Abstract
Acute myocardial infarction (AMI) can rarely arise from non-lipid-rich coronary plaques. This study sought to compare the clinical outcomes after percutaneous coronary intervention (PCI) between AMI showing maximum lipid-core burden index in 4 mm (maxLCBI4mm) < 400 and ≥ 400 in the infarct-related lesions assessed by near-infrared spectroscopy-intravascular ultrasound (NIRS-IVUS). We investigated 426 AMI patients who underwent NIRS-IVUS in the infarct-related lesions before PCI. Major adverse cardiovascular events (MACE) were defined as the composite of cardiac death, non-fatal MI, clinically driven target lesion revascularization (TLR), clinically driven non-TLR, and congestive heart failure requiring hospitalization. 107 (25%) patients had infarct-related lesions of maxLCBI4mm < 400, and 319 (75%) patients had those of maxLCBI4mm ≥ 400. The maxLCBI4mm < 400 group had a younger median age at onset (68 years [IQR: 57–78 years] vs. 73 years [IQR: 64–80 years], P = 0.007), less frequent multivessel disease (39% vs. 51%, P = 0.029), less frequent TIMI flow grade 0 or 1 before PCI (62% vs. 75%, P = 0.007), and less frequent no-reflow immediately after PCI (5% vs. 11%, P = 0.039). During a median follow-up period of 31 months [IQR: 19–48 months], the frequency of MACE was significantly lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group (4.7% vs. 17.2%, P = 0.001). MaxLCBI4mm < 400 was an independent predictor of MACE-free survival at multivariable analysis (hazard ratio: 0.36 [confidence interval: 0.13–0.98], P = 0.046). MaxLCBI4mm < 400 measured by NIRS in the infract-related lesions before PCI was associated with better long-term clinical outcomes in AMI patients.
Similar content being viewed by others
Introduction
Most acute myocardial infarctions (AMI) are caused by thrombotic occlusion of coronary atherosclerotic lesions. Atherosclerotic lesions have lipid deposits in the core region of the plaque. Previous autopsy studies have demonstrated that 50–70% of coronary thrombosis resulted from the rupture of a plaque with a large lipid-core1. However, 20–40% of coronary thrombosis resulted from non-ruptured plaques, generally with small or no evidence of a lipid-core1. Compared with AMI arising from the lipid-rich plaque, AMI arising from the non-lipid-rich plaque (defined as a lipid-free or small lipid-containing plaque) may have different clinical features and prognosis.
Developments of intravascular imaging have enabled evaluation of infarct-related coronary lesions of AMI in the clinical setting. However, intracoronary thrombi often obscure the lesion, preventing precise determination of the underlying mechanisms of AMI. Recently, near-infrared spectroscopy (NIRS) combined with intravascular ultrasound (IVUS) has emerged as an imaging technique that can accurately identify lipids in the coronary artery wall even in the presence of thrombi. Several NIRS studies have demonstrated that infarct-related lesions generally exhibit high lipid composition and rarely low lipid composition2,3. In NIRS, maximum lipid-core burden index in 4 mm (maxLCBI4mm) of 400 is used to differentiate between lipid-rich plaque and non-lipid-rich plaque4. The present study sought to compare the clinical outcomes after percutaneous coronary intervention (PCI) between AMI with maxLCBI4mm < 400 and ≥ 400 in the infarct-related lesions assessed by NIRS.
Methods
Study population
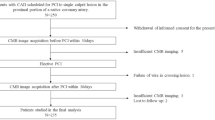
This was a multicentre retrospective registry of NIRS-IVUS in AMI patients. Between May 2016 and March 2021, 997 AMI patients underwent PCI for de novo native coronary artery lesions. NIRS-IVUS before PCI using a second-generation drug-eluting stent was performed in 461 patients (Supplementary Table 1). Of these, 35 patients were excluded due to technical failure to deliver NIRS-IVUS catheter (n = 20) and poor NIRS-IVUS images (n = 15). Thus, 426 patients constituted the final study population (Fig. 1). AMI was defined as a type 1 MI according to the universal definition5. The infarct-related lesion was identified by the operator at the time of angiography on the basis of angiographic lesion morphology, such as total or subtotal occlusion, as well as electrocardiographic findings and echocardiographic results. This study was carried out in accordance with the Declaration of Helsinki. This study was approved by the institutional review board at Wakayama Medical University (3384). The requirement for written informed consent was waived by the institutional review board at Wakayama Medical University because of the retrospective design of the study. All methods were carried out in accordance with relevant guidelines and regulations.
Patient selection. From 997 patients with AMI, 426 patients who underwent NIRS-IVUS before primary PCI were selected for analysis. AMI: acute myocardial infarction, DES: drug-eluting stent, IVUS: intravascular ultrasound, LCBI: lipid core burden index, NIRS: near-infrared spectroscopy, PCI: percutaneous coronary intervention.
NIRS-IVUS imaging
NIRS-IVUS was performed before PCI at the operator's discretion using the TVC Imaging System (MC8 with a 40-MHz TVC Insight catheter [n = 146] or MC10 with a 50-MHz Dualpro catheter [n = 280], InfraReDx, Burlington, Massachusetts, USA). There were no pre-determined inclusion/exclusion criteria for the use of NIRS-IVUS. In case with TIMI (Thrombolysis in Myocardial Infarction) flow grade 0 or 16, balloon angioplasty with a small balloon ≤ 2.0 mm in diameter (n = 319 [75%]) and/or aspiration thrombectomy with a 5.1-F aspiration catheter (n = 132 [31%]) was performed prior to the NIRS-IVUS imaging. The NIRS-IVUS catheter was advanced distally to the infarct-related lesion over a 0.014-inch conventional angioplasty guidewire. The NIRS-IVUS imaging core was retracted to the coronary ostia at a rate of 0.5 mm/s for the 40-MHz TVC Insight catheter or 2.0 mm/s for the 50-MHz Dualpro catheter using an automatic pullback device. Color-coded NIRS spectral data co-registered with IVUS images were acquired during pullback and stored digitally for offline analysis.
NIRS-IVUS analysis
NIRS-IVUS analysis was performed using CAAS Intra Vascular (Pie Medical Imaging, Maastricht, the Netherlands) in an independent core laboratory (Wakayama Medical University, Wakayama, Japan), blinded to clinical information and angiography findings. In IVUS, plaque rupture (defined by an intra-plaque cavity that communicated with the lumen with an overlying residual fibrous cap fragment)7, attenuated plaque (defined by a signal reduction behind hypoechoic plaque without calcium), and convex calcium (defined by an intraluminal protrusion > 0.5 mm in thickness, with a bright echo, convex shape, irregular surface, and acoustic shadowing) were evaluated3. External elastic membrane (EEM), lumen, and plaque burden (defined as [EEM area—lumen area]/EEM area × 100) were measured on a cross-section at 1 mm longitudinal intervals8. Minimum lumen area (MLA) was determined in the infarct-related lesion. Reference site was set at a cross-section adjacent to the infarct-related lesion that had the largest lumen and plaque burden of < 50%8. Lesion length was defined as a distance between the proximal and distal reference sites8. Positive remodeling was defined by a remodeling index (calculated as EEM area at the MLA site/the proximal reference EEM area) > 1.059.
NIRS data were automatically displayed on a chemogram demonstrating the distribution of lipid within the coronary artery in a longitudinal view. In the chemogram, lipid content was quantified as the LCBI, defined as the fraction of valid pixels indicating lipid at the probability > 0.6 within the scanned region multiplied by 1000. The LCBIvessel was defined as the value of LCBI in the entire scanned region within the infarct-related vessel2. The maxLCBI4mm was defined as the maximum value of the LCBI for any 4 mm region in the infarct-related lesion2.
PCI
PCI was performed in a standard manner using a second-generation drug-eluting stent (Supplementary Table 2). During PCI, patients received intravenous heparin (a bolus of 100 IU/kg and additional doses aimed at achieving activated clotting time of 250–300 s). Thrombolysis was not performed for any patient. Glycoprotein IIb/IIIa inhibitors were not used because they were not approved in Japan. Dual antiplatelet therapy with aspirin and a thienopyridine (clopidogrel or prasugrel) was continued at least 6 months after PCI.
Angiography
Angiograms before and immediately after PCI were analysed using QAngio XA ver 7.1 (Medis, Leiden, the Netherlands) in an independent core laboratory (Wakayama Medical University, Wakayama, Japan), blinded to clinical information and NIRS-IVUS findings. Quantitative coronary angiography included the reference lumen diameter, minimum lumen diameter, and percent diameter stenosis. Multivessel disease was defined as having angiographical stenoses with more than 50% diameter stenosis in two or three major epicardial coronary arteries. TIMI flow grade before PCI, no-reflow (defined as TIMI flow grade 0, 1 or 2) immediately after PCI and distal embolization (defined as a distal filling defect with an abrupt cut-off in the infarct-related artery) immediately after PCI were evaluated according to established definitions6. Balloon-to-artery ratio was defined as maximum balloon diameter divided by reference lumen diameter.
Clinical outcomes
Clinical follow-up data of patients were collected from their medical records. The adjudication of major adverse cardiovascular events (MACE) was based on the discussions with three experienced cardiologists (Y.S, T.Ku, and S.F) who were blinded to the NIRS-IVUS results. MACE was defined as the composite of cardiac death10, non-fatal MI (including culprit plaque [CP] -related MI and non-culprit plaque [NCP] -related MI)5, clinically driven target lesion revascularization (TLR)10, clinically driven non-TLR10, or congestive heart failure (CHF) requiring hospitalization11. TLR was defined as a repeat revascularization for the lesions treated with baseline PCI. Non-TLR was defined as a revascularization for non-infarct-related lesions that were identified in baseline coronary angiography. Scheduled revascularization for non-infarct-related lesions was not considered as an adverse event. TLR/non-TLR was considered to be clinically-driven if revascularization was performed on a patient who had a positive result on a functional ischemia study and ischemic symptoms such as chest pain10.
Statistical analysis
Statistical analysis was performed by using JMP 13.0 (SAS Institute, Cary, North Carolina, USA). Categorical variables were presented as frequency and percentages, with comparison with chi-square statistics or the Fisher exact test (if the expected cell value was < 5). Continuous variables were presented as median and interquartile range (IQR) and compared using the Mann–Whitney U test. The Kaplan–Meier method and log-rank test was used to compare the cumulative rate of MACE between the groups. Univariate Cox regression analysis was performed to evaluate for clinical, angiographic and NIRS-IVUS variables before PCI that were associated with MACE. The variables with P-values < 0.1 in the univariate analysis were then included in multivariable Cox regression analysis. Although both maxLCBI4mm and LCBIvessel met the criteria for inclusion in the multivariable Cox regression analysis, only maxLCBI4mm was chosen for inclusion because maxLCBI4mm more directly represents the tissue characteristics of infarct-related lesions. Results were reported as hazard ratios (HRs) with 95% confidence intervals (CIs). A P-value < 0.05 was considered statistically significant.
Results
Patient clinical characteristics
In NIRS before PCI, 107 (25%) patients had infarct-related lesions with maxLCBI4mm < 400 (279 [IQR: 158–332]), and 319 (75%) patients had infarct-related lesions with maxLCBI4mm ≥ 400 (710 [IQR: 556–827]). Patient clinical characteristics at baseline were not different between the maxLCBI4mm < 400 group and the maxLCBI4mm ≥ 400 group, except for age (68 years [IQR: 57–78 years] vs. 73 years [IQR: 64–80 years], P = 0.007) (Table 1 and Supplementary Table 3).
Angiographic findings and procedural characteristics
Angiographic findings and procedural characteristics are shown in Table 2. The distribution of infarct-related coronary artery was not different between the two groups. Reference lumen diameter, minimum lumen diameter, and percent diameter stenosis before PCI were similar between the two groups. The frequency of TIMI flow grade 0 or 1 before PCI (62% vs. 75%, P = 0.007) and multivessel disease (39% vs. 51%, P = 0.029) was significantly lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group.
Stent diameter was not different between the two groups. Stent length was significantly shorter in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group (18 mm [IQR: 16–24 mm] vs. 24 mm [IQR: 18–33 mm], P < 0.001). Maximum balloon diameter and balloon-to-artery ratio were similar between the two groups. Minimum lumen diameter and percent diameter stenosis immediately after PCI were not different between the two groups. The frequency of no-reflow was significantly lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group (5% vs. 11%, P = 0.039). The frequency of distal embolization was similar between the two groups.
IVUS findings
IVUS findings before PCI are shown in Table 3. The frequency of plaque rupture (4% vs. 59%, P < 0.001) and attenuated plaque (23% vs. 77%, P < 0.001) was significantly lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group. The frequency of convex calcium was significantly higher in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group (14% vs. 6%, P = 0.008). Lesion length was significantly shorter in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group (17 mm [IQR: 14–22 mm] vs. 22 mm [IQR: 16–30 mm], P < 0.001). Reference EEM area was similar between the two groups. MLA was significantly larger in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group (2.5 mm2 [IQR: 2.1–3.2mm2] vs. 2.2 mm2 [IQR: 1.9–2.6mm2], P < 0.001). At the MLA site, EEM area (15.4 [IQR: 11.9–19.0] vs. 16.5 [IQR: 13.2–19.7], P = 0.039) and plaque burden (84% [IQR: 79–86%] vs. 87% [IQR: 83–89%], P < 0.001) were significantly smaller in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group. The frequency of positive remodeling (23% vs. 71%, P < 0.001) was significantly lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group.
NIRS findings
The analyzed length of NIRS in the maxLCBI4mm < 400 group and the maxLCBI4mm ≥ 400 group was 65 mm [IQR: 55–75 mm] and 66 mm [IQR: 57–79 mm], respectively (P = 0.348). The value of LCBIvessel was significantly different between the maxLCBI4mm < 400 group and the maxLCBI4mm ≥ 400 group (78 [IQR: 41–109] and 188 [IQR: 132–245], P < 0.001).
Clinical outcomes
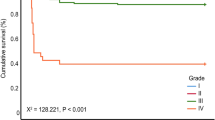
Clinical outcomes are shown in Table 4. Follow-up duration in the maxLCBI4mm < 400 group and the maxLCBI4mm ≥ 400 group was 35 months [IQR: 18–52 months] and 30 months [IQR: 19–46 months], respectively (P = 0.222). The frequency of MACE was significantly lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group (4.7% vs. 17.2%, P = 0.001) (Fig. 2). The frequency of cardiac death (0% vs. 5.0%, P = 0.018) and non-fatal MI (0% vs. 4.4%, P = 0.028) was significantly lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group. The frequency of CP-related MI was not different between the two groups (0% vs. 1.3%, P = 0.245). The frequency of NCP-related MI was numerically lower in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group, but the differences did not reach statistical significance. (0% vs. 3.1%, P = 0.064). The frequency of TLR, non-TLR, and CHF requiring hospitalization was not different between the two groups.
Regarding the prediction of MACE, diabetes mellitus, prior MI, Killip class 4, peak creatine kinase myocardial band > 200 IU/L, left ventricular ejection fraction < 40%, multivessel disease, maxLCBI4mm < 400, attenuated plaque, lesion length > 20 mm, and positive remodeling were variables with a p-value < 0.10 in univariate Cox regression analysis (Supplementary Table 5). The multivariable analysis including these variables revealed that Killip class 4 was an independent predictor of MACE (HR: 4.79, [CI: 1.88–12.25], P = 0.001) and the maxLCBI4mm < 400 was an independent predictor of MACE-free survival (HR: 0.36 [CI: 0.13–0.98], P = 0.046) (Table 5).
Discussion
The major findings in the present NIRS-IVUS study were that the AMI patients with maxLCBI4mm < 400 in infarct-related coronary lesions had a younger median age at onset, less frequent multivessel disease, less frequent TIMI flow grade 0 or 1 before PCI, less frequent no-reflow immediately after PCI, and better long-term clinical outcomes compared with those with maxLCBI4mm ≥ 400. In addition, maxLCBI4mm < 400 in infarct-related coronary lesions was an independent predictor of MACE-free survival.
Infarct-related lesions with low LCBI
Most infarct-related lesions of AMI show high LCBI in NIRS. A previous NIRS study demonstrated that the infarct-related plaques had a median maxLCBI4mm of 523 (IQR: 445–821) and a threshold of maxLCBI4mm > 400 distinguished infarct-related plaques from non-infarct-related plaques with a sensitivity of 85% and specificity of 98%2. Infarct-related plaques with low maxLCBI4mm are likely to have different pathologies compared with those with high maxLCBI4mm. An autopsy study demonstrated that pathological intimal thickening without lipid-core is the basis of plaque erosion rather than plaque rupture1. A case report showed that maxLCBI4mm in plaque erosion was zero12. Our recent clinical study revealed that maxLCBI4mm was significantly smaller in plaque erosion compared with plaque rupture and maxLCBI4mm < 426 identified plaque erosion with sensitivity 92%, specificity 97%3. Therefore, the maxLCBI4mm < 400 group in the present study may consist mostly of plaque erosion.
Acute results of PCI
Acute results of PCI for a lipid-free or small lipid-containing plaques in AMI appears to be associated with better clinical outcomes than those for lipid-rich plaques. A NIRS-IVUS study showed that lower maxLCBI4mm in the infarct-related lesions was associated with smaller infarction size and less frequent coronary microvascular obstruction assessed by magnetic resonance imaging after PCI13. In the present study, the maxLCBI4mm < 400 group had a lower frequency of no-reflow phenomenon during PCI than the maxLCBI4mm ≥ 400 group. The favourable acute results of PCI for a lipid-free or small lipid-containing plaques in AMI might have a positive effect on long-term outcomes.
Long-term clinical outcomes
Pathohistological characteristics of infarct-related lesions are associated with long-term clinical outcomes in AMI patients treated with PCI. Previous OCT studies have demonstrated that AMI arising from a lipid-free or small lipid-containing plaque with intact fibrous cap (i.e. OCT-derived plaque erosion) had a lower frequency of MACE (including death, MI, revascularization, and CHF) during a follow-up period of > 1 year compared with AMI arising from lipid-rich plaque with disrupted fibrous-cap14,15,16. In line with these OCT studies, the present NIRS-IVUS study showed that lower maxLCBI4mm in the infarct-related plaques was associated with better long-term clinical outcomes in AMI patients. One reason for this association is that maxLCBI4mm in the infarct-related plaques may reflect the amount of remaining vascular lipid after PCI in three coronary arteries. In the present study, multivessel disease was less frequent in the maxLCBI4mm < 400 group compared with the maxLCBI4mm ≥ 400 group. The extent of coronary atherosclerosis might influence long-term outcomes. Based on our results, the patients with maxLCBI4mm ≥ 400 in the infarct-related plaques might require stricter clinical follow-up and aggressive treatment of coronary risk factors to prevent future MACE. Recent clinical studies have shown that the intensive lipid-lowering therapy with proprotein convertase subtilisin kexin type 9 inhibitor (PCSK9i) increased fibrous cap thickness and decreased lipid core volume in coronary atheroma17,18. In patients with maxLCBI4mm ≥ 400 in the infarct-related plaques, aggressive use of PCSK9i should be considered to stabilize atherosclerotic plaques and prevent future cardiovascular events.
Limitations
There are several limitations that should be acknowledged. First, NIRS-IVUS was performed at the operator’s discretion, which might have led to selection bias. Second, there are limits to diagnosing plaque erosion using only LCBI in NIRS. Plaques with maxLCBI4mm < 400 are not necessarily plaque erosion. Third, the present study did not use OCT. OCT allows accurate assessment of the morphological characteristics of the lesion responsible for AMI. The combined imaging of OCT and NIRS will be a promising technique for identifying plaque erosion. Fourth, balloon angioplasty and aspiration thrombectomy before imaging may have induced iatrogenic fibrous cap disruption and reduced the lipid composition in plaque rupture, although meticulous care was taken to avoid excessive mechanical trauma. Fifth, there were no data on NIRS-IVUS immediately after PCI. Therefore, the present study was unable to evaluate the clinical significance of changes in maxLCBI4mm during PCI. Sixth, there were no data on NIRS-IVUS in the non-infarct-related vessel. The presence or absence of lipid-rich plaque in non-infarct-related vessels might affect the prognosis of the patient. Seventh, different types of stents were used in the present study. However, all were current generation drug-eluting stents, 80% of which were everolimus-eluting stent, and there were no statistical differences in stent type between the two groups. Finally, the patients receiving thienopyridine at follow-up tended to be more frequent in the maxLCBI4mm < 400 group than in the maxLCBI4mm ≥ 400 group, which may have influenced our results regarding clinical outcome.
Conclusions
MaxLCBI4mm < 400 measured by NIRS in the infract-related lesions before PCI was associated with better long-term clinical outcomes in AMI patients.
Clinical perspectives
Competency in medical knowledge
maxLCBI4mm < 400 measured by NIRS in the infract-related lesions before PCI was associated with better long-term clinical outcomes in AMI patients.
Translational outlook
NIRS might be useful in the risk stratification of AMI patients undergoing PPCI, because maxLCBI4mm < 400 measured by NIRS in the infract-related lesions before PCI is associated with better long-term clinical outcomes in AMI patients.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary file.
Abbreviations
- AMI:
-
Acute myocardial infarction
- CHF:
-
Congestive heart failure
- EEM:
-
External elastic membrane
- IVUS:
-
Intravascular ultrasound
- LCBI:
-
Lipid core burden index
- maxLCBI4mm :
-
Maximum lipid core burden index in 4 mm
- MLA:
-
Minimum lumen area
- NIRS:
-
Near-infrared spectroscopy
- PCI:
-
Percutaneous coronary intervention
- TLR:
-
Target lesion revascularization
References
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Madder, R. D. et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 6, 838–846 (2013).
Terada, K. et al. NIRS-IVUS for differentiating coronary plaque rupture, erosion, and calcified nodule in acute myocardial infarction. JACC Cardiovasc. Imaging 14, 1440–1450 (2021).
Waksman, R. et al. Identification of patients and plaques vulnerable to future coronary events with near-infrared spectroscopy intravascular ultrasound imaging: A prospective, cohort study. Lancet 394, 1629–1637 (2019).
Thygesen, K. et al. Fourth universal definition of myocardial infarction. J. Am. Coll. Cardiol. 72, 2231–2264 (2018).
Sharma, V. et al. Myocardial blush and microvascular reperfusion following manual thrombectomy during percutaneous coronary intervention for ST elevation myocardial infarction: Insights from the TOTAL trial. Eur. Heart. J 37, 1891–1898 (2016).
Kotani, J. et al. Intravascular ultrasound analysis of infarct-related and non-infarct-related arteries in patients who presented with an acute myocardial infarction. Circulation 107, 2889–2893 (2003).
Mintz, G. S. et al. American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American college of cardiology task force on clinical expert consensus documents. J. Am. Coll. Cardiol. 37, 1478–1492 (2001).
Schoenhagen, P. et al. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: An intravascular ultrasound study. Circulation 101, 598–603 (2000).
Garcia-Garcia, H. M. et al. Standardized end point definitions for coronary intervention trials: The academic research consortium-2 consensus document. Circulation 137, 2635–2650 (2018).
Yancy, C. W. et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J. Am. Coll. Cardiol. 70, 776–803 (2017).
Usui, E. et al. Multimodality imaging of possible healed plaque erosion pathologically validated by directional coronary atherectomy specimens. JACC Cardiovasc. Interv. 15, 2225–2227 (2022).
Terada, K. et al. Near-infrared spectroscopy to predict microvascular obstruction after primary percutaneous coronary intervention. EuroIntervention 17, e999–e1006 (2021).
Higuma, T. et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: Incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc. Interv. 8, 1166–1176 (2015).
Niccoli, G. et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur. Heart J. 36, 1377–1384 (2015).
Yonetsu, T. et al. Plaque morphologies and the clinical prognosis of acute coronary syndrome caused by lesions with intact fibrous cap diagnosed by optical coherence tomography. Int. J. Cardiol. 203, 766–774 (2016).
Räber, L. et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: The PACMAN-AMI randomized clinical trial. JAMA 327, 1771–1781 (2022).
Nicholls, S. J. et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc. Imaging 15, 1308–1321 (2022).
Funding
This work was partially supported by JSPS KAKENHI Grant Number JP17K01417.
Author information
Authors and Affiliations
Contributions
K.T. performed data analysis and wrote the first draft of the manuscript. M.T., N.W., and T.Ka. collected clinical data and images. A.K. and Y.I. performed image analysis. T.Ku., Y.S., and S.F. adjudicated the adverse cardiovascular events. R.M. reviewed and revised the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Madder has received speaker honoraria and research support from Infraredx and serves on the advisory board of SpectraWave. All other authors have no relationships relevant to the contents of this paper to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Terada, K., Wakana, N., Kubo, T. et al. Clinical outcomes of acute myocardial infarction arising from non-lipid-rich plaque determined by NIRS-IVUS. Sci Rep 13, 11544 (2023). https://doi.org/10.1038/s41598-023-38578-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38578-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.