Abstract
Murine pneumonia models for ESKAPE pathogens serve to evaluate novel antibacterials or to investigate immunological responses. The majority of published models uses intranasal or to a limited extent the intratracheal instillation to challenge animals. In this study, we propose the aerosol delivery of pathogens using a nebulizer. Aerosol delivery typically results in homogeneous distribution of the inoculum in the lungs because of lower particle size. This is of particular importance when compounds are assessed for their pharmacokinetic and pharmacodynamic (PK/PD) relationships as it allows to conduct several analysis with the same sample material. Moreover, aerosol delivery has the advantage that it mimics the ‘natural route’ of respiratory infection. In this short and concise study, we show that aerosol delivery of pathogens resulted in a sustained bacterial burden in the neutropenic lung infection model for five pathogens tested, whereas it gave a similar result in immunocompetent mice for three out of five pathogens. Moreover, a substantial bacterial burden in the lungs was already achieved 2 h post inhalation. Hence, this study constitutes a viable alternative for intranasal administration and a refinement of murine pneumonia models for PK/PD assessments of novel antibacterial compounds allowing to study multiple readouts with the same sample material.
Similar content being viewed by others
Introduction
There is a high quest for novel antibacterials, especially against the so-called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp), as the rise in multidrug-resistance poses a true public health threat whereas at the same time the antibacterial development pipeline runs dry1,2,3. This still holds true despite the fact that recently, new antibacterials, including those with new modes of action like cefiderocol4,5, have reached the market or are under late-stage clinical evaluation6. Neutropenic as well as acute lung infection models are common experimental systems to provide a proof-of-concept and to explore PK/PD relationships of novel antibacterials7. Most frequently, the infection is introduced using an intranasal instillation8,9. Additional techniques consist of oropharyngeal or intratracheal instillation10,11. Nevertheless, all aforementioned techniques have several limitations: (1) The volume of the inoculum is restricted and narrow. For intranasal administration (depending on the country) only 30 to 50 µl are permitted to be used on one nostril; similarly, oropharyngeal as well as the intratracheal administration allow only 50–70 µl for instillation. (2) The success of the infection is highly dependent on the experimenter, as that person needs to assure that equal volumes are introduced9. (3) Finally, as the success of the infection depends on the breathing rate of the animal, it is possible that the infection of the lung does not occur homogeneously, but that only one lung lobe is infected12,13. This limits the use of different parts of the lung of the same mouse for analysis requiring different sample preparation, e.g. cfu determination procedures differ from RNA preparation and extraction procedures. It is known that aerosol delivery results in a more homogeneous distribution of particles within the lungs14. The aim of this study was to investigate if sustained infections were established upon aerosol infection with several pathogens of the ESKAPE group, which overcomes the aforementioned constraints of intranasal or intratracheal instillation, and to validate the models using a positive control antibacterial, i.e. levofloxacin, to demonstrate that they work in the same manner as models using intranasal or intratracheal instillation7,15.
Results
Aerosol delivery in the neutropenic pneumonia model
First, we deployed the standard neutropenic pneumonia model7, but instead of intranasal infection we used aerosol delivery of several ESKAPE pathogens, namely S. aureus, K. pneumoniae, P. aeruginosa, A. baumannii (two different strains) and S. pneumoniae. We chose one strain per pathogen which is well defined and frequently used for neutropenic animal models. Aerosol delivery was performed under ketamine/xylazine anesthesia which enabled that the mice were breathing slowly resulting in less inter-animal variation. Mice were held in upright position and infected individually using the Aeroneb® lab nebulizer device with a defined amount of inoculum (Supplemental video files S1–S3), which was placed carefully over mouth and nose of the individual animal in a dedicated infection cage. Afterwards, remaining droplets of the inoculum, which might have been deposited on the whiskers and/or the fur of the individual animals, were removed using a tissue before placing the anesthetized animal back to its original cage and on a warming mat until it was awake again.
To determine which inoculum size was needed to achieve a sustainable burden after 24 h, three different inocula were tested for every strain (Table S1). As expected, an increase of the inoculum resulted in an increased bacterial burden for S. aureus (Fig. 1a). However, 2 × 109 cfu/ml as inoculum only gave around 1 log10 unit increased burden compared to 4 × 108 cfu/ml. In case of S. pneumoniae, the increase of bacterial burden at 24 h post infection was also only slightly augmenting with a higher inoculum (Fig. 1b). For K. pneumoniae, the highest bacterial burden at 24 h post infection was achieved with an inoculum dose of 2 × 109 cfu/ml (Fig. 1c). For A. baumannii strain ATCC 19606 and P. aeruginosa only a moderate bacterial burden was achieved after 24 h and not much difference was observed between the three different inocula (Fig. 1d,e). However, inoculum preparation for those two strains in presence of 0.01% mucin resulted in a much higher terminal bacterial burden using the highest inoculum. Finally, with the highest inoculum as infection dose, a good terminal bacterial burden at 24 h post infection was achieved for the five different bacterial species assessed here (Fig. S1a). Whereas both Gram-positive bacterial strains of S. pneumoniae and S. aureus showed a bacterial burden of around 5 log10 cfu/g tissue 24 h after infection, the three Gram-negative tested strains of K. pneumoniae, P. aeruginosa and A. baumannii exhibited a bacterial burden greater than 6 log10 cfu/g tissue. Moreover, only a small standard deviation with respect to bacterial burden was seen for the strains of K. pneumoniae, P. aeruginosa, S. aureus and S. pneumoniae. This demonstrates that aerosolized delivery did not result in high variability (Table S2).
Bacterial burden after aerosol delivery of different ESKAPE pathogens in the standard neutropenic pneumonia model during inoculum titration. Bacterial burden after 24 h after aerosolized delivery of three different inocula is shown for S. aureus (a), S. pneumoniae (b), K. pneumoniae (c), P. aeruginosa (d), A. baumannii (e) in the neutropenic pneumonia model.
Next, we wanted to validate the models using a positive control antibacterial, in our case levofloxacin. Moreover, we determined bacterial burden at 2, 5 and 24 h post infection for all five pathogens. For S. aureus, we did observe that from the two-hour time point, bacteria grew by around 1 log10 unit up to five hours. Furthermore, bacterial burden in the vehicle-treated groups at 5 and 24 h was nearly similar. The positive control group with levofloxacin treatment decreased bacterial burden significantly at 5 and 24 h, although a higher decrease was observed at 24 h (Fig. 2a). For S. pneumoniae, a slight increase of bacterial burden from two towards five and, finally, to 24 h was seen in the vehicle-treated group. Again, levofloxacin reduced bacterial burden significantly for both time points assessed (Fig. 2b). In case of K. pneumoniae, there was not much growth observed in the vehicle-treated group from two towards five hours. However, a substantial increase in bacterial burden was seen at 24 h. Levofloxacin did only slightly reduce bacterial burden at 5 h, whereas this reduction was significant at 24 h (Fig. 2c). Similar to K. pneumoniae, also for P. aeruginosa not much growth from two to five hours with respect to bacterial burden was observed in the vehicle-treated group, but a substantial growth was seen at 24 h. Levofloxacin did significantly reduce bacterial burden at 5 and 24 h compared to the vehicle-treated groups at the same time points (Fig. 2d). Similar to S. pneumoniae, growth from two towards five and 24 h was observed for A. baumannii strain ATCC 19606. Levofloxacin did reduce bacterial burden at 5 h, but missed significance, whereas the reduction was significant at 24 h (Fig. 2e). To get a first idea if the method might also be suited for strains of the same bacterial species, we used A. baumannii strain NCTC 13301 with the same inoculum (in presence of mucin) as established for the strain ATCC 19606 (Fig. 2f). Similar growth was observed as seen for the strain ATCC 19606 giving first hints that the aerosolized delivery of the pathogen is not limited to particular strains.
Validation of neutropenic pneumonia models with levofloxacin at different time points. Bacterial burden after 2, 5 and 24 h after aerosolized delivery is shown for S. aureus (a), S. pneumoniae (b), K. pneumoniae (c), P. aeruginosa (d), A. baumannii strain ATCC 19606 (e) and A. baumannii strain NCTC 13301 (f). Animals were treated with vehicle (black) or with levofloxacin (grey). Levofloxacin did reduce bacterial burden at 24 h significantly for all pathogens tested; at 5 h levofloxacin did only reduce bacterial burden for S. aureus, S. pneumoniae and P. aeruginosa significantly. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Finally, we show that the neutropenic lung infection models with the five pathogens (and the specific strains) tested here are validated in a similar manner as neutropenic lung infection models with intranasal instillation of the inoculum. Thus, the model we propose here can be equally used to assess the efficacy of novel anti-infectives.
Using aerosol delivery in acute pneumonia models
In a second step, we aimed to evaluate if a sustainable burden until 24 h was also achieved with nebulization when immunocompetent mice were used. Mice were infected in a similar manner as described for the neutropenic models using aerosolized delivery of the respective bacterial inoculum. First, different inocula were used (Table S1) to establish an infection. The infection with both Gram-positive strains of S. aureus and S. pneumoniae was established with mean log10 cfu/g tissue values of 4.3 and 4.6, respectively (Fig. S1b, Table S2). For both pathogens the highest inoculum dose (Table S2) was achieving the highest terminal bacterial burden with an acceptable standard deviation (Fig. 3a,b). However, no infection was established for the strains of P. aeruginosa and A. baumannii used in this study. Twenty-four hours post infection, bacteria were already cleared and no sustained bacterial burden was found in the lungs. Therefore, aerosolized delivery was not suited to induce an acute infection for P. aeruginosa and A. baumannii. By contrast, the infection with aerosolized delivery for the Gram-negative pathogen K. pneumoniae was successful and a good bacterial burden of around 7 log10 cfu/g tissue was detected 24 h post infection (Fig. S1b, Table S2). Moreover, with increasing inoculum dose an increased bacterial burden was observed (Fig. 3c).
Bacterial burden after aerosol delivery of different ESKAPE pathogens in the acute pneumonia model during inoculum titration. Bacterial burden after 24 h after aerosolized delivery of three different inocula is shown for S. aureus (a), S. pneumoniae (b), K. pneumoniae (c) in the acute pneumonia model.
Similar to the neutropenic models, we aimed for a validation using the positive control antibacterial levofloxacin. Levofloxacin did reduce bacterial burden with S. aureus compared to the vehicle-treated group by 2 log10 units (Fig. 4a), which was a similar magnitude of reduction upon infection with S. pneumoniae (Fig. 4b). For K. pneumoniae, the mean reduction in bacterial burden in the levofloxacin group was around 4 log10 units compared to the vehicle-treated group (Fig. 4c).
Validation of acute pneumonia models with levofloxacin. Bacterial burden after 24 h after aerosolized delivery is shown for S. aureus (a), S. pneumoniae (b), K. pneumoniae (c). Animals were treated with vehicle (left) or with levofloxacin (LVX, right). Levofloxacin did reduce bacterial burden at 24 h significantly for all pathogens tested. *p < 0.05, **p < 0.01, ***p < 0.001.
In summary, the aerosolized delivery worked for three out of five pathogens tested. Moreover, we demonstrate using the positive control antibacterial levofloxacin as comparator that the model is entirely validated and usable to assess the efficacy of novel anti-infectives.
Discussion
Neutropenic and immunocompetent, acute murine pneumonia models are established systems to provide a proof-of-concept as well as research primary pharmacology of novel antibacterials13,16,17,18,19,20,21,22. They help to understand PK/PD relationships and translate doses to obtain a good probability of target attainment in human clinical trials23,24,25. So far, the majority of these models predictive for efficacy in humans uses intranasal instillation of the pathogen9,10.
Herein, we show that aerosolized delivery of pathogens to induce pneumonia can be a viable alternative to conventional intranasal and intratracheal administration. In this study, we demonstrate that an infection was successfully established for five different strains in the neutropenic murine pneumonia model. Moreover, we showed that a high inoculum was needed to achieve a sustained bacterial burden for the five pathogens assessed. This was in line with previous reports of the inoculum size needed for murine pneumonia models9. Additionally, both P. aeruginosa and A. baumannii needed 0.01% mucin during inoculum preparation to achieve high terminal bacterial burden. In the context of an assessment of human-derived antibodies against P. aeruginosa, we have shown recently, that histopathological analysis showed an increased inflammation in the lungs in untreated animals26. This observation was in agreement with previous reports on histopathology results of murine pneumonia models7,13,15. It is known that mucin interacts with P. aeruginosa and that it attenuates its virulence and can impact its ability to form biofilms27,28,29. It has also been shown that mucin impacts the virulence of A. baumannii, in particular in the context of intraperitoneal infections30,31. Similarly, it has been demonstrated that mucin addition helps to establish intraperitoneal infections with Gram-negative pathogens, including P. aeruginosa and A. baumannii32. However, in that study mucin was not added during inoculum titration32. We hypothesize that low amount of mucin addition might have contributed to successful establishment of infection in the neutropenic models for P. aeruginosa and A. baumannii, whereas that was not possible in the acute lung infection models. Nevertheless, deciphering the exact mechanism of mucin addition during inoculum preparation was not within the scope of this study, but might be subject to further research. Additionally, it is known for P. aeruginosa as well as for A. baumannii that the establishment of acute lung infection models is challenging in general: For both, the size of the inoculum is critical33,34. If the inoculum is chosen too low, then rapid clearance of the infection in immunocompetent mice occurs as a result of the innate immune response in case of P. aeruginosa34,35,36. As a higher inoculum results in a more mucoid solution, a nebulization is not possible. Thus, aerosolized delivery has a limitation with regard to viscosity37. Therefore, in such cases intranasal or intratracheal administration of the pathogen is needed to enable an acute lung infection10,38. Nevertheless, aerosolized delivery of the pathogen was possible in case of three tested strains in this study. Finally, the models for all the pathogens tested in this study were validated with a positive control antibacterial, levofloxacin, demonstrating that they are suited to be used to also evaluate novel anti-infectives in a similar manner as models using intranasal instillation for infection9.
In general, aerosolized delivery of the inoculum has several advantages, which also contributes to a low standard deviation of terminal bacterial lung burden observed. One of these advantages is certainly that delivery is experimenter-independent because the flow rate and aerosol size is solely dependent on the nebulizer used37. The mesh nebulizer we used, i.e. Aeroneb® lab nebulizer, a vibrating mesh nebulizer, with the small nebulizer unit usually produces particle sizes between 2.5 and 4 µm (according to the manufacturer’s specifications). Moreover, nebulization was performed under controlled conditions, as required for animal experiments, in a temperature range of 21–22 °C and a relative humidity of 45–65%, despite the fact that the manufacturer also allows greater ranges for temperature and relative humidity. Choosing a nebulizer, which keeps the specifications (according to the manufacturer) at varying environmental conditions, renders the infection with aerosolized delivery more robust. As environmental conditions will be similar for murine pneumonia models worldwide because of animal welfare regulations, we did not change environmental conditions for the nebulization process. Furthermore, we used a small-sized nebulizer device making delivery of the inoculum simple without the need for extensive or huge special equipment. Additionally, intratracheal and intranasal administration as more invasive techniques might cause differences in the breathing rates, in case of intranasal administration as a result of obstruction of one nostril39. This might result in particle sizes not being defined, in contrast to a vibrating mesh nebulizer, causing higher standard deviation of the bacterial burden as observed by Bergamini and colleagues for intranasal and intratracheal administration11. We hypothesize that the particles generated by the nebulizer used in this study are much smaller than the ones generated through extensive breathing of the animal after intranasal administration. The particle size distribution generated by the vibrating mesh nebulizer might be more suitable for disposition in the respiratory tract as it has been shown that particles between < 5 µm deposit more easily in the respiratory tract, whereas those < 1 µm can be exhaled again40,41. By contrast, a lower standard deviation within groups is always advisable, as it will allow reducing group sizes with respect to statistical testing42. One might hypothesize that an evaporation process took place causing bacteria to be destroyed. However, for the Aeroneb® lab nebulizer used in this study, it has been observed, that smaller droplet sizes, below 2.5 µm, through evaporation were only achieved by varying experimental conditions extensively, such as by heating of the aerosol43,44,45,46. We chose the Aeroneb® lab nebulizer, because it is a well-characterized and specified system. It was out of the scope of this study to perform an in-depth characterization of the aerodynamic properties of the nebulization device going beyond the extensive characterizations already performed by the manufacturer. We did show that bacteria were growing in vivo starting at a burden of around 4 log10 units to a burden of 6–8 log10 units within 22 h. Thus, this suggested that bacteria were not destroyed by the nebulization process, but were still alive. A similar growth of bacteria was also seen in murine pneumonia models after intranasal instillation10. Finally, this contradicts the hypothesis that bacteria were destroyed by an evaporation process.
Thus, we believe that the low standard deviation we observe for the majority of pathogens tested in this study might be attributed to a homogenous particle size distribution of the bacterial inoculum resulting in low inter-animal variability. The low standard variation in combination with the device we used, only taking several seconds of aerosolization time, is the unique contribution compared to similar methods in literature. There is no need to involve large nebulization chambers and invest in highly specialized apparatus and training of personnel as described previously13,47,48,49. In the context of viral infections, nebulizers have already been used and were able to produce a sustained infection50. Moreover, in one study even the same Aeroneb® was used for assessment of a drug’s efficacy51. Nevertheless, the major part of studies needs higher volumes and longer time periods for inoculation. Bowling and colleagues have assessed differences between the former ‘gold standard’, the collison nebulizer, and the Aeroneb® lab nebulizer and determined less loss of pathogens during delivery in case of viral infections. With respect to initiation of bacterial inoculation, they were experiencing difficulties52. It has to be highlighted here that Bowling and colleagues used a chamber for inoculation. By contrast, we directly put the nebulizer over mouth and nose of the individual animal, which, presumably, causes less loss of the pathogen.
With the caveat that only one strain was used for the majority of the pathogens tested, sustained bacterial burden in lung tissue was obtained for the strains of the pathogens studied in the neutropenic pneumonia models as well as for the majority of strains in the acute pneumonia models. A good disposition of bacteria at the target site, i.e. lung, was observed at 2 h post infection. Furthermore, we were able to show that we get a similar terminal bacterial burden when using the same inoculum, but different strains of A. baumannii. This demonstrates that the method presented here is not restricted to specific strains, but has broader applicability, although inoculum titrations might be necessary for different strains to establish the model with aerosolized delivery.
Additionally, it is expected that due to the disposition of particles after aerosol delivery14, different analysis from the same sample material is enabled, such as RNA level determination of bacterial biomarkers beside cfu determination. It has been shown for the Aeroneb® we used in this study that a homogenous distribution of particle sizes and deposition into the lung is achieved14,53,54,55,56. Although it is mainly well documented that drug particles are well distributed across the lung when using an Aeroneb® device56, we argue that the same can be expected in a similar manner for bacterial deposition as it has already been shown in the context of respiratory syncytial virus (RSV)50. This gave us confidence that our assumption of a homogenous deposition of bacteria after aerosolized delivery is correct.
In summary, this small study showed that aerosol delivery for infection constitutes a suitable alternative for intranasal and intratracheal instillation. Mimicking the ‘natural route of infection’ this delivery method represents a refinement of the standard murine pneumonia models and encourages to consider this route for delivery, in particular when the lung tissue is needed for multiple readouts.
Methods
Bacterial strains
The following bacterial strains were used for in vivo studies: S. aureus ATCC 33591, K. pneumoniae ATCC 43816, P. aeruginosa ATCC 27853, A. baumannii ATCC 19606, A. baumannii NCTC 13301, S. pneumoniae ATCC 700905.
Preparation of the inoculum for infection with S. aureus ATCC 33591 and K. pneumoniae ATCC 43816
Inoculi were prepared as described previously with the modification that 0.9% NaCl-solution was used instead of PBS57. For the neutropenic and the acute lung infection model an inoculum of 2 × 109 cfu/ml was used for S. aureus ATCC 33591 as well as for K. pneumoniae ATCC 43816.
Preparation of the inoculum for infection with P. aeruginosa ATCC 27853
The inoculum was prepared as follows: on day -1 the P. aeruginosa strain was streaked out onto a blood agar plate and incubated at 37 °C. Then one single colony was inoculated into LB medium (diluted 1:6 in water) containing 0.01% mucin and incubated at 120 rpm and 37 °C. On day 0 bacteria were centrifuged for 15 min at 4,000 rpm and washed twice in 0.9% NaCl-solution. Then they were adjusted to an OD of 10. For infection with P. aeruginosa an inoculum of 5 × 109 cfu/ml was used.
Preparation of the inoculum for infection with A. baumannii ATCC 19606 and NCTC 13301
The respective strain was streaked out from a glycerol culture onto a blood agar plate and incubated at 37 °C overnight. A few colonies were inoculated in a mixture of MHB diluted in water (one part MHB and five parts water) with 0.01% mucin and incubated for 14–15 h at 120 rpm and 37 °C. The following day, the culture was centrifuged at 4000 rpm and 4 °C for 15 min when it reached an OD600 between 0.5 and 0.6. Then it was washed twice with 0.9% NaCl-solution and centrifuged for 10 min at 4000 rpm and 4 °C. Finally, the pellet was resuspended in 0.9% NaCl-solution and the OD600 was adjusted to 10. For infection with A. baumannii an inoculum of 2 × 109 cfu/ml was used.
Preparation of the inoculum for infection with S. pneumoniae ATCC 700905
The strain was streaked out from a glycerol culture onto a blood agar plate and incubated at 37 °C overnight. A few colonies were inoculated in THY medium and incubated overnight at 120 rpm and 37 °C. The following day the overnight culture is diluted 1:100 in THY incubated at 120 rpm and 37 °C until it reached an OD600 of around 0.5–0.6. Then it was washed twice with 0.9% NaCl-solution and centrifuged for 10 min at 4000 rpm and 4 °C. Finally, the pellet was resuspended in 0.9% NaCl-solution and the OD600 was adjusted to 5. For infection with S. pneumoniae an inoculum of 3 × 109 cfu/ml was used.
Mice
The animal studies were conducted in accordance with the recommendations of the European Community (Directive 2010/63/EU, 1st January 2013). All animal procedures were performed in strict accordance with the German regulations of the Society for Laboratory Animal Science (GV- SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). Animals were excluded from further analysis if sacrifice was necessary according to the humane endpoints established by the ethical board. All experiments were approved by the ethical board of the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Oldenburg, Germany. Animals were kept in individually ventilated cages with a 10h/14h dark/light cycle and had access to food and water ad libitum. The animals were housed in individually ventilated cages in an animal facility with a room temperature of 21–22 °C and a relative humidity in the range of 45–65%. The study is reported in accordance with the ARRIVE guidelines.
Neutropenic and acute pneumonia models
General
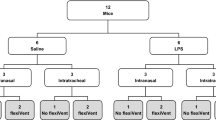
Female, eight weeks-old, CD-1 mice (Charles River, Germany) were used for both models (n = 6 animals per study per strain for the 24 h-time point and n = 2 animals for the 2 h-time point). In case of the neutropenic pneumonia model, animals were rendered neutropenic by administration of 150 mg/kg and 100 mg/kg cyclophosphamide intraperitoneally on day-4 and-1, respectively. On the day of infection (day 0), mice received 15 µl (neutropenic models) or 50 µl (acute models) of the respective strain administered via an Aeroneb® nebulizer device (Kent Scientific) under anesthesia with 100 mg/kg ketamine and 10 mg/kg xylazine (both administered intraperitoneally). Nebulization procedures were performed within a BSL-2 safety cabinet in an animal facility with a room temperature of 21–22 °C and a relative humidity of 45–65%. Two hours (in case of the neutropenic models) and 24 h after infection (neutropenic and acute models), mice were euthanized, blood was removed from the heart and lungs were aseptically removed. Whole blood was collected into Eppendorf tubes coated with 0.5 M EDTA and immediately spun down at 13.000 rpm for 10 min at 4 °C. The plasma was transferred into a new Eppendorf tube and then stored at − 80 °C until analysis. Organs were homogenized in 0.9% NaCl-solution and plated onto agar plates in duplicates in serial dilutions and incubated at 37 °C for 24 h.
Inoculum titration for model development
To establish the inoculum for both the neutropenic and acute lung infection models, three inocula were tested. The inocula were prepared as described in the section for preparation of the inoculum for the respective strain. For the inoculum titration 1/5, 1/2 and the inoculum size as described in the section ‘preparation of the inoculum’ were used, corresponding to ‘inoculum 1’ for 1/5 of the original inoculum, to ‘inoculum 2’ for the 1/2 of the original inoculum and ‘inoculum 3’ for the original inoculum (Table S1). In case of A. baumannii strain ATCC 19606 as well as for P. aeruginosa, the first tests were performed without addition of mucin during inoculum preparation.
Assessment of bacterial growth over time and validation of the model with the positive control
Levofloxacin 100 mg/kg IP served as a positive control antibacterial. The animal dose in mice of 100 mg/kg corresponds to a human equivalent dose of 8.1 mg/kg58. For S. aureus, A. baumannii ATCC 19606 and P. aeruginosa, it was administered once daily at t = 2 h. For K. pneumoniae and S. pneumoniae it was administered three times daily at t = 2, 6 and 10 h. Levofloxacin was not used for the A. baumannii strain NCTC 13301 as levofloxacin was not sensitive enough according to literature59. Moreover, bacterial burden in lung tissue for the neutropenic models was assessed at time points t = 2, 5 and 24 h, whereas bacterial burden in the acute models was assessed at time points t = 24 h.
Statistical testing
Statistical testing was performed using GraphPad Prism 9.5.1 software. For the acute infection models a two-tailed unpaired t-test was performed. For the neutropenic infection models a mixed-effect analysis with Fisher’s LSD test was performed for determination of significance at t = 24 h between the vehicle and the levofloxacin group.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018).
Miethke, M. et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. https://doi.org/10.1038/s41570-021-00313-1 (2021).
Walesch, S. et al. Fighting antibiotic resistance—strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 24, e56033 (2022).
Lee, Y. R. & Yeo, S. Cefiderocol, a new siderophore cephalosporin for the treatment of complicated urinary tract infections caused by multidrug-resistant pathogens: Preclinical and clinical pharmacokinetics, pharmacodynamics: Efficacy and Safety. Clin. Drug Investig. 40, 901–913 (2020).
Kaye, K. S., Naas, T., Pogue, J. M. & Rossolini, G. M. Cefiderocol, a siderophore cephalosporin, as a treatment option for infections caused by carbapenem-resistant enterobacterales. Infect. Dis. Ther. 13, 874176 (2023).
Khalid, K. & Rox, K. All roads lead to Rome: Enhancing the probability of target attainment with different pharmacokinetic/pharmacodynamic modelling approaches. Antibiotics 12, 690 (2023).
Andes, D. R. & Lepak, A. J. In vivo infection models in the pre-clinical pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Curr. Opin. Pharmacol. 36, 94–99 (2017).
Medina, E. Murine Model of Pneumococcal Pneumonia. In Mouse Models for Drug Discovery: Methods and Protocols (eds Proetzel, G. & Wiles, M. V.) 405–410 (Humana Press, UK, 2010). https://doi.org/10.1007/978-1-60761-058-8_22.
Arrazuria, R. et al. Expert workshop summary: Advancing toward a standardized murine model to evaluate treatments for antimicrobial resistance lung infections. Front. Microbiol. 13, 988725 (2022).
Arrazuria, R. et al. Variability of murine bacterial pneumonia models used to evaluate antimicrobial agents. Front. Microbiol. 13, 988728 (2022).
Bergamini, G. et al. Mouse pneumonia model by Acinetobacter baumannii multidrug resistant strains: Comparison between intranasal inoculation, intratracheal instillation and oropharyngeal aspiration techniques. PLOS ONE 16, e0260627 (2021).
Southam, D. S., Dolovich, M., O’Byrne, P. M. & Inman, M. D. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L833–L839 (2002).
Mizgerd, J. P. & Skerrett, S. J. Animal models of human pneumonia. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L387–L398 (2008).
Brain, J. D., Knudson, D. E., Sorokin, S. P. & Davis, M. A. Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ. Res. 11, 13–33 (1976).
Zhao, M., Lepak, A. J. & Andes, D. R. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg. Med. Chem. 24, 6390–6400 (2016).
Byrne, J. M. et al. FDA public workshop summary: Advancing animal models for antibacterial drug development. Antimicrob. Agents Chemother. https://doi.org/10.1128/aac.01983-20 (2020).
Rouse, M. S., Hein, M. M., Anguita-Alonso, P., Steckelberg, J. M. & Patel, R. Ceftobiprole medocaril (BAL5788) treatment of experimental Haemophilus influenzae, Enterobacter cloacae, and Klebsiella pneumoniae murine pneumonia. Diagn. Microbiol. Infect. Dis. 55, 333–336 (2006).
Louie, A. et al. Combination treatment with meropenem plus levofloxacin is synergistic against Pseudomonas aeruginosa infection in a murine model of pneumonia. J. Infect. Dis. 211, 1326–1333 (2015).
Thabit, A. K., Crandon, J. L. & Nicolau, D. P. Pharmacodynamic and pharmacokinetic profiling of delafloxacin in a murine lung model against community-acquired respiratory tract pathogens. Int. J. Antimicrob. Agents 48, 535–541 (2016).
Kidd, J. M., Abdelraouf, K. & Nicolau, D. P. Comparative efficacy of human-simulated epithelial lining fluid exposures of tedizolid, linezolid and vancomycin in neutropenic and immunocompetent murine models of staphylococcal pneumonia. J. Antimicrob. Chemother. 74, 970–977 (2019).
Wicha, W. W., Strickmann, D. B. & Paukner, S. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob. Chemother. 74, 11–18 (2019).
Abdelraouf, K. & Nicolau, D. P. In vivo pharmacokinetic/pharmacodynamic evaluation of cefepime/taniborbactam combination against cefepime-non-susceptible Enterobacterales and Pseudomonas aeruginosa in a murine pneumonia model. J. Antimicrob. Chemother. 78, 692–702 (2023).
Craig, W. A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26, 1–12 (1998).
Ambrose, P. G. et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 44, 79–86 (2007).
Martinez, M. N., Papich, M. G. & Drusano, G. L. Dosing regimen matters: The Importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 56, 2795–2805 (2012).
Simonis, A. et al. Discovery of highly neutralizing human antibodies targeting Pseudomonas aeruginosa. Cell 186, 5098-5113.e19 (2023).
Co, J. Y. et al. Mucins trigger dispersal of Pseudomonas aeruginosa biofilms. Npj Biofilms Microbiomes 4, 1–8 (2018).
Wheeler, K. M. et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 4, 2146–2154 (2019).
Hoffman, C. L., Lalsiamthara, J. & Aballay, A. Host mucin is exploited by Pseudomonas aeruginosa to provide monosaccharides required for a successful infection. mBio https://doi.org/10.1128/mbio.00060-20 (2020).
Ohneck, E. J. et al. Mucin acts as a nutrient source and a signal for the differential expression of genes coding for cellular processes and virulence factors in Acinetobacter baumannii. PLOS ONE 13, e0190599 (2018).
Harris, G., Holbein, B. E., Zhou, H., Xu, H. H. & Chen, W. Potential mechanisms of mucin-enhanced acinetobacter baumannii virulence in the mouse model of intraperitoneal infection. Infect. Immun. https://doi.org/10.1128/iai.00591-19 (2019).
Park, J. Y. et al. Establishment of experimental murine peritonitis model with hog gastric mucin for carbapenem-resistant gram-negative bacteria. Infect. Chemother. 49, 57–61 (2017).
Palmer, L. D., Green, E. R., Sheldon, J. R. & Skaar, E. P. Assessing Acinetobacter baumannii virulence and persistence in a murine model of lung infection. Methods Mol. Biol. Clifton NJ 1946, 289–305 (2019).
Bobrov, A. G. et al. Evaluation of Pseudomonas aeruginosa pathogenesis and therapeutics in military-relevant animal infection models. APMIS 130, 436–457 (2022).
van Heeckeren, A. M. & Schluchter, M. D. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 36, 291–312 (2002).
Koh, A. Y., Priebe, G. P., Ray, C., Van Rooijen, N. & Pier, G. B. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect. Immun. 77, 5300–5310 (2009).
Ghazanfari, T., Elhissi, A. M. A., Ding, Z. & Taylor, K. M. G. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int. J. Pharm. 339, 103–111 (2007).
Pan, X. & Wu, W. Murine acute pneumonia model of Pseudomonas aeruginosa lung infection. Bio-Protoc. 10, e3805 (2020).
Xie, J., Xi, Y., Zhang, Q., Lai, K. & Zhong, N. Impact of short term forced oral breathing induced by nasal occlusion on respiratory function in mice. Respir. Physiol. Neurobiol. 205, 37–41 (2015).
Darquenne, C. Aerosol deposition in health and disease. J. Aerosol Med. Pulm. Drug Deliv. 25, 140–147 (2012).
Jabbal, S., Poli, G. & Lipworth, B. Does size really matter? Relationship of particle size to lung deposition and exhaled fraction. J. Allergy Clin. Immunol. 139, 2013-2014.e1 (2017).
Berben, L., Sereika, S. M. & Engberg, S. Effect size estimation: Methods and examples. Int. J. Nurs. Stud. 49, 1039–1047 (2012).
Worth Longest, P. et al. Production of inhalable submicrometer aerosols from conventional mesh nebulizers for improved respiratory drug delivery. J. Aerosol. Sci. 51, 66–80 (2012).
Longest, P. W., Walenga, R. L., Son, Y.-J. & Hindle, M. High-efficiency generation and delivery of aerosols through nasal cannula during noninvasive ventilation. J. Aerosol Med. Pulm. Drug Deliv. 26, 266–279 (2013).
Pritchard, J. N., Hatley, R. H., Denyer, J. & von Hollen, D. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther. Deliv. 9, 121–136 (2018).
Gerde, P., Nowenwik, M., Sjöberg, C.-O. & Selg, E. Adapting the aerogen mesh nebulizer for dried aerosol exposures using the preciseinhale platform. J. Aerosol Med. Pulm. Drug Deliv. 33, 116–126 (2020).
Laurenzi, G. A., Berman, L., First, M. & Kass, E. H. A quantitative study of the deposition and clearance of bacteria in the murine lung*. J. Clin. Invest. 43, 759–768 (1964).
Davis, J. K. et al. Development of an aerosol model of murine respiratory mycoplasmosis in mice. Infect. Immun. 54, 194–201 (1986).
Skerrett, S. J. et al. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 276, 715–727 (1999).
Grosz, D. D. et al. Sucrose stabilization of respiratory syncytial virus (RSV) during nebulization and experimental infection. BMC Res. Notes 7, 158 (2014).
Lieber, C. M. et al. 4’-Fluorouridine mitigates lethal infection with pandemic human and highly pathogenic avian influenza viruses. PLOS Pathog. 19, e1011342 (2023).
Bowling, J. D., O’Malley, K. J., Klimstra, W. B., Hartman, A. L. & Reed, D. S. A vibrating mesh nebulizer as an alternative to the collison three-jet nebulizer for infectious disease aerobiology. Appl. Environ. Microbiol. 85, e00747-e819 (2019).
Watts, A. B., McConville, J. T. & Williams, R. O. Current therapies and technological advances in aqueous aerosol drug delivery. Drug Dev. Ind. Pharm. 34, 913–922 (2008).
Zhang, G., David, A. & Wiedmann, T. S. Performance of the vibrating membrane aerosol generation device: Aeroneb micropump nebulizer™. J. Aerosol. Med. 20, 408–416 (2007).
Cannon, C. L. et al. In vitro and murine efficacy and toxicity studies of nebulized SCC1, a methylated caffeine-silver(I) complex, for treatment of pulmonary infections. Antimicrob. Agents Chemother. 53, 3285–3293 (2009).
Rajapaksa, A. E. et al. Pulmonary deposition of radionucleotide-labeled palivizumab: Proof-of-concept study. Front. Pharmacol. 11, 1291 (2020).
Rox, K. Influence of tramadol on bacterial burden in the standard neutropenic thigh infection model. Sci. Rep. 12, 19606 (2022).
Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016).
Anane, Y. A., Apalata, T., Vasaikar, S., Okuthe, G. E. & Songca, S. Molecular detection of carbapenemase-encoding genes in multidrug-resistant acinetobacter baumannii clinical isolates in South Africa. Int. J. Microbiol. 2020, 7380740 (2020).
Acknowledgements
KR and EM thank Andrea Ahlers, Janine Schreiber and Jennifer Wolf for excellent technical assistance. KR and EM received support from the German Centre for Infection Research (DZIF; TTU 09.710 PK/PD unit).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
K.R. and E.M. conceived the study. K.R. designed and conducted the studies, analyzed the data and wrote the manuscript. Both authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rox, K., Medina, E. Aerosolized delivery of ESKAPE pathogens for murine pneumonia models. Sci Rep 14, 2558 (2024). https://doi.org/10.1038/s41598-024-52958-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-52958-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.