with heart failure, and its efficacy was even better than GFR and serum creatinine16,17. A large study in China found that BUN levels were positively associated with the risk of developing T2DM in Chinese adults18. Studies in T2DM patients showed that the increased BUN level will significantly increase the risk of diabetes retinopathy and diabetes nephropathy19,20. Elevated BUN indicated poor prognosis for patients in the ICU13,21,22. In the ICU, T2DM patients with CKD had circulatory dysfunction and neuroendocrine system dysfunction, which further aggravated kidney injury. At this time, high levels of BNU may predict a worse prognosis for patients.
ALB is not only a nutritional marker, but also plays an important role in anti-inflammatory, antioxidant and others aspects23. As an important antioxidant in plasma, ALB inhibits apoptosis of renal tubular cells by clearing oxygen free radicals24. It was found that ALB not only improves renal perfusion and glomerular filtration by prolonging renal vasodilation, but also selectively inhibits the expression of tumor necrosis factor-α-induced vascular cell adhesion molecule 1 and the activation of nuclear factor kB and monocyte adhesion in human endothelial cells to prevent kidney injury13,25.In T2DM patients, ALB level was negatively correlated with the incidence of diabetes retinopathy26. Besides, a study also showed that hypoproteinemia significantly accelerated the risk of renal failure in patients with diabetes nephropathy27. Low ALB level was caused by insufficient nutrition intake and a state of inflammatory stress in ICU patients. Numerous studies had suggested that hypoalbuminemia was a risk factor for poor prognosis in ICU patients28,29,30.
High BAR levels are caused by high BUN or low ALB. BAR has been proven to be a more reliable predictor than BUN or ALB. In our study, ROC curve also showed the AUC value of BAR was significantly higher than the ALB and BUN. Studies suggest that high BAR can significantly increase the mortality of patients with sepsis, acute myocardial infarction, acute pulmonary embolism, heart failure and others11,21,31,32. A study found that high BAR significantly increased in-hospital mortality and the incidence of AKI for patients with cerebral hemorrhage in the ICU13. Our study also found similar results. We found that when BAR > 21.3, patients' stay in the ICU, hospital mortality, and 30-day and 90-day mortality were significantly increased. After adjusting for confounders such as serum creatinine, CKD and others, elevated BAR was still positively associated with poor prognosis in patients undergoing cardiac surgery33. Similarly, in our study, after controlled for confounding variables by multivariate Cox regression analysis, we found that high BAR was an independent risk factor for 90-day mortality. Besides, in subgroup analysis, we demonstrated that BAR was an effective predictor of 90-day mortality in T2DM patients with CKD under various specific conditions. Therefore, in clinical practice, we may be able to reduce the BUN value by improving glomerular filtration rate (such as increasing renal perfusion by maintaining normal volume load), and also by increasing albumin (infusion of human serum albumin, etc.) to reduce BAR, thereby reducing the risk of 90 day mortality in patients. In routine clinical practice, BAR could be calculated easily and quickly, and is more stable and conducive to clinical application compared with a single indicator.
In our study, several limitations should be highlighted to interpret the results as follows: First, this study as a single-centric retrospective study, we couldn’t avoid to selection bias. Second, the data used in the manuscript were recorded from 2001 to 2012, which were old. Third, due to the limited contents of this database, some unrecorded clinical information was missing, may affect the outcome. Fourth, this study only included BUN and albumin records of patients at their first admission, so that the prognostic impact of dynamic changes in BAR was still unclear. Finally, the underlying mechanism of BAR affected the prognosis could not be determined. Therefore, it is necessary to establish a large multicenter prospective study to confirm the above results and further to investigate the mechanism.
Conclusion
A higher BAR was significantly associated with an increased risk of 90-day mortality. BAR could be served as a prognostic predictor for 90-day mortality in T2DM patients with CKD in ICU, due to its inexpensive and readily available nature.
Data availability
Original data used in this study is from the MIMIC-III database: MIMIC III (https://physionet.org/content/mimiciii/1.4/, version 1.4). The author (S.L.) obtained access to this database (certification number: 42883491) and was responsible for extracting the data. If needed, related data can be provided by contacting G.H. and S.L.
References
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157, 107843 (2019).
Tuttle, K. R. et al. Incidence of chronic kidney disease among adults with diabetes, 2015–2020. N. Engl. J. Med. 387, 1430–1431 (2022).
Garla, V., Kanduri, S., Yanes-Cardozo, L. & Lién, L. F. Management of diabetes mellitus in chronic kidney disease. Minerva Endocrinol. 44, 273–287 (2019).
Wen, C. P. et al. Diabetes with early kidney involvement may shorten life expectancy by 16 years. Kidney Int. 92, 388–396 (2017).
Pan, X. et al. The burden of diabetes-related chronic kidney disease in China from 1990 to 2019. Front. Endocrinol. 13, 892860 (2022).
Ahmed, I., Kaifi, H. M., Tahir, H. & Javed, A. Malnutrition among patients with type-2 diabetes mellitus. Pak. J. Med. Sci. 39, 64–69 (2023).
Slee, A. & Reid, J. Disease-related malnutrition in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care. 25, 136–141 (2022).
Tanaka, S. et al. Impact of blood urea nitrogen to creatinine ratio on mortality and morbidity in hemodialysis patients: The Q-Cohort Study. Sci. Rep. 7, 14901 (2017).
Seki, M. et al. Blood urea nitrogen is independently associated with renal outcomes in Japanese patients with stage 3–5 chronic kidney disease: A prospective observational study. BMC Nephrol. 20, 115 (2019).
Cheng, T. et al. The level of serum albumin is associated with renal prognosis and renal function decline in patients with chronic kidney disease. BMC Nephrol. 24, 57 (2023).
Zou, X. L., Feng, D. Y., Wu, W. B., Yang, H. L. & Zhang, T. T. Blood urea nitrogen to serum albumin ratio independently predicts 30-day mortality and severity in patients with Escherichia coli bacteraemia. Med. Clin. (Barc). 157, 219–225 (2021).
Huang, D. et al. Blood urea nitrogen to serum albumin ratio (BAR) predicts critical illness in patients with coronavirus disease 2019 (COVID-19). Int. J. Gen Med. 14, 4711–4721 (2021).
Yang, F. et al. Prognostic value of blood urea nitrogen to serum albumin ratio for acute kidney injury and in-hospital mortality in intensive care unit patients with intracerebral haemorrhage: A retrospective cohort study using the MIMIC-IV database. BMJ Open. 13, e069503 (2023).
Johnson, A. E. et al. MIMIC-III, a freely accessible critical care database. Sci. Data. 3, 160035 (2016).
Levin, A. & Stevens, P. E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 85, 49–61 (2014).
Miura, M. et al. Prognostic impact of blood urea nitrogen changes during hospitalization in patients with acute heart failure syndrome. Circ. J. 77, 1221–1228 (2013).
Kazory, A. Emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am. J. Cardiol. 106, 694–700 (2010).
Li, S. N. et al. Association between blood urea nitrogen and incidence of type 2 diabetes mellitus in a Chinese population: A cohort study. Endocr. J. 68, 1057–1065 (2021).
Zhong, J. B. et al. A closer association between blood urea nitrogen and the probability of diabetic retinopathy in patients with shorter type 2 diabetes duration. Sci. Rep. 13, 9881 (2023).
Hu, Y., Shi, R., Mo, R. & Hu, F. Nomogram for the prediction of diabetic nephropathy risk among patients with type 2 diabetes mellitus based on a questionnaire and biochemical indicators: a retrospective study. Aging (Albany NY). 12, 10317–10336 (2020).
Lin, Z. et al. Blood urea nitrogen to serum albumin ratio as a new prognostic indicator in critical patients with chronic heart failure. ESC Heart Fail. 9, 1360–1369 (2022).
Giri, M. et al. Blood urea nitrogen is associated with in-hospital mortality in critically ill patients with acute exacerbation of chronic obstructive pulmonary disease: A propensity score matching analysis. J. Clin. Med. 11, 6709 (2022).
Fanali, G. et al. Human serum albumin: From bench to bedside. Mol. Aspects Med. 33, 209–290 (2012).
Roche, M., Rondeau, P., Singh, N. R., Tarnus, E. & Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 582, 1783–1787 (2008).
Zhang, W. J. & Frei, B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc. Res. 55, 820–829 (2002).
Wang, G. X. et al. The correlation between serum albumin and diabetic retinopathy among people with type 2 diabetes mellitus: NHANES 2011–2020. PLoS ONE. 17, e0270019 (2022).
Zhang, J. et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J. Diabetes Res. 2019, 7825804 (2019).
Chao, P. et al. Serum albumin and the short-term mortality in individuals with congestive heart failure in intensive care unit: an analysis of MIMIC. Sci. Rep. 12, 16251 (2022).
Bekhit, O. E., Yousef, R. M., Abdelrasol, H. A. & Mohammed, M. A. Serum albumin level as a predictor of outcome in patients admitted to pediatric intensive care units. Pediatr. Emerg. Care. 37, e855–e860 (2021).
Liang, M. et al. The association between lactate dehydrogenase to serum albumin ratio and the 28-day mortality in patients with sepsis-associated acute kidney injury in intensive care: a retrospective cohort study. Ren. Fail. 45, 2212080 (2023).
Zhao, D. et al. Predictive value of blood urea nitrogen to albumin ratio in long-term mortality in intensive care unit patients with acute myocardial infarction: A propensity score matching analysis. Int. J. Gen. Med. 15, 2247–2259 (2022).
Fang, J. & Xu, B. Blood urea nitrogen to serum albumin ratio independently predicts mortality in critically ill patients with acute pulmonary embolism. Clin. Appl. Thromb. Hemost. 27, 10760296211010240 (2021).
Ye, L. et al. Elevated blood urea nitrogen to serum albumin ratio is an adverse prognostic predictor for patients undergoing cardiac surgery. Front. Cardiovasc. Med. 9, 888736 (2022).
Funding
This work was supported by Project of Jiangmen Science and Technology Bureau, Grant No. 2022YL01052 and 2023YL01037.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.L., F.L. and G.H.; methodology, S.L., C.Q., X.L. and G.H.; software, S.L., X.L., and C.Q; validation, S.L., G.H., W.L. and C.Q.; formal analysis, X.L., S.L., F.L. and C.Q.; investigation, G.H., S.L., F.L. and X.L.; resources, S.L.; data curation, X.L., S.L., W.L., G.H. and C.Q.; writing—original draft preparation, all authors; writing—review and editing, C.Q., S.L., and G.H.; visualization, S.L. and F.L.; supervision, G.H. and X.L.; project administration, S.L.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Qiu, C., Li, W. et al. Blood urea nitrogen to serum albumin ratio as a new prognostic indicator in type 2 diabetes mellitus patients with chronic kidney disease. Sci Rep 14, 8002 (2024). https://doi.org/10.1038/s41598-024-58678-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58678-4
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.