Abstract
A novel magnetic silica/graphene oxide nanocomposite supported ionic liquid/manganese complex (Fe3O4@SiO2-NH2/GO/IL-Mn) is prepared, characterized and its catalytic application is investigated. The Fe3O4@SiO2-NH2/GO/IL-Mn catalyst was synthesized via chemical immobilization of graphene oxide on Fe3O4@SiO2 nanoparticles followed by modification with ionic liquid/Mn complex. This nanocomposite was characterized by using SEM, TGA, FT-IR, PXRD, EDX, TEM, nitrogen adsorption–desorption, and VSM analyses. The catalytic application of Fe3O4@SiO2-NH2/GO/IL-Mn was studied in the synthesis of tetrahydrobenzo[b]pyrans (THBPs) in water solvent at RT. This nanocatalyst was successfully recovered and reused at least eight times without a significant decrease in its activity.
Similar content being viewed by others
Introduction
Carbon-based materials are very attractive among chemists due to their high efficiency as a support for different catalysts and also their good conductivity1,2,3. One of the most important allotropes of carbon is graphene oxide (GO)4,5 which has a two-dimensional and single-layer structure and involves hydroxyl, carboxylic acid, and epoxy groups on its surface6,7. The properties of graphene oxides, such as very good specific surface area, biocompatibility, high flexibility, and lightness, endow them with strong potential for applications in catalytic processing8,9,10. However, GO accumulates in salt solutions and biological media. Therefore, to overcome this problem and also for easy separation of GO, recently, the immobilization of graphene oxide on magnetic nanoparticles has been considered11,12. In fact, the unique properties of magnetic NPs such as high surface area, availability, easy separation, and recoverability from the environment, make them attractive candidates to composite with GO. Some reports in this matter are TiO2/Fe3O4/GO13, Ag3PO4-Fe3O4-GO14, PEG/Fe3O4/GO-NH215, Fe3O4/GO-COOH16, Fe3O4/GO/CS17, MOF@Fe3O4@GO18, Fe3O4-GO-(o-MWCNTs)hybrid19, Fe3O4/GO/chitosan20 and γ-PGA-Fe3O4-GO-(o-MWCNTs)21. Moreover, several organic functional groups have also been used to modify GO for practical applications22. Some reported examples in this matter are GO@IL/MoO2(acac)223, Cu–NiAAPTMS@GO24, GO@melamine25, plydopamine@GO/cellulose26, Al2O3/GO cellulose27, GO-TCT-DETA28, and Mn-UiO-66@GO-NH229.
An important process in chemistry is multicomponent reaction (MCR), in which at least three starting materials are used to synthesis valuable organic compounds30,31,32. As example, this process has been effectively used for the synthesis of tetrahydrobenzo[b]pyrans (THBPs)33,34 with excellent biological activities such as antiviral, anticancer, and dementia35,36. Although, to date, many catalytic systems have been used for the synthesis of THBPs, however, the most of them suffer from drawbacks of high catalyst loading, the use of toxic organic solvents, high reaction temperature, and non-recoverability of the catalyst. Therefore, the preparation of a novel and powerful catalytic system to overcome the aforementioned limitations is an important challenge in this matter.
In view of the above, herein, we report the synthesis and characterization of a novel magnetic silica/graphene oxide nanocomposite supported ionic liquid/Mn complex (Fe3O4@SiO2-NH2/GO/IL-Mn). This is effectively applied as an efficient and recoverable catalyst in the synthesis of THBPs.
Experimental section
Preparation of Fe3O4@SiO2-NH2
For the synthesis of Fe3O4@SiO2-NH2, firstly, Fe3O4 nanoparticles were prepared according to a known method37. Then, 0.5 g of Fe3O4 was added in a solution containing 30 mL of ethanol, 20 mL of distilled water, and 10 mL of ammonia (25%). After that, 70 μL of 3-aminopropyltriethoxysilane (APTES) and 70 μL of tetraethoxysilane (TEOS) were added and the resulted mixture was stirred at 35 °C for 3 h. Finally, the product was separated by using a magnet, washed with distilled water and ethanol, dried at 75 °C for 7 h and denoted as Fe3O4@SiO2-NH2.
Preparation of Fe3O4@SiO2-NH2/GO
The Fe3O4@SiO2-NH2/GO nanocomposite was prepared as follows. First, 0.3 g of GO was suspended in 20 mL of distilled water for 10 min. Then, 0.5 g of Fe3O4@SiO2-NH2 was added and the obtained mixture was vigorously stirred at 70 °C for 2 h. Finally, the product was separated by using a magnet, washed with distilled water and ethanol, dried at 75 °C for 7 h and denoted as Fe3O4@SiO2-NH2/GO.
Preparation of Fe3O4@SiO2-NH2/GO/IL
For the preparation of Fe3O4@SiO2-NH2/GO/IL, firstly, 1 g of Fe3O4@SiO2-NH2/GO nanocomposite was suspended in 50 mL of toluene and sonicated for 20 min at RT. Then, 0.2 mmol of 1-methyl-3-(3-trimethoxysilylpropyl)imidazolium chloride (Im) was added and the obtained mixture was stirred under reflux conditions for 24 h. The product was separated by using a magnet, washed with ethanol, dried at 70 °C for 6 h and denoted as Fe3O4@SiO2-NH2/GO/IL.
Preparation of Fe3O4@SiO2-NH2/GO/IL-Mn
For this, 1 g of Fe3O4@SiO2-NH2/GO /IL was dispersed in 20 mL of DMSO under ultrasonic irradiation. Then, 0.5 mmol of Mn(OAc)3.4H2O salt was added and the resulting mixture was stirred at 80 °C for 2 h. The product was separated by using a magnet, washed with ethanol, dried at 70 °C for 6 h and denoted as Fe3O4@SiO2-NH2/GO/IL-Mn.
Synthesis of THBPs using Fe3O4@SiO2-NH2/GO/IL-Mn nanocatalyst
For this purpose, the Fe3O4@SiO2-NH2/GO/IL-Mn catalyst (0.8 mol%), malononitrile (1 mmol), benzaldehyde (1 mmol) and dimedone (1 mmol) were added in distilled water (10 mL). The resulting mixture was vigorously stirred at RT. The progress of the reaction was monitored by using TLC. After the completion of the reaction, the catalyst was separated by using a magnet. Then, ethyl acetate (20 mL) was added to the residue and the obtained mixture was washed three times with water in a decanter to remove some impurities. Finally, the obtained ethyl acetate solution was placed in an ice bath to crystalize/precipitate the desired pure products.
IR, 1H-NMR and 13C-NMR data of THBPs
2-Amino-4-(3-nitrophenyl)-7,7-dimethyl-5-oxo-6,6,8,8-tetrahydro-4H-chromene-3-carbonitrile
White solid; yield: 85%; M. P.: 211–212 °C (210–21235), IR (KBr, cm−1): 3420, 3339 (NH2, stretching vibration), 3181 (= C–H, stretching vibration sp2), 2958 (C–H, stretching vibration sp3), 2186 (CN, stretching vibration), 1673 (C=O, stretching vibration), 1604, 1488 (C=C, Ar stretching vibration sp2), 1245 (C–O, stretching vibration). 1H-NMR (300 MHz, CDCl3): δ (ppm) 0.99 (s, 3H), 1.09 (s, 3H), 2.15 (d, 1H, J = 15 Hz), 2.33 (d, 1H, J = 15 Hz), 2.59 (s, 2H), 4.46 (s, 1H), 7.24 (s, 2H), 7.63–7.75 (m, 2H), 8.2 (s, 1H), 8.3 (d, 1H, J = 9 Hz). 13C-NMR (75 MHz, CDCl3): δ (ppm) 27.6, 28.7, 32.5, 35.9, 40.4, 50.5, 56.9, 112.4, 120.1, 121.2, 122.3, 130.1, 134.8, 147.3, 148.7, 159.5, 164.1, 196.1.
2-Amino-4-(4-methylyphenyl)-7,7-dimethyl-5-oxo-6,6,8,8-tetrahydro-4Hchromene-3-carbonitrile.
White solid; yield: 85%; M. P.: 217–219 °C (218–22038), IR (KBr, cm−1): 3424, 3328 (NH2, stretching vibration), 3036 (=C–H, stretching vibration sp2), 2960 (C–H, stretching vibration sp3), 2192 (CN, stretching vibration), 1670 (C=O, stretching vibration), 1561, 1471 (C=C, Ar stretching vibration sp2), 1241 (C–O, stretching vibration).1H-NMR (300 MHz, CDCl3):1.08 (s, 3H), 1.15 (s, 3H), 2.10 (d, 1H, J = 6 MHz), 2.25 (d, 1H, J = 15.2 MHz), 2.25 (s, 3H), 2.52 (s, 2H), 4.43 (s, 1H), 7.05–7.14 (m, 4H), 7.28 (s, 2H) 13C-NMR (75 MHz, CDCl3): δ (ppm) 21.2, 27.9, 29.1, 33.1, 35.1, 41.2, 50.9, 64.1, 114.2, 118.7, 127.5, 129.4, 137.0, 140.2, 157.4, 161.5, 196.0
2-Amino-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-6,6,8,8-tetrahydro-4H-chromene-3-carbonitrile
White solid; yield: 90%; M. P.: 199–201 °C (196–19839), IR (KBr, cm−1): 3432, 3332 (NH2, stretching vibration), 3100 (=C–H, stretching vibration sp2), 2958 (C–H, stretching vibration sp3), 2190 (CN, stretching vibration), 1666 (C=O, stretching vibration), 1527, 1419 (C=C, Ar stretching vibration sp2), 1249 (C–O, stretching vibration).1H-NMR (300 MHz, CDCl3): δ (ppm) 1.05 (s,3H), 1.14 (s, 3H), 2.20 (d, 1H, J = 3.4 Hz), 2.23 (d, 1H, J = 3.4 Hz), 2.45 (s, 2H), 3.75 (s, 3H), 4.37 (s, 1H), 4.50 (s, 2H, NH2), 6.80 (d, 2H, J = 8.6 Hz), 7.15 (d, 2H, J = 8.6 Hz). 13C NMR (75 MHz, CDCl3): δ (ppm) 27.8, 28.10, 32.4, 34.6, 40.6, 51.2, 63.9, 113.3, 114.6, 115.2, 128.8, 133.5, 135.3, 157.5, 158.4, 161.3, 195.9.
Results and discussion
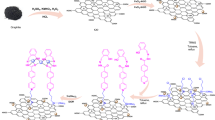
The preparation of the Fe3O4@SiO2-NH2/GO/IL-Mn nanocomposite includes four steps (Fig. 1). Firstly, the magnetic Fe3O4 nanoparticles were modified with TEOS and APTES to give Fe3O4@SiO2-NH2 NPs. Secondly, this material was chemically reacted with GO to give Fe3O4@SiO2-NH2/GO nanocomposite. Thirdly, the Im-based ionic liquid was chemically grafted on the surface of Fe3O4@SiO2-NH2/GO to deliver the Fe3O4@SiO2-NH2/GO/IL material. Finally, the last product was treated with manganese acetate to give the Fe3O4@SiO2-NH2/GO/IL-Mn nanocatalyst.
The functional groups of the GO, Fe3O4@SiO2-NH2 and Fe3O4@SiO2-NH2/GO/IL-Mn materials were determined by using a Fourier transform infrared (FT-IR) spectrometer (Fig. 2). For all samples, the strong peak at 3394 cm−1 is due to the O–H bonds of the material surface (Fig. 2a–c)40. Moreover, the peaks at 1724, 1519, 1288 and 1049 cm−1 are, respectively, associated to carboxyl C=O, aromatic C=C, epoxy C–O and alkoxy C–O bonds of GO (Fig. 2a–c)41. For the Fe3O4@SiO2-NH2 and Fe3O4@SiO2-NH2/GO/IL-Mn materials, the signals at 2825 and 2923 cm−1 are attributed to the C–H bonds of the aliphatic groups (Fig. 2b and c)42. Moreover, for the latter materials, the peak at 593 cm−1 is assigned to the Fe–O bond (Fig. 2b and c)43. For Fe3O4@SiO2-NH2/GO/IL-Mn, the signal at 1627 cm−1 is attributed to C=N bond of ionic liquids (Fig. 2c)41,44. In addition, for both Fe3O4@SiO2-NH2 and Fe3O4@SiO2-NH2/GO/IL-Mn nanomaterials, the strong signals at 1083 and 1215 cm−1 are assigned to the Si–O-Si vibrations45,46.
The surface morphology of Fe3O4@SiO2-NH2/GO/IL-Mn was studied by using SEM technique. The spherical nanoparticles of Fe3O4@SiO2 NPs and also the graphene oxide layers were clearly seen in the SEM image (Fig. 3). This confirms the successful formation of the Fe3O4@SiO2-NH2/GO composite during applied conditions.
The TEM analysis of the designed catalyst was also performed to investigate its structure. This analysis showed the catalyst to be composed of spherical Fe3O4@SiO2 NPs and GO layers (Fig. 4).
The EDX analysis showed the signals of carbon, nitrogen, oxygen, silicon, manganese and iron elements in the prepared nanocomposite (Fig. 5). This is in good agreement with the FT-IR results, confirming the successful immobilization of IL-Mn complex on Fe3O4@SiO2-NH2/GO composite.
The EDX-mapping analysis of the Fe3O4@SiO2-NH2/GO/IL-Mn nanocatalyst is shown in Fig. 6. As seen, all desired elements of C, O, N, Fe, Si and Mn are very well distributed in the material. This is also in good agreement with the FT-IR and EDX results, indicating the successful formation of the designed Fe3O4@SiO2-NH2/GO/IL-Mn nanocomposite.
The powder XRD analysis of Fe3O4@SiO2-NH2/GO/IL-Mn showed six signals at 2θ of 30, 35.5, 43.1, 54, 57.2, and 63.5 degree, corresponding to the Miller indices of 220, 311, 400, 422, 511 and 440, respectively (Fig. 7). These signals are attributed to the spinel structure of magnetic iron oxide NPs,47,48 confirming the high stability of the magnetite NPs during modification processes. Also, the peak at 2θ = 19° is related to silica layer of the designed catalyst49,50.
According to the VSM analysis, the saturation magnetization of the designed Fe3O4@SiO2-NH2/GO/IL-Mn material was found to be 40 emu/g (Fig. 8), confirming its high magnetic properties. This characteristic is very important in the fields of adsorption and catalysis.
Thermal stability of the Fe3O4@SiO2-NH2/GO/IL-Mn nanocatalyst was investigated by using thermal gravimetric analysis (TGA, Fig. 9). The first weight loss at temperatures between 10 to 110 °C (3%) is related to the removal of water and alcoholic solvents39. The second weight loss at 111–210 °C (4%) is attributed to the removal of the parts of functional groups that are located on the surface of the material. The main weight loss at temperatures more than 220 °C is related to the complete removal of the ionic liquids and also some parts of GO.
The nitrogen adsorption–desorption isotherms of the Fe3O4@SiO2-NH2/GO/IL-Mn nanocomposite showed a type II curve with a pronounced H3 hysteresis loop, according to the IUPAC classification51. The BET specific surface area and total pore volume of the material were calculated to be about 386.5 m2/g and 0.35 cm3/g, respectively. In addition, the BJH pore size distribution analysis showed a peak with good intensity centered at average pore diameter of about 4.8 nm (Fig. 10).
After preparation and characterization, the catalytic activity of Fe3O4@SiO2-NH2/GO/IL-Mn was investigated in the synthesis of THBPs at room temperature (RT). For this, the reaction between benzaldehyde, dimedone and malononitrile was selected as a test model (Table 1). The effect of various parameters such as catalyst loading and solvent was investigated to obtain the best conditions. In the absence of a catalyst, no product was obtained after 3 h, proving the catalyst is necessary for the development of this reaction (Table 1, entry 1). After addition of the catalyst, the reaction was progressed effectively and the best result was obtained in the presence of 0.8 mol% of Fe3O4@SiO2-NH2/GO/IL-Mn (Table 1, entries 2–4). It is important to note that increasing the amount catalyst to 1 mol% did not result in a significant change in the reaction yield (Table 1, entry 5). In order to demonstrate the effect of the Mn-centers on the catalytic process, the catalytic activity of Mn-free Fe3O4@SiO2-NH2/GO/IL nanocomposite was also investigated. This experiment showed that the Mn-free material gave no yield of the desired product, verifying the process is actually catalyzed by catalytic Mn sites (Table 1, entry 6). This catalytic system was also significantly affected by the solvent. Yields of 58%, 82%, 53% were obtained in toluene, EtOH and also under solvent-free media, respectively. Pleasingly, in water, the best yield was obtained (Table 1, entry 4). Accordingly, 0.8 mol% of catalyst, water solvent and RT were identified as the optimal conditions (Table 1, entry 4).
With the optimum conditions in hand, various aldehyde derivatives containing both electron withdrawing and electron donating substituents were used as substrate (Table 2). All of these aldehydes delivered the desired products in high yield at short time. It was also found that Fe3O4@SiO2-NH2/GO/IL-Mn offers high turnover number (TON) and turnover frequency (TOF) for all products, confirming the high ability of the present catalytic system to synthesis a wide range of biologically active THBPs.
The recoverability and reusability of Fe3O4@SiO2-NH2/GO/IL-Mn were also investigated in the reaction model. For this, after finishing of the reaction, the catalyst was easily separated by using a magnet. Then, it was reused in the next run under the same conditions as the first run. These steps were repeated and it was found that the catalyst could be recovered and reused for at least eight times with no significant decrease in efficiency (Fig. 11). These findings confirm high performance and very good stability of the designed catalyst under applied conditions.
Next, a leaching test was performed in the model reaction to investigate the nature of the Fe3O4@SiO2-NH2/GO/IL-Mn nanocatalyst under the applied conditions. For this, after the conversion was about 45% complete, the catalyst was magnetically removed. Then, the progress of catalyst-free residue was monitored. Interestingly, after 120 min, no notable conversion was observed. This proves no leaching of Mn species in the reaction solution under the applied conditions and also the heterogeneous nature of the designed catalyst.
Furthermore, the reactivity of the catalyst was investigated under optimal conditions. For this purpose, the model reaction was carried out and its progress was monitored using TLC. After the completion of the reaction, the starting materials were again added to the reaction vessel in the same proportion as the first run. These steps were repeated and the results showed that the activity of the Fe3O4@SiO2-NH2/GO/IL-Mn nanocatalyst is maintained for at least seven runs without a significant decrease in performance (Table 3).
In the next, in order to study the chemical and structural stability of the catalyst under applied conditions, the FT-IR and XRD analyses of the recovered catalyst were performed after fifth run. As shown in Fig. 12, the FT-IR spectrum of the recovered Fe3O4@SiO2-NH2/GO/IL-Mn showed a pattern similar to the FT-IR of fresh nanocatalyst, proving the high stability of the designed material under the applied reaction conditions.
The PXRD of the recovered Fe3O4@SiO2-NH2/GO/IL-Mn also illustrated six peaks at 2θ of 30, 35.5, 43.1, 54, 57.2, and 63.5, which are in good agreement with the PXRD pattern of the fresh nanocatalyst, proving the high stability of the crystalline structure of Fe3O4 NPs during the reaction process (Fig. 13).
Finally, the performance of Fe3O4@SiO2-NH2/GO/IL-Mn nanocomposite was compared with some previous catalytic systems in the synthesis of THBPs (Table 4). The results showed that our catalyst is better in terms of reaction conditions, catalyst loading and recovery times. These findings may be attributed to the magnetic nature of Fe3O4@SiO2-NH2/GO/IL-Mn as well as the positive effect of chemically immobilized ionic liquids in the stabilization of the catalytically active Mn-species.
A plausible mechanism for the synthesis of THBPs using Fe3O4@SiO2-NH2/GO/IL-Mn is outlined in Fig. 14. At first, the malononitrile and the Mn-activated aldehyde are condensed through the Knoevenagel condensation to give intermediate 1. Intermediate 2 is then delivered via a Michael-type addition between the enol form of dimedone and intermediate 1. An intramolecular cyclo-condensation is performed on intermediate 2 to give intermediate 3. Finally, the intermediate 3 is converted to the desired product 4 through a tautomerization process58.
Conclusion
In this study, for the first time, a manganese-containing IL-modified Fe3O4@SiO2-NH2/GO nanocomposite was prepared, characterized and used as a novel catalyst for the synthesis of THBPs. The high chemical and thermal stability of the designed catalyst were confirmed by using FT-IR, TGA and EDX analyses. The PXRD and VSM analyses showed high magnetic properties of the designed catalyst. The SEM and TEM analyses also confirmed the successful formation of the Fe3O4@SiO2-NH2/GO composite. The Fe3O4@SiO2-NH2/GO/IL-Mn catalyst was effectively used in the synthesis of THBPs and gave the desired products in high yields. The leaching test and also the recoverability and reactivity studies clearly showed high performance and stability of the catalyst under applied conditions.
Data availability
All data and materials are included in the manuscript.
References
Zare, P., Aleemardani, M., Seifalian, A., Bagher, Z. & Seifalian, A. M. Graphene oxide: Opportunities and challenges in biomedicine. Nanomaterials 11, 1083 (2021).
Liu, L. et al. Phase change materials with Fe3O4/GO three-dimensional network structure for acoustic-thermal energy conversion and management. Chem. Eng. J. 426, 130789 (2021).
Darvishi, K., Amani, K. & Rezaei, M. Preparation, characterization and heterogeneous catalytic applications of GO/Fe3O4/HPW nanocomposite in chemoselective and green oxidation of alcohols with aqueous H2O2. Appl. Organomet. Chem. 32, e4323 (2018).
Liu, C., Liu, Y., Dang, Z., Zeng, S. & Li, C. Enhancement of heterogeneous photo-Fenton performance of core-shell structured boron-doped reduced graphene oxide wrapped magnetical Fe3O4 nanoparticles: Fe (II)/Fe (III) redox and mechanism. Appl. Surf. Sci. 544, 148886 (2021).
Liu, Z. et al. Fe3O4@ graphene oxide@ Ag particles for surface magnet solid-phase extraction surface-enhanced Raman scattering (SMSPE-SERS): From sample pretreatment to detection all-in-one. ACS Appl. Mater. Interfaces 8, 14160–14168 (2016).
Yu, W., Sisi, L., Haiyan, Y. & Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 10, 15328–15345 (2020).
Seabra, A. B., Paula, A. J., de Lima, R., Alves, O. L. & Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 27, 159–168 (2014).
Agarwal, V. & Zetterlund, P. B. Strategies for reduction of graphene oxide—A comprehensive review. Chem. Eng. J. 405, 127018 (2021).
Rao, C., Biswas, K., Subrahmanyam, K. & Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 19, 2457–2469 (2009).
Razaq, A. et al. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 15, 1012 (2022).
Kumar, N. et al. Top-down synthesis of graphene: A comprehensive review. FlatChem 27, 100224 (2021).
Tiwari, S. K., Sahoo, S., Wang, N. & Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 5, 10–29 (2020).
Hamdy, G., El-Sabbagh, I. A. & Taher, F. Highly efficient sorption of thorium (IV) onto a ternary magnetic TiO2/Fe3O4/GO nanocomposite. Mater. Today Proc. 42, 2218–2226 (2021).
Muthukumar, P., Alex, V., Pannipara, M., Al-Sehemi, A. G. & Anthony, S. P. Fabricating highly efficient Ag3PO4-Fe3O4-GO ternary nanocomposite photocatalyst: Effect of Fe3O4-GO preparation methods on photocatalytic activity. Mater. Res. Bull. 141, 111337 (2021).
He, L. et al. Removal of Ca2+ and Mg2+ from oilfield wastewater using reusable PEG/Fe3O4/GO-NH2 nanoadsorbents and its efficiency for oil recovery. J. Environ. Chem. Eng. 9, 104653 (2021).
He, L. et al. A reusable Fe3O4/GO-COOH nanoadsorbent for Ca2+ and Cu2+ removal from oilfield wastewater. Chem. Eng. Res. Des. 166, 248–258 (2021).
John, J. A., Samuel, M. S. & Selvarajan, E. Immobilized cellulase on Fe3O4/GO/CS nanocomposite as a magnetically recyclable catalyst for biofuel application. Fuel 333, 126364 (2023).
Jia, W., Du, A., Fan, Z. & Shi, L. Goat milk-derived short chain peptides: Peptide LPYV as species-specific characteristic and their versatility bioactivities by MOF@ Fe3O4@ GO mesoporous magnetic-based peptidomics. Food Res. Int. 164, 112442 (2023).
Wang, L. et al. Anionic polypeptide poly (γ-glutamic acid)-functionalized magnetic Fe3O4-GO-(o-MWCNTs) hybrid nanocomposite for high-efficiency removal of Cd (II), Cu (II) and Ni (II) heavy metal ions. Chem. Eng. J. 346, 38–49 (2018).
Yadav, M., Rhee, K. Y., Park, S. J. & Hui, D. Mechanical properties of Fe3O4/GO/chitosan composites. Compos. Part B Eng. 66, 89–96 (2014).
Zhang, Q., Hou, Q., Huang, G. & Fan, Q. Removal of heavy metals in aquatic environment by graphene oxide composites: A review. Environ. Sci. Pollut. Res. 27, 190–209 (2020).
Farjadian, F. et al. Recent developments in graphene and graphene oxide: Properties, synthesis, and modifications: A review. ChemistrySelect 5, 10200–10219 (2020).
Kargar, S., Elhamifar, D. & Zarnegaryan, A. Ionic liquid modified graphene oxide supported Mo-complex: A novel, efficient and highly stable catalyst. Surf. Interfaces 23, 100946 (2021).
Rana, S., Maddila, S. & Jonnalagadda, S. B. Synthesis and characterization of Pd (II) dispersed over diamine functionalized graphene oxide and its scope as a catalyst for selective oxidation. Catal. Sci. Technol. 5, 3235–3241 (2015).
Chen, J. et al. Syntheses of magnetic GO@ melamine formaldehyde resin for dyes adsorption. Mater. Res. Express 6, 086103 (2019).
Liu, Y. et al. Construction of a mesoporous polydopamine@ GO/cellulose nanofibril composite hydrogel with an encapsulation structure for controllable drug release and toxicity shielding. ACS Appl. Mater. Interfaces 12, 57410–57420 (2020).
Singh, N., Kumari, S., Goyal, N. & Khan, S. Al2O3/GO cellulose based 3D-hydrogel for efficient fluoride removal from water. Environ. Nanotechnol. Monit. Manag. 15, 100444 (2021).
Ma, L. et al. Reinforcing carbon fiber epoxy composites with triazine derivatives functionalized graphene oxide modified sizing agent. Compos. Part B Eng. 176, 107078 (2019).
Eltaweil, A. S., Elshishini, H. M., Ghatass, Z. F. & Elsubruiti, G. M. Ultra-high adsorption capacity and selective removal of Congo red over aminated graphene oxide modified Mn-doped UiO-66 MOF. Powder Technol. 379, 407–416 (2021).
Khumalo, M. R., Maddila, S. N., Maddila, S. & Jonnalagadda, S. B. A facile and one-pot synthesis of new tetrahydrobenzo [b] pyrans in water under microwave irradiation. BMC Chem. 13, 1–7 (2019).
Hassanzadeh, F., Daneshvar, N., Shirini, F. & Mamaghani, M. Introduction of a new bis-derivative of succinimide (Bis-Su) as a sustainable and efficient basic organo-catalyst for the synthesis of arylidene malononitrile and tetrahydrobenzo [b] pyran derivatives under green conditions. Res. Chem. Intermed. 46, 4971–4984 (2020).
Memar, F. O., Khazdooz, L., Zarei, A. & Abbaspourrad, A. Green synthesis of pyrano [3, 2-b] pyran derivatives using nano Si–Mg–fluorapatite catalyst and the evaluation of their antibacterial and antioxidant properties. Med. Chem. Res. 29, 1792–1803 (2020).
Mohammadi, A. A., Asghariganjeh, M. R. & Hadadzahmatkesh, A. Synthesis of tetrahydrobenzo [b] pyran under catalysis of NH4Al(SO4)2· 12H2O (Alum). Arab. J. Chem. 10, S2213–S2216 (2017).
Badiger, K. B., Sannegowda, L. K. & Kamanna, K. Microwave-assisted one-pot synthesis of tetrahydrobenzo [b] pyrans in the presence of WEWFPA and their electrochemical studies. Organ. Commun. 15, 148–166 (2022).
Kangani, M., Hazeri, N. & Maghsoodlou, M. T. A mild and environmentally benign synthesis of tetrahydrobenzo [b] pyrans and pyrano [c] chromenes using pectin as a green and biodegradable catalyst. J. Chin. Chem. Soc. 63, 896–901 (2016).
Saadati-Moshtaghin, H. R. & Zonoz, F. M. Preparation and characterization of magnetite-dihydrogen phosphate as a novel catalyst in the synthesis of tetrahydrobenzo [b] pyrans. Mater. Chem. Phys. 199, 159–165 (2017).
Elhamifar, D., Ramazani, Z., Norouzi, M. & Mirbagheri, R. Magnetic iron oxide/phenylsulfonic acid: A novel, efficient and recoverable nanocatalyst for green synthesis of tetrahydrobenzo [b] pyrans under ultrasonic conditions. J. Colloid Interface Sci. 511, 392–401 (2018).
Norouzi, M., Elhamifar, D., Mirbagheri, R. & Ramazani, Z. Synthesis, characterization and catalytic application of a novel ethyl and boron sulfonic acid based bifunctional periodic mesoporous organosilica. J. Taiwan Inst. Chem. Eng. 89, 234–244 (2018).
Shaker, M. & Elhamifar, D. Magnetic Ti-containing phenylene-based mesoporous organosilica: A powerful nanocatalyst with high recoverability. Colloids Surf. A Physicochem. Eng. Aspects 608, 125603 (2021).
Zhang, L.-Y. et al. Control synthesis of magnetic Fe3O4–chitosan nanoparticles under UV irradiation in aqueous system. Curr. Appl. Phys. 10, 828–833 (2010).
Norouzi, M. & Elhamifar, D. Ionic liquid-modified magnetic mesoporous silica supported tungstate: A powerful and magnetically recoverable nanocatalyst. Compos. Part B Eng. 176, 107308 (2019).
Karimi, B., Maleki, A., Elhamifar, D., Clark, J. H. & Hunt, A. J. Self-assembled organic–inorganic hybrid silica with ionic liquid framework: A novel support for the catalytic enantioselective Strecker reaction of imines using Yb (OTf) 3–pybox catalyst. Chem. Commun. 46, 6947–6949 (2010).
Mirbagheri, R. & Elhamifar, D. Magnetic ethyl-based organosilica supported Schiff-base/indium: A very efficient and highly durable nanocatalyst. J. Alloys Compd. 790, 783–791 (2019).
Kargar, S., Elhamifar, D. & Elhamifar, D. Ionic liquid/high-density polyethylene composite supported molybdenum complex: A powerful, highly stable and easy recoverable catalyst. J. Polym. Res. 29, 484 (2022).
Li, W., Zhang, B., Li, X., Zhang, H. & Zhang, Q. Preparation and characterization of novel immobilized Fe3O4@SiO2@ mSiO2–Pd (0) catalyst with large pore-size mesoporous for Suzuki coupling reaction. Appl. Catal. A Gen. 459, 65–72 (2013).
Hou, S. et al. Synthesis of core–shell structured magnetic mesoporous silica microspheres with accessible carboxyl functionalized surfaces and radially oriented large mesopores as adsorbents for the removal of heavy metal ions. RSC Adv. 7, 51993–52000 (2017).
Zhang, Y. et al. Benzoboroxole-functionalized magnetic core/shell microspheres for highly specific enrichment of glycoproteins under physiological conditions. Small 10, 1379–1386 (2014).
Liu, G., Wang, D., Zhou, F. & Liu, W. Electrostatic self-assembly of Au nanoparticles onto thermosensitive magnetic core-shell microgels for thermally tunable and magnetically recyclable catalysis. Small 11, 2807–2816 (2015).
Wang, Y. et al. Highly selective fluorescent chemosensor for Zn2+ derived from inorganic-organic hybrid magnetic core/shell Fe3O4@SiO2 nanoparticles. Nanoscale Res. Lett. 7, 1–13 (2012).
Lee, J. et al. Simple synthesis of functionalized superparamagnetic magnetite/silica core/shell nanoparticles and their application as magnetically separable high-performance biocatalysts. Small 4, 143–152 (2008).
Sing, K., Everett, D., Raw, H. & Ra, R. J. & Siemieniewska, T. Reporting physisorption data for gas/solid system with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985).
Abaeezadeh, S., Elhamifar, D., Norouzi, M. & Shaker, M. Magnetic nanoporous MCM-41 supported ionic liquid/palladium complex: An efficient nanocatalyst with high recoverability. Appl. Organomet. Chem. 33, e4862 (2019).
Alinezhad, H., Tarahomi, M., Maleki, B. & Amiri, A. SO3H-functionalized nano-MGO-D-NH2: Synthesis, characterization and application for one-pot synthesis of pyrano [2, 3-d] pyrimidinone and tetrahydrobenzo [b] pyran derivatives in aqueous media. Appl. Organomet. Chem. 33, e4661 (2019).
Khazaei, A., Gholami, F., Khakyzadeh, V., Moosavi-Zare, A. R. & Afsar, J. Magnetic core–shell titanium dioxide nanoparticles as an efficient catalyst for domino Knoevenagel–Michael-cyclocondensation reaction of malononitrile, various aldehydes and dimedone. RSC Adv. 5, 14305–14310 (2015).
Mane, V. U., Chavan, S. M., Choudhari, B. & Mane, D. V. Microwave assisted synthesis of tetrahydrobenzo [b] pyrans via one pot multicomponent reaction using [Et3NH][HSO4] as ionic liquid catalyst. J. Pharm. Chem. Biol. Sci. 6, 311–319 (2019).
Sameri, F., Mobinikhaledi, A. & Bodaghifard, M. A. Preparation of core/shell CaO@SiO2-SO3H as a novel and recyclable nanocatalyst for one-pot synthesize of dihydropyrano [2, 3-c] pyrazoles and tetrahydrobenzo [b] pyrans. Silicon, 14, 1–12 (2021).
Ataie, F., Davoodnia, A. & Khojastehnezhad, A. Graphene oxide functionalized organic-inorganic hybrid (GO–Si–NH2–PMo): An efficient and green catalyst for the synthesis of tetrahydrobenzo [b] pyran derivatives. Polycycl. Aromat. Compds. 41, 781–794 (2021).
Taheri, K., Elhamifar, D., Kargar, S. & Zarnegaryan, A. Graphene oxide supported ionic liquid/Fe complex: A robust and highly stable nanocatalyst. RSC Adv. 13, 16067–16077 (2023).
Acknowledgements
The authors thank the Yasouj University and the Iran National Science Foundation (INSF) for supporting this work.
Author information
Authors and Affiliations
Contributions
F.D.: writing—original draft, investigation, resources, formal analysis. D.E.: Conceptualization, writing—review and editing, supervision, visualization. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dadvar, F., Elhamifar, D. Magnetic silica/graphene oxide nanocomposite supported ionic liquid–manganese complex as a powerful catalyst for the synthesis of tetrahydrobenzopyrans. Sci Rep 13, 19354 (2023). https://doi.org/10.1038/s41598-023-46629-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46629-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.