Abstract
For patients with upper limb melanoma, the significance of specific death is more important than that of all-cause death, and traditional survival analysis may overestimate the mortality rate of patients. Therefore, the nomogram model for predicting the specific mortality risk of melanoma in the upper limbs was developed. A population with melanoma in the upper limbs, diagnosed from 2010 to 2015, were selected from the National Cancer Institute database of Surveillance, Epidemiology, and End Results (SEER). The independent predictive factors of specific death were confirmed by the competing risk model of one-factor analysis and multi-factor analysis, and the nomogram was constructed according to the independent predictive factors. 17,200 patients with upper limb melanoma were enrolled in the study (training cohort: n = 12,040; validation cohort: n = 5160). Multi-factor analysis of the competing risk model showed that age, marital status, gender, tumor stage, T stage, M stage, regional lymph node surgery information, radiotherapy, chemotherapy, mitotic cell count, ulcer and whether there were multiple primary cancers, were independent factors affecting the specific death of upper limb melanoma patients (P < 0.05). The nomogram has good predictive ability regarding the specific mortality risk of melanoma in the upper limbs, and could be of great help to formulate prognostic treatment strategies and follow-up strategies that are conducive to survival.
Similar content being viewed by others
Introduction
Melanoma originates from melanocytes, and is one of the most aggressive malignant tumors. They easily recur or metastasize. According to the characteristics of the pathogenesis, course and prognosis of melanoma, melanoma mainly includes the following four subtypes: Superficial Spreading Melanoma (SSM), Lentigo Maligna Melanoma (LMM), Nodular Melanoma (NM) and Acral Lentiginal Melanoma (ALM)1. Among these four subtypes, the incidence of acral melanoma is the highest in Asian countries. Among the white population, acral melanoma accounts for 1–7% of all malignant melanomas, but among Asian people, acral melanoma accounts for more than 50%. The most common primary sites are palms, toes, fingertips and nails2. Population based studies have shown that, compared with non-acral melanoma, acral melanoma has a lower survival rate and a worse prognosis3. Because melanomas often metastasize through lymph or blood, and lack effective intervention measures, they are usually found in late stages. However, the survival rate of advanced or metastatic melanoma is extremely low. The overall survival period of this kind of rapidly progressive disease is 6–9 months. The 1-year survival rate is 30%—60%, and the 5-year survival rate is only 16%4. Therefore, early prediction and diagnosis of acral melanoma is conducive to reducing mortality and improving disease survival rate through long-term effective prognosis and control.
At present, studies on the mortality risk of acral melanoma mainly focus on all-cause death. However, for this kind of disease with good early prognosis, it is found in long-term follow-up that the risk of non-cancer events affecting the survival and prognosis of patients with acral melanoma is also increasing, such as nervous system diseases, heart disease, leukemia, accidents, etc.5. However, the current risk research tools often do not have population variables reflecting such other causes of death, which reduces the accuracy of all-cause mortality prediction tools. The traditional survival analysis only focuses on one endpoint event, and if the competing events are not considered, the risk of death may be overestimated. In a practical sense, considering specific death from other causes is more instructive for clinicians and caregivers than all-cause death. Therefore, for patients with melanoma, it is very important to carry out risk stratification for specific death at an early stage. At present, there is a lack of relevant tool to predict the specific death risk of melanoma in the upper limbs. Therefore, the present study analyzed the independent influencing factors of melanoma specific death in the upper limbs based on the competing risk model, and developed a predictive nomogram model for the specific death risk of melanoma in the upper limbs, which could help broaden researchers’ train of thoughts regarding risk prediction and prognosis research for these patients.
Methods
Data collection
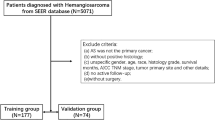
The patients who were definitely diagnosed with melanoma by pathology/histology from 2010 to 2015 in the database of Surveillance, Epidemiology, and End Results (SEER) were included as the subjects of this study. Inclusion criteria: (1) The primary site was on the upper limbs; (2) Melanoma was clearly diagnosed by pathology or histology; (3) The diagnosis was made from 2010 to 2015. Patients’ demographic information (age, marital status, gender, race) and clinical information (affected side, tumor stage, T stage, N stage, M stage, operation information of primary tumor, operation information of regional lymph nodes, operation of distant metastasis site, radiotherapy, chemotherapy, Breslow thickness, LDH, number of mitotic cells, ulcer, number of positive lymph nodes, bone metastasis, brain metastasis, liver metastasis, lung metastasis, tumor diameter, number of tumors at diagnosis, whether multiple primary cancers are involved, survival time, cause of death and survival status) were collected. At the same time, these were excluded: (1) survival time < 1 month; (2) Patients with unclear tumor stage, Breslow thickness, affected side, surgical information, mitotic cell number, ulcer and other information at the time of diagnosis; (3) Unknown cause of death (COD). The flow chart of the specific screening process is shown in Fig. 1.
Construction of competing risk model
In this study, a nomogram of competing risk model was constructed. Therefore, in the data, the death cases caused by other reasons were defined as competing events, the cases that were still alive at the end of the follow-up period were defined as right censoring events, and the cases that passed away from melanoma were defined as interest events. 17,200 samples were randomly divided into a training cohort (70%) and a validation cohort (30%) according to a ratio of 7:3. In the training cohort, single factor analysis and multi-factor analysis of competing risk model were used to screen the independent influencing factors of upper limb melanoma specific death. Variables were discarded due to more than 50% missing values or singular regression problems. According to these independent influencing factors, a risk prediction nomogram model was constructed. The concordance index (c-index) and the areas under the ROC curve (AUC) at different time points were used to evaluate the prediction accuracy of the nomogram, and a larger value indicated a higher prediction accuracy. The calibration curve was used to assess the calibration of the predictive nomogram, and it represents the agreement between the observed and predicted probabilities through 1000 iterations of resampling.
Statistical methods
R software (Version 4.2.1) and SPSS 26.0 software were used for statistical analysis. Firstly, continuous variables (age, Breslow thickness, number of mitotic cells) were expressed under the form of mean ± standard deviation, and data of the two groups were tested by independent variance T-test. Count data were expressed by case frequency (%), and chi-square test was used for comparison between groups. The patients were randomized into a training cohort, which was used to identify prognostic factors associated with cause-specific death in patients with upper limb melanoma and construct the nomogram, and a validation cohort, which was used to validate the accuracy and effectiveness of the model. Nomograms are common tools for prognostic assessment of tumors, and through nomograms, the complex regression equations are transformed into visual charts, making the results of prediction models more readable and easier for patient assessment. Secondly, traditional survival analysis and competing risk model were used to compare the specific death risk at different time points. After the model was constructed, we calculated the c-index and AUC values in the training and validation cohorts to evaluate the effectiveness and reliability of the prediction model and employed the calibration curve to assess the consistency between the model and the actual situation. P < 0.05 was considered as statistically significant.
Results
Clinical characteristics
A total of 17,200 patients were included in this study, and randomly assigned to the training cohort (N = 12,040) and the validation cohort (N = 5160). The average age of patients in the training cohort was 63.41 ± 15.204 years old, 8199 (68.10%) were married, 6482 (53.84%) were male, and 11,933 (99.11%) were of the white race. The average age of patients in the validation cohort was 36.11 ± 15.544 years old; 3542 (68.64%) were married patients, 2795 (54.17%) were male patients, and 5101 (98.86) were patients of white race. 1548 (12.9%) patients died of other causes in the training cohort, and 638 (12.4%) patients died of other causes in the validation cohort. Regarding other variables (Table 1), there was no statistically significant difference between the clinical characteristics of patients in the training cohort and the validation cohort (P > 0.05).
Building a competing risk model
The results from single factor competing risk model analysis showed that age, marital status, gender, race, tumor stage, T stage, N stage, M stage, primary tumor operation information, regional lymph node operation information, distant metastasis site surgery, radiotherapy, chemotherapy, Breslow thickness, LDH, mitotic cell count, ulcer, number of positive lymph nodes, bone metastasis, brain metastasis, liver metastasis, lung metastasis, tumor diameter, whether multiple primary cancers were involved; all these may cause specific death of melanoma in the upper limbs (Table 2). By substituting these variables into the analysis of the multifactor competing risk model, it was concluded that age, marital status, sex, tumor stage, T stage, M stage, regional lymph node operation information, radiotherapy, chemotherapy, mitotic cell count, ulcer, number of positive lymph nodes, bone metastasis, brain metastasis, liver metastasis, lung metastasis and whether there were multiple primary cancers, were independent influencing factors for the specific death of upper limb melanoma patients (Table 2). Based on these independent influencing factors, a nomogram for predicting the specific mortality risk of melanoma in the upper limbs was constructed (Fig. 2).
Validation of the competing risk model
In this study, the c-index and AUC were used to evaluate the prediction accuracy of the nomogram, and the calibration curve was used to evaluate the calibration of the predictive nomogram. Through the construction of the prediction model of upper limb melanoma specific death, the C-index of the prediction model in the training cohort was 0.894, and the areas under the ROC curve (Fig. 3) of the training cohort at 1, 3 and 5 years was 0.926 (95% CI 0.907–0.945), 0.915 (95% CI 0.903–0.927) and 0.896 (95% CI 0.883–0.910) respectively. At the same time, the calibration curve of the prediction model showed that the predicted risk of the model corresponded well with the actual risk. (Fig. 4). Therefore, the risk prediction model had a quite satisfying prediction effect in the training cohort.
Similarly, the risk prediction model also showed good prediction effect in the validation cohort. The C-index of the prediction model in the validation cohort was 0.875. The AUC of the validation cohort for 1 year, 3 years and 5 years were 0.911 (95% CI 0.887–0.934), 0.903 (95% CI 0.890–0.916) and 0.885 (95% CI 0.871–0.899) respectively (Fig. 3). At the same time, the calibration curve of the prediction model showed that the model had good calibration in the validation cohort (Fig. 4).
Comparison between traditional survival analysis and competing risk model in mortality estimation
Because the traditional survival analysis only takes into account a single end point event, therefore when the traditional survival analysis estimates the morbidity and mortality, other causes of death are excluded. However, in this study, there is a competitive relationship between the death of patients due to other reasons and melanoma specific death. If the traditional survival analysis is used, it will lead to deviation in the prediction and estimation of the results; overestimating the mortality of melanoma patients, and underestimating the survival possibility of the patients. According to the comparison, at different time points, between the traditional survival analysis and the competing risk model in estimating the cumulative mortality of melanoma in the upper limbs in this study (Table 3), the mortality estimated by the traditional survival analysis and the competing risk model were significantly different. The mortality estimated by the traditional survival analysis was higher than that of the competing risk model.
Discussion
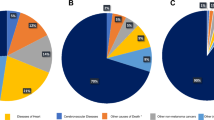
Based on the SEER database, the study found that the number of deaths from other causes in upper limb melanoma patients accounted for 12.7% of the total number. In order to avoid prediction bias caused by deletion, the study object could not exclude these patients who died from other causes. Therefore, it was impossible to build a specific death risk prediction based on traditional survival analysis. For this reason, this study used the competing risk model to construct the risk prediction nomogram of upper limb melanoma. Through internal and external validation, it was concluded that the nomogram had very ideal risk assessment accuracy (the C-index in the training cohort was 0.894, and the C-index in the validation cohort was 0.875). At the same time, the study found that there was considerable difference between the mortality estimated by the traditional survival analysis and the competing risk model, which also confirmed Lacny et al.6 conclusion that the traditional survival analysis overestimates the cumulative morbidity in the clinical field.
At present, predictive nomograms of melanoma have been widely established. Jun Tian et al. used the Kaplan–Meier method of survival analysis and COX proportional-hazards regression model to build a nomogram, based on three long non-coding RNAs (lncRNAs), to predict the overall survival rate of skin melanoma. The AUC of the validation cohort for 3, 5, and 10 years were 0.717, 0.724, and 0.633, respectively7. Deng et al. used the traditional survival analysis method, by combining autophagy related gene characteristics and clinical parameters, to construct and validate the prognostic nomogram (C-index, 0.717) of melanoma patients. The AUC values for 3 and 5 years were 0.790 and 0.7608. Sandra L. Wong et al. developed a nomogram to predict the positive probability of sentinel lymph nodes in melanoma patients (C-index, 0.694)9. Verver et al., based on Cox regression analysis, constructed and validated a nomogram of recurrence of sentinel node negative patients and melanoma specific mortality, with a c-index of 0.7610. Most studies use the results of traditional survival analysis to construct all-cause mortality nomograms. Although they can be used to predict the cumulative survival rate of melanoma, they are not accurate enough. Li et al., based on the competing risk model, developed a nomogram on cancer specific mortality of patients with metastatic cutaneous melanoma. The results showed that the AUC values at 6 months, 12 months and 18 months in the training cohort were 0.706, 0.700 and 0.706 respectively, and 0.702, 0.670 and 0.656 in the validation cohort respectively11. More accurate prediction models are needed for the competing survival risk of patients with melanoma in the upper limbs. Our study, based on the competing risk model, constructed the risk prediction nomogram of upper limb melanoma specific death. The C-index in the training cohort was 0.894, and the AUC in the training cohort for 1, 3, and 5 years was 0.926, 0.915, and 0.896 respectively. The results showed that, regarding the construction of melanoma risk prediction, the competing risk model was more effective.
This study found that the death risk of melanoma increases with age, which is consistent with some already published research results. Age is a key factor in the occurrence of all cancers. With age, the risk of gene mutation that may cause cancer increases12. Older patients with melanoma are more likely to have thicker tumors, higher mitotic rates, and are more prone to ulceration5. Marital status is also one of the predictors of the nomogram in this study. The cumulative survival rate of unmarried melanoma patients was lower than that of married patients. Previous studies have shown that compared with single status patients (single, widowed or divorced), married patients (including men and women) had a 35% lower risk of death after melanoma diagnosis13. This may be because married patients have better social support and living environment11. At the same time, we also found that women have a higher cumulative survival rate, which is consistent with other research results12. The survival rate of women in the early stages of melanoma (stage I and II and stage III) is higher than that of men14. The incidence of acral melanoma is related to race; the incidence rate in colored people is significantly higher than that in Caucasians15. TNM staging is one of the important independent factors for melanoma risk prediction, and is an internationally recognized prognostic factor of cancer. It is the most commonly used staging and prognostic assessment tool in clinical practice. AJCC staging provides the most important initial classification for melanoma patients16, which is of great significance for the prediction of melanoma specific death risk10. A large number of studies12,17,18 emphasized the importance of mitotic rate for the prognosis of melanoma. Statistically, except for thickness, the mitotic rate is the most effective survival predictor. One or more mitotic rates per square meter are related to the significant reduction of survival rate17. Our research also confirmed this point. At the same time, we also realized that ulcer is an important independent influencing factor of melanoma specific death5. The presence of ulcer may reflect the relatively rapid growth of melanoma. Previous research results showed that in the presence of ulcer, the five-year survival rate of stage I–II melanoma decreased from 80 to 55%, and that of stage III melanoma decreased from 53 to 12%19. When bone metastasis appear in melanoma, the survival rate of patients will be significantly reduced. The research results of Melissa A Wilson et al. proved this point. Clinically, melanoma will be difficult to treat when it spreads to the skeletal system20. Melanoma brain metastasis (MBM) is very common, and is related to a particularly poor prognosis. They directly lead to 60–70% of melanoma patients' death21. Our research also proved that brain metastasis of melanoma can seriously affect the survival rate of patients. The univariate competing risk model suggested that chemotherapy was a risk factor for upper limb melanoma. Previous studies have shown that metastatic melanoma has a very low response rate to single-agent chemotherapy, and multiple chemotherapy methods improve the response rate but do not significantly improve the overall survival (PMID: 12407508, PMID: 12407507). For advanced malignant melanomas, radiotherapy and chemotherapy are still relatively ideal treatment methods22, which can reduce the recurrence risk of patients to a certain extent, and prolong their survival period23. More studies are needed to clarify whether chemotherapy alone can improve the survival rate in melanoma.
Melanoma is a cancer with good prognosis at early stages. The 10-year metastasis free survival rate is 91.8–99.5%24. Therefore, in the long-term follow-up process, there may be deaths due to other causes, such as heart disease, nervous system disease, leukemia, etc. In our study, 69.5% of upper limb melanoma patients died due to other reasons. When predicting the death risk of upper limb melanoma, these competing influencing outcomes should be considered. This study explored disease mortality based on traditional survival analysis and competing risk model for different end points. The results showed that the mortality estimated by traditional survival analysis was higher than that of the competing risk model. At the same time, in the competing risk model, the mortality caused by other reasons was higher than the specific mortality. Therefore, in this study, the traditional survival analysis may have overestimated the mortality of melanoma in the upper limbs due to the deletion of outcome variables. This is consistent with the results of other diseases that used different analysis methods to estimate mortality5,25. Therefore, when using the competing risk model to predict the mortality risk of melanoma in upper limbs, this study obtained good evaluation efficacy.
The nomogram of upper limb melanoma specific death developed in this study has good predictive effect. Compared with the traditional survival analysis and prediction tools, it also has better predictability. However, as this study was based on one same database for data validation, it did not use real clinical external data for prospective validation. This kind of studies will continue to be completed in follow-up work.
Conclusion
Under the circumstance that competing events accounted for a large proportion, competing risk model seems to be a more reasonable tool for studying diseases’ specific death. The risk prediction nomogram constructed in this study, based on this method, had better accuracy and good prediction capacity for predicting the specific death risk of melanoma in upper limbs. This model can provide a reference basis for melanoma risk stratification, and can also help to formulate prognostic treatment strategies and follow-up strategies conducive to survival.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Redi, U. et al. Acral lentiginous melanoma: A retrospective study. J. Cosmet. Dermatol. 20, 1813–1820. https://doi.org/10.1111/jocd.13737 (2021).
Nakamura, Y. & Fujisawa, Y. Diagnosis and management of acral lentiginous melanoma. Curr. Treat. Options Oncol. 19, 42. https://doi.org/10.1007/s11864-018-0560-y (2018).
Bian, S. X. et al. Acral lentiginous melanoma-Population, treatment, and survival using the NCDB from 2004 to 2015. Pigment Cell Melanoma Res. 34, 1049–1061. https://doi.org/10.1111/pcmr.12999 (2021).
Hao, M., Zhao, G., Du, X., Yang, Y. & Yang, J. Clinical characteristics and prognostic indicators for metastatic melanoma: Data from 446 patients in north China. Tumour Biol. 37, 10339–10348. https://doi.org/10.1007/s13277-016-4914-4 (2016).
Shen, W., Sakamoto, N. & Yang, L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: A population-based analysis. BMC Cancer 16, 413. https://doi.org/10.1186/s12885-016-2438-3 (2016).
Lacny, S. et al. Kaplan-Meier survival analysis overestimates cumulative incidence of health-related events in competing risk settings: A meta-analysis. J. Clin. Epidemiol. 93, 25–35. https://doi.org/10.1016/j.jclinepi.2017.10.006 (2018).
Tian, J., Yang, Y., Li, M. Y. & Zhang, Y. A novel RNA sequencing-based prognostic nomogram to predict survival for patients with cutaneous melanoma: Clinical trial/experimental study. Medicine 99, e18868. https://doi.org/10.1097/md.0000000000018868 (2020).
Deng, G. et al. Nomogram based on autophagy related genes for predicting the survival in melanoma. BMC Cancer 21, 1258. https://doi.org/10.1186/s12885-021-08928-9 (2021).
Wong, S. L., Kattan, M. W., McMasters, K. M. & Coit, D. G. A nomogram that predicts the presence of sentinel node metastasis in melanoma with better discrimination than the American Joint Committee on Cancer staging system. Ann. Surg. Oncol. 12, 282–288. https://doi.org/10.1245/aso.2005.05.016 (2005).
Verver, D. et al. Development and validation of a nomogram to predict recurrence and melanoma-specific mortality in patients with negative sentinel lymph nodes. Br. J. Surg. 106, 217–225. https://doi.org/10.1002/bjs.10995 (2019).
Li, W., Xiao, Y., Xu, X. & Zhang, Y. A novel nomogram and risk classification system predicting the cancer-specific mortality of patients with initially diagnosed metastatic cutaneous melanoma. Ann. Surg. Oncol. 28, 3490–3500. https://doi.org/10.1245/s10434-020-09341-5 (2021).
Xiao, Y., Peng, S., Hu, Y., Zhang, J. & Cao, X. Development and validation of prognostic nomogram in patients with nonmetastatic malignant melanoma: A SEER population-based study. Cancer Med. 9, 8562–8570. https://doi.org/10.1002/cam4.3318 (2020).
Maas, J. A. et al. Marital status and survival in patients diagnosed with melanoma. Dermatol. Res. Pract. 2020, 2485401. https://doi.org/10.1155/2020/2485401 (2020).
Enninga, E. A. L. et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 6, 2203–2212. https://doi.org/10.1002/cam4.1152 (2017).
Kuchelmeister, C., Schaumburg-Lever, G. & Garbe, C. Acral cutaneous melanoma in caucasians: Clinical features, histopathology and prognosis in 112 patients. Br. J. Dermatol. 143, 275–280. https://doi.org/10.1046/j.1365-2133.2000.03651.x (2000).
Soong, S. J. et al. Predicting survival outcome of localized melanoma: An electronic prediction tool based on the AJCC Melanoma Database. Ann. Surg. Oncol. 17, 2006–2014. https://doi.org/10.1245/s10434-010-1050-z (2010).
Scolyer, R. A. et al. Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am. J. Surg. Pathol. 27, 1571–1576. https://doi.org/10.1097/00000478-200312000-00011 (2003).
Gimotty, P. A. et al. Thin primary cutaneous malignant melanoma: A prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J. Clin. Oncol. 22, 3668–3676. https://doi.org/10.1200/jco.2004.12.015 (2004).
Paladugu, R. R., Benfield, J. R., Pak, H. Y., Ross, R. K. & Teplitz, R. L. Bronchopulmonary Kulchitzky cell carcinomas A new classification scheme for typical and atypical carcinoids. Cancer 55, 1303–1311 (1985).
Albrecht, K. J., Nashan, D., Meiss, F., Bengel, J. & Reuter, K. Shared decision making in dermato-oncology: Preference for involvement of melanoma patients. Melanoma Res. 24, 68–74 (2014).
Gutzmer, R. et al. Melanoma brain metastases: Interdisciplinary management recommendations 2020. Cancer Treat. Rev. 89, 102083. https://doi.org/10.1016/j.ctrv.2020.102083 (2020).
Seth, R. et al. Systemic therapy for melanoma: ASCO guideline. J. Clin. Oncol. 38, 3947–3970. https://doi.org/10.1200/jco.20.00198 (2020).
Weise, A. M. & Flaherty, L. E. New options for the adjuvant treatment of cutaneous melanoma?. Curr. Oncol. Rep. 16, 409. https://doi.org/10.1007/s11912-014-0409-x (2014).
Leiter, U., Buettner, P. G., Eigentler, T. K. & Garbe, C. Prognostic factors of thin cutaneous melanoma: An analysis of the central malignant melanoma registry of the German dermatological society. J. Clin. Oncol. 22, 3660–3667. https://doi.org/10.1200/jco.2004.03.074 (2004).
Cui, H. et al. Nomogram predicts the role of primary tumor surgery on De Novo stage-IV breast cancer patients: A SEER-based competing risk analysis model. Front. Oncol. 12, 819531. https://doi.org/10.3389/fonc.2022.819531 (2022).
Author information
Authors and Affiliations
Contributions
Mingju Gao and Xinping Bai wrote the main manuscript and fully participated in all analyses. Bingwei Wu contributed to the study concept and design. Mingju Gao and Xinping Bai participated in literature search, data extraction, and quality assessment. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, M., Wu, B. & Bai, X. Establishment and validation of a nomogram model for predicting the specific mortality risk of melanoma in upper limbs based on the SEER database. Sci Rep 14, 9623 (2024). https://doi.org/10.1038/s41598-024-57541-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57541-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.