Abstract
This research aims to advance knowledge on the impact of four processing methods on volatile compounds from insect-based baked products (cookies) to provide insights on consumer acceptance. Samples were exposed to double step enzyme digestive test, volatiles characterized through headspace analysis, while semi-trained panelists were recruited for the sensory test. Blanched and boiled samples of R. differens had considerably higher digestibility (83.42% and 81.61%, respectively) (p < 0.05) than toasted and deep-fried samples. Insect-based cookie products integrated with blanched and boiled R. differens meal expressed higher digestibility (80.41% and 78.73%, respectively) that was comparable to that of commercial cookie products (control cookies-CTRC with 88.22%). Key volatile compounds common between the various cookie products included, nonanal, octanal, methyl-pyrazine, hexanal, tetradecane, 2-pentylfuran, 2-heptanone, 2E-octenal, 2E-heptenal and dodecane. Among the volatile compounds, pleasant aromas observed were 2E,4E-dodecadienal, pentanal, octanal, methyl pyrazine, furfurals, benzaldehyde, and 2-pentyl furan, which were more pronounced in cookies fortified with boiled, toasted and deep-fried R. differens meal. There was a greater resemblance of sensory characteristics between control cookies and those fortified with deep-fried R. differens. These findings underscore the significant influence of aroma compounds on consumer acceptability and preference for insect-based baked food products, which allows for future process-modification of innate aromas of insect-based meals to produce high-valued pleasant consumer driven market products.
Similar content being viewed by others
Edible insects portray enormous nutritional potential accentuating excellent sources of proteins, lipids, certain vitamins, and minerals, such as calcium, iron, or zinc1 and healthy unsaturated fatty acids2. This rich nutritional profile has attracted the attention of researchers and the food industry for their possible application in the development of foods with improved nutritional qualities to promote human nutrition. This initiative has however, experienced challenges with regards to consumer acceptance of insect-based products3, characterized by food neophobia and disgust4. Moreover, even if the insect-based products are accepted, their nutritional copiousness does not necessarily signify their high digestibility5. It is therefore paramount to understand the bio-accessibility of the proteins, especially after processing to guarantee nutritional benefits to the consumers.
Digestibility determines whether physiologically active and nutritionally valuable molecules are released from the food matrix into the gastrointestinal tract, making them available for intestinal absorption5. Despite edible insects’ high digestibility of 76–98%6, factors such as species differences, chitin levels and processing techniques significantly influence it7. From literature, toasted and dried R. differens demonstrated higher digestibility as opposed to termites8, boiled and oven cooked mealworms displayed higher digestibility9 and toasting of mopane worms remarkably declined their digestibility10.
Food neophobia is a personality trait characterized by the avoidance of novel or unfamiliar meals, whereas disgust is associated with implicit attitudes that are impacted by people's implicit associations with a food's disgust-inducing characteristics4. Of great influence to acceptability of edible insects is disgust, as it has been cited the major deterrent factor to entomophagy adoption11,12,13. Disgust is provoked by sensory characteristics such as aroma, texture and general appearance3. Development of insect-based products with modified sensory properties aligned to the gastronomic customs of consumers has enhanced consumer preference and familiarity to these products to some extent4. However, reduced ranking of sensory attributes has been witnessed when the insect-based products are compared against their insect-free counterparts14,15,16.
Distaste of insect-based products, galvanized by aroma and flavours, is the key reason behind consumer prejudice of these products marked by reluctant consideration into diets17,18. Flavours in edible insects are derived from the pheromone on their surfaces, the environment where they grow or are bred, the feeds they feed/fed on and fermentation products19,20,21. Therefore, research directed towards the modification of these sensory properties of edible insects into consumer-pleasant properties in a bid to comply with the FAO advocacy for insects is pertinent.
A distinctive study by Ssepuuya et al.22 expressed boiling R. differens to enhance the levels of aroma compounds hexanal and 2-pentylfuran and further toasting to markedly elevate the levels of heptanal, octanal, nonanal, 2-heptanone, 2-nonanone, 2-decanone and limonene. Cheseto et al.23 equally reported a number of ketones and aldehydes in cookies baked with different insect oils including R. differens oil. Considerable concentrations of these chemical compounds were associated with increased consumer acceptability.
This study purposely sought to integrate R. differens flours processed through blanching, boiling, toasting and deep-frying into cookies to assess how they impact protein digestibility, aroma compounds and sensory characteristics. Cookies are cereal-based bakery products that are quite famous and well-liked all around the world, and their formulations with low concentrations of insect flours have proven to be better acceptable and familiarized by consumers24.
Results
In vitro protein digestibility of processed R. differens and the respective cookies
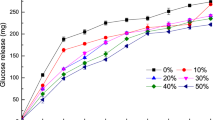
The percentage in vitro protein digestibility of R. differens after exposure to different processing methods are illustrated in Fig. 1. Both blanched and boiled R. differens had considerably higher protein digestibility (p < 0.05) than toasted and deep-fried samples. For the cookies, higher digestibility (p < 0.05) was depicted in the CTRC with DFRC recording the lowest (Fig. 2). However, there was no discernible difference in digestibility between BCRC and BLRC.
In vitro protein digestibility (%) of the processed R. differens based cookies. Bars with same small letters following each other are not significantly different (p < 0.05). CTRC Control cookies with eggs; BCRC Blanched R. differens-based cookies; BLRC Boiled R. differens-based cookies; TSRC Toasted R. differens-based cookies; DFRC Deep fried R. differens-based cookies.
Sensory evaluation of the cookies
The sensory attributes; colour (F(5,852) = 11.8, p < 0.001), flavour (F(5,852) = 14.3, p < 0.001), mouthfeel (F(5,852) = 6.2, p < 0.001), texture (F(5,852) = 7.8, p < 0.001) and overall acceptability (F(5,852) = 14.7, p < 0.001) varied significantly across the five cookie types (Table 1). The texture ratings of CTRC samples were significantly (p < 0.05) higher than the other cookie types with the colour and overall acceptability ratings being significantly (p < 0.05) higher than BCRC, BLRC and TSRC only. The consumers rated the colour, texture, flavour, and overall acceptability of both CTRC and DFRC equally. In addition, when compared to other cookies, BCRC recorded significantly (p < 0.05) lower preference of flavour attributes. A two-dimensional Principal Component Analysis (PCA) explaining 97.6% of the variation differentiated the cookies based on their sensory scores and portrayed the correlation between the sensory characteristics of the cookies (Fig. 3). The PCA revealed strong positive correlation between mouthfeel and overall acceptability.
Principal component analysis (PCA) biplot showing the variation of sensory attribute scores among the cookies enriched with differentially processed R. differens. CTRC = Control cookies with eggs; BCRC = Blanched R. differens-based cookies; BLRC = Boiled R. differens-based cookies; TSRC = Toasted R. differens-based cookies; DFRC = Deep fried R. differens-based cookies.
Volatile organic compounds of the cookies
Chemical characterization of headspace components of the cookies revealed 79 major distinct volatile organic compounds (VOCs) presented in supplementary Table 1 (Table S1). The VOCs dominantly comprised of hydrocarbons, aldehydes, monoterpenes, and ketones. The stress value of < 0.1 (0.081) from the Non-Metric Multidimensional Scaling (NMDS) ordination (Fig. 4A) indicated a good representation of the dissimilarities and correct grouping of the VOCs from the cookies enriched with differently processed R. differens (Fig. 4B). The major compounds that led to the differentiation of the cookies enriched with differently processed R. differens are nonanal, octanal, methyl-pyrazine, hexanal, tridecane, 2-pentylfuran, 2-heptanone, (E)-2-octenal, (E)-2-heptenal, and dodecane (Fig. 4A and C) with their respective total ion chromatogram presented in Fig. 4D and peak areas presented in Table 2.
(A) Non-metric multidimensional scaling plot (NMDS) clustering the different R. differens-based cookies based on the type of volatile they emit, analysis of similarities (ANOSIM). (B) Shepard plot showing the great ordination of the NMDS analysis (stress value < 0.1). (C) Histogram displaying the contribution of the 10 most important volatiles to the differentiation of all the different enriched cookies. CTRC = Control cookies with eggs; BCRC = Blanched R. differens-based cookies; BLRC = Boiled R. differens-based cookies; TSRC = Toasted R. differens-based cookies; DFRC = Deep fried R. differens-based cookies. (D) Overlayed Total ion Chromatogram (TIC) indicating some of the identified violates in C. Each cookie chromatogram is represented by a different colour.
Discussion
Protein digestibility is a measure of a protein's or its structural subunits' (peptides and amino acids) bio-accessibility in the gut relative to what is consumed. The processed insects' in vitro protein digestibility varied from 72.32 to 83.42%, which corroborates prior reports indicating 76–96% in edible insects28. Edible insect proteins are slightly less digestible than eggs (95%) and beef (98%) but are more digestible than plant proteins20. In the present study, the blanched R. differens expressed highest digestibility followed by boiled, toasted and then deep-fried R. differens thereby exposing the impactful consequences of their respective processes. Thermal processing has been reported to either increase or decrease protein digestibility depending on processing conditions and circumstances. Kinyuru et al.8 found that toasting and drying reduced protein digestibility of R. differens while unaltering that of edible winged termites (Macrotermes subhylanus).
Denaturation temperatures improve native protein digestibility by unfolding the polypeptide chain and making the protein more accessible to digestive enzymes8. The decreased protein digestibility exhibited in toasted and deep-fried samples may be due to exposure of proteins to dry heat, which promotes the formation of disulphide linkages, hampering digestive enzymes accessibility. Furthermore, the low moisture and high temperature conditions characterizing toasting and deep frying processes, intensified Maillard reactions which may have utilized available proteins and amino acids, thereby reducing the amount of digestible proteins29. Also, the greater fat content of deep-fried and toasted R. differens compared to blanched and boiling samples30 may have promoted the formation of protein-lipid oxidation products complexes posing a hindrance to enzyme-protein accessibility5. Antinutrients (tannins and phytates), chitin, and the experimental approach used in terms of enzymes applied could all play a role in explaining the differences in digestibility identified in edible insect proteins7. Although there is still inadequate information on the anti-nutrient content of R. differens, analysis of its closely related species S. prasiniferum and C. trachypterus revealed that their levels were within acceptable ranges31 hence, may have not significantly influenced the digestibility. In this respect, due to its soft body nature, R. differens has a low chitinous content, which could have had little impact on digestion5.
The protein digestibility of the enriched cookies paralleled the pattern observed in their processed R. differens counterparts. As a result, it is possible that the elements that limit or enhance digestibility in processed R. differens also played a role in the protein digestibility of the cookies. This is consistent with findings made by Akullo et al.29 which ascribed differences in protein digestibility of crackers to the influence of termite processing conditions. In other studies, increasing the quantity of Bambara ground nut flours with high protein digestibility boosted the protein digestibility of non-wheat cookies32. Contrastingly, the enhanced cookies' protein digestibility was marginally lower than that of their processed R. differens counterparts. This could possibly be attributed to the processed R. differens having a higher digestible protein content (7.8–44.7%)30 compared to the cookies (nutrients dilution effect). Other factors such as such as physical parameters and enzyme(s) have been shown to affect digestibility. For instance, Abdel-Aal33 found that biscuits had improved digestibility in a two-step enzyme metabolism but decreased digestibility in a one-step enzyme metabolism. The former was associated to increased protein accessibility by enzymes during baking, while the latter was linked to a pH change caused by the biscuit mix's buffering capacity. The inclusion of eggs in the formulation with the omission of the insect flours resulted in a higher digestibility of control cookies (CTRC). Eggs are known to have a high protein digestibility (95%)20. This is explained by their lack of chitin, which correlates adversely with protein digestibility7 as well as the presence of highly soluble proteins.
Despite the fact that a significant level of insect familiarity has been gained as a result of increased awareness and incorporation into modern food products, a significant number of consumers remain opposed to the idea. Authors have established that there is a general trend of a negative association between increasing levels of insect incorporation into products and their acceptability15,34,35,36,37,38. Contrary to Bawa et al.39, Ojinnaka et al.37 and Adeboye et al.34 reporting a significant difference in flavour perception of 10% insect enriched cookies compared to the control, the current study found no significant differences in BLRC, TSRC, and DFRC, but not BCRC (Table 1). This may be attributed to the raw insects' original terrible flavours being transformed into new, more attractive flavours as a result of the processing. BCRCs were developed using blanched R. differens, a process characterized by short-lived hot water treatment, which may have been inadequate for total transformation of the flavour compounds. Consumers' aversion to edible insects has been highlighted as the most common barrier to their acceptance. The primary elicitors have been identified as sensory qualities of insects such as flavour, appearance, and texture40,41 and determines whether an insect-based product is acceptable or not37. However, flavours were very weakly correlated to overall acceptability in this study (Fig. 3B), most likely due to the unpleasant flavours, which were not considered to gauge the acceptability. CTRC's colour rankings differed greatly from BCRC, BLRC, and TSRC. This could be due to the demonstrated higher levels of accessible proteins, peptides, and amino acids in blanched, boiled, and toasted R. differens30 catalyzing Maillard reactions37,42, responsible for darker colours in BCRC, BLRC and TSRC (Fig. 1). Notably, there was no statistically significant difference in colour scores between CTRC and DFRC, which may be due to the deep-frying temperatures degradation of proteins and amino acids in the R. differens30 employed in the DFRC formulation. The exoskeleton of the ground R. differens integrated in the insect-based cookies explain the significant variations in texture scores between the CTRC and the insect-based cookies37. The mouthfeel of the cookies substantially correlated positively with overall acceptance (R = 0.99), indicating that the majority of the panellists relied on physical sensation of the cookies while in the mouth to judge their general acceptability. CTRC was the most popular choice in general hence consistent with findings from other researchers15,29,37. The use of a 5-point hedonic scale was adopted due to its less complexity and suitability for naïve assessors. However, future studies should employ 9-point hedonic scales for comprehensive product sensory evaluations and deduction of consumer trends.
Volatile organic compounds (VOCs), which contribute to aroma, flavour, and taste, are one of the features that influence the perception and acceptance of foods, including edible insects. Acids, alcohols, aldehydes, alkenes, amines, terpenes, ketones, and esters are among the aroma chemicals previously discovered in insects19,22,23 which translate to savory, umami, buttery, meaty, bacony, sweet, herbal, or fruity flavours43. The main VOCs that contributed significantly to cookie difference are consistent with the profiles found in boiled and roasted R. differens22 and R. differens oil23. The higher concentrations of hexanal and 2-pentylfuran in BLRC cookies prepared with boiled R. differens underpins the findings by Ssepuuya et al.22 revealing that boiling R. differens enhances the two compounds. The detection of limonene in all the cookies and 1-heptanol in the BLRC is in agreement with a previous study which found limonene and heptanol as the predominant volatiles in raw R. differens22. Similarly, the pronounced levels of hexanal and 2-pentylfuran in BLRC are consistent with previous research that found the two aroma compounds to be the most prevalent VOCs in boiled R. differens22. High methyl pyrazine concentrations associated with TSRC and DFRC (formulated with toasted and deep-fried R. differens, respectively) maybe hypothesized to emanate from oxidation decarboxylation of reducing sugars and amino acids during frying process to generate Strecker aldehydes and α-amrinones. The resultant compounds subsequently condense to form alkane pyrazines, hence serving as dominant aroma compounds in toasted and deep-fried R. differens and in their respective cookie products44.
Unsaturated fatty acid breakdown has been reported to produce 2E,4E-decadienal, and 2E-heptenal45. In this study, the higher 2E,4E-decadienal found in TSRC and DFRC could be attributed to their emergence during high-temperature toasting and deep-frying of R. differens. BCRC were characterized with low concentrations of nonanal, hexanal, 2E,4E-dodecadienal, pentanal, and octanal are aldehydes which are associated with fat, meaty flavour, nutty, sweet, and almond-like aroma26,46 and methyl pyrazine, furfurals, benzaldehyde and 2-pentyl furan which are associated with desirable flavours; nutty, cocoa, roasted meat, almond-like and sweet. This may have contributed to the low sensory scores regarding flavour of the BCRC compared with the other cookie types. BCRC were formulated with R. differens processed by blanching, an ephemeral processing technique, which may have resulted in insufficient chemical interactions to produce adequate flavour profiles. This is apparent from Fig. 4A displaying no VOC associated with BCRC from the identified influential profiles in Fig. 4C. In another study, fermentation of Allomyrina dichotoma larvae with Saccharomyces cerevisiae significantly reduced indole, a faecal odour compound, while simultaneously introducing new aroma compounds such as 2-undecanone, 2-methyl-1-butanol, 2-nonanone, 3-methyl-1-butanol, isopentyl acetate, and ethyl acetate and enhancing others47. Therefore, edible insects processing can be a prospective strategy adoptable to manipulate insect aroma from native and undesirable profiles to new pleasant profiles in order to advance entomophagy. The current study is by no means exhaustive. Therefore, future use of other techniques such as Gas chromatography–olfactometry-mass spectrometry for identification of aroma active compounds would be crucial.
Conclusion
The high levels of protein digestibility observed in R. differens meals was clearly mirrored in the value-added cookie products. Most of the volatile compounds produced by the baked cookie products fortified with R. differens meal were associated with attractive aroma. These findings suggest that different processing conditions could be used to obtain diversified insect-based food products contributing to increased consumer satisfaction. This groups of volatile compounds; acetoin, pyrazine, (E)-3-penten-2-one, 2,3-butanediol, 2,3,5-trimethyl hexane, methylpyrazine, furfural, 2,4-dimethyl heptanone, 2-heptanone, benzaldehyde, eicosane and (2E,4Z)-decadienal in R. differens fortified cookies highlights the unique specificity of these products from conventional cookie bakery product. Given that aroma and flavour of novel food products could be a deterrent factor to consumers, further investigations are needed for quality control.
Materials and methods
Acquisition and processing of R. differens
Fresh and sorted R. differens of 20 kg, with the ovipositors, appendages and wings removed, were purchased from Masaka (0°20′ 28.0′′ S 31° 44′10.0′′ E) and Kampala (0.3476° N, 32.5825° E), Uganda in 2019. The samples were packed into sterile sample collection plastic containers, placed in cool boxes, covered with flaked ice (4–7 °C) and transported to International Centre of Insect Physiology and Ecology (icipe) laboratory. Blanching, boiling, toasting and deep-frying were adopted for processing 700 g each of R. differens and oven-dried (SDO-225, Wagtech International, Thatcham, UK) at 60 ℃ for 24 h to a moisture content of < 15% according to procedures delineated by Ochieng et al.30. The samples were milled using a three-speed Waring laboratory blender, (Camlab, Over, UK) and screened through a 0.1 mm stainless steel laboratory sieve. They were then subsequently vacuum packed in sterile zip loc bags, labelled accordingly and temporarily stored at −4 ℃ awaiting formulation.
Formulation and baking of cookies
Cookies enriched with the processed R. differens as well as the control were formulated according to a method described by Aziah et al.48, with a few modifications. Wheat was substituted with the processed R. differens at 10% (w/w) based on a consistently demonstrated marginal acceptability of sensory characteristics of bakery products previously formulated with insects flours at 10% inclusion level15,34,35,36,37,38. Cookies with no insects added, contained eggs, serving as the control. About 172.2 g of sugar and 3.4 g of salt were sieved and mixed with 408.2 g of wheat flour, already premixed with food grade improvers, for 5 min. Approximately 172.2 g of shortening was added and mixed in a bakery mixer (BJY-BM10, Berjaya, Malaysia) for 15 min to produce a creamy mixture. About 84 g of whisked eggs (for control) or processed R. differens flours was added and mixed for another 10 min. The mixture was then hand-kneaded for 5 min to obtain a firm consistent dough of approximately 180 g each. The dough was rolled out on a wooden board using a rolling pin to a thickness of 5 mm and cut into 5 cm diameter circles. The cut-out cookie doughs were arranged on greased baking trays at 50 mm apart and baked for 15 min at 180 °C, 30% dryness in a preheated oven (BISTROT 665; BestFor®, Ferrara, Italy). Approximately 180 cookies were prepared from each formulated dough with each cookie type represented in Fig. 5. Three cookies, from each treatment were randomly selected and immediately taken for headspace volatiles trapping. Cookies (143) from each dough, intended for sensory study, was packed in 2-inch mini plastic zip loc bags and coded accordingly. The remaining cookies were kept in a cold room at −10 ℃ for a successive digestibility test.
In vitro protein digestibility of the processed R. differens flours and their respective cookies
Determination of in vitro protein digestibility of the processed R. differens and their respective cookies were conducted according to Chavan et al.49 and modified by Wang et al.50. Samples of 1 g each were weighed into 50 mL centrifuge tubes, which were then filled with 20 mL of 0.10 M HCl. Likewise, 50 mg of pepsin (Porcine gastric mucosa, ≥ 250 units/mg solid, Sigma-Aldrich) suspended in 1 mL of 0.01 M HCl were added and mixed. The mixture was then gently shaken for 3 h in a water bath shaker (GYROMAX, Amerex Instruments, Inc, CA, USA) at 37 °C. Subsequently, a mix of 10 mL distilled water and 10 mL of 0.1 M phosphate buffer (pH 8.0) containing 5 mg of trypsin (porcine pancreas, lyophilized powder, BioReagent, 1000–2000 BAEE units/mg solid, Sigma-Aldrich) was introduced, and the mixture subjected to a 37 ℃ water bath for 3 h under continuous agitation. Ten millilitres of trichloroacetic acid (TCA) was infused to purposefully terminate the enzymes activity followed by centrifugation (Eppendorf AG, Hamburg, Germany, 2500 g, 20 ℃) at 14 000 rpm for 10 min. The supernatant was discarded, and the residue dried in an oven at 105 ℃ for 3 h. The nitrogen content of dried residue (0.5 g) was measured using the Kjeldahl technique. The difference between the total quantity of protein in the samples and the remaining protein after enzyme digestion was divided by the total protein in the samples to compute the protein digestibility. The protein content of blanched, boiled, toasted and deep-fried R. differens (40.1, 43.1, 44.7 and 7.8%, respectively)30 and those of cookies; CTRC, BCRC, BLRC, TSRC and DFRC (11.09, 10.90, 10.99 and 6.78%, respectively) were considered as initial protein conents for computation of % digestibility.
Sensory evaluation of the cookies
The cookies' appearance, taste, mouthfeel, texture, and general acceptability were evaluated for consumer preference. A 5-point hedonic scale was used where; 5 denoted like extremely, 4 denoted like, 3 denoted neither like or dislike, 2 denoted dislike and 1 denoted dislike extremely51, the ranking test evaluated differences in intensity of the sensory properties among samples using comparable intervals between the categories. The experiment randomly enrolled a team of 143 semi-trained panellists, comprised of 72 males and 73 females, of age ranging 18–50 years. The panellists were selected based on their experience in food products description and knowledge of cookies. Individual temporary booths made of paperboards for segregation of assessors were equipped with pens and questionnaires for data collection and processing in a sensory laboratory room that almost practically resembled ISO requirements as part of the examinations were set up52. The samples (coded as NJM for CTRC, VPK for BCRC, HQT for BLRC, UAL for TSRC and YHP for DFRC) cookie samples were served to the panellists at room temperature. Alongside the samples, the panellists were given a cup of room-temperature clean water for palate cleansing before commencement of the test and between every tasting done. They were instructed to consent to the study, carefully read the instructions and focus on the texture and colour of the cookies first before proceeding to taste. Panellists were required to score the samples against attributes provided in the evaluation forms. The scores were compiled and analyzed.
Ethical approval
This research was approved by the Institutional Animal Care and Use Committee (IACUC) of Kenya Agricultural and Livestock Research Organization (KALRO)-Veterinary Science Research Institute (VSRI); Muguga North upon compliance with all provisions vetted under and coded: KALRO-VSRI/IACUC028/16032022. This study was reviewed and approved by Egerton University and the National Council for Science Technology and Innovation in Kenya (NACOSTI/P/21/8303). Further, an informed consent was obtained from all the participants and/or their legal guardians via the statement "I am aware that my responses are confidential, and I agree to participate in this survey as well as affirming that I can withdraw from the survey at any time without giving a reason. The products tested were safe for consumption". The appropriate protocols for protecting the rights and privacy of all participants during the execution of the research were utilized. All the experiments were performed in accordance with relevant guidelines and regulations.
GC–MS analysis of the volatile organic compounds
The volatile compounds were determined according to previous methods23,53. Ground cookie samples (10 g) were precisely weighed into 250 mL quick fit chamber Agricultural Research Service (ARS) (Gainesville, FL, USA). A push–pull Gast pump (Gast Manufacturing Inc., Benton Harbor, MI, USA), was used to pump an activated charcoal-filtered and humidified air over the samples at a flowrate of 340 mL/min as the volatiles simultaneously adhered on GC grade dichloromethane (DCM)-precleaned Super-Q traps (30 mg, Analytical Research System, Gainesville, FL, USA) at 170 mL/min flow rate, sustained by Vacuubrand CVC2 vacuum pump (Vacuubrand, Wertheim, Germany) for 24 h. Trapped volatiles were then eluted with 200 µL of GC-grade DCM (Merck, Darmstadt, Germany) into 250 µL conical point glass inserts (Supelco, Bellefonte, PA, USA) fitted into 2 mL glass vials and immediately queued for GC–MS analysis.
The volatiles were identified by a GC–MS on an HP 7890A series gas chromatograph (Agilent Technologies, Wilmington, NC, USA) attached to an HP 5975C mass spectrometer (Agilent Technologies, Wilmington, NC, USA) operated in electron ionization mode of 70 eV. A non-polar HP-5MS capillary column (30 m 0.25 mm i.d.; 0.25 m film thickness; J & W Scientific, Folsom, CA, USA) was fitted to the instrument. Helium was employed as the carrier gas at a rate of 1.2 mL min-1. One microliter of each sample was, in a splitless mode, injected at 35 °C for 5 min, then adjusted to 280 °C at 10 °C min-1. The injector and detector were maintained isothermal at 280 °C for 35 min while temperature of the ion source was 230 ℃. Electron ionization mass spectra were recorded at 70 eV spanning a mass range of 38–550 Daltons over a scan period of 0.73 scans s-1 (Da). Authentic standard hexanal was run in the GC–MS in full scan mode to generate a linear calibration curve (peak area vs. concentration) with the following equation: \([\mathrm{y }= 203482\mathrm{x}- 451578]\) to yield R2 = 0.9997. To identify volatile compounds, their retention periods and mass fragmentation spectra were compared to authentic standards (those available). Others were tentatively identified using Adams, Chemoecol, and the National Institute of Standards and Technology mass spectrum library matching (NIST) (MSD Chemstation E.02.00.493, MS HP, USA). All assays were done in triplicates.
Statistical analysis
All descriptive and quantitative data were statistically analyzed using R Studio software version 1.3.1093–154. The data sets were verified for normal distribution using the Shapiro–Wilk test (p > 0.05). The effects of processing on the digestibility of processed R. differens and the related cookies, as well as the distribution of volatile organic components and consumer acceptability of the cookies, were studied using one way analysis of variance (ANOVA). Tukey's and Bonferroni’s multiple comparison tests with p < 0.05 were used to differentiate the means. The variations in the sensory scores of the developed cookies were evaluated using Principal Component Analysis (PCA). One-way analysis of similarities (ANOSIM) with the Bray–Curtis dissimilarity matrix was used to examine the chemical profiles of various enriched cookie volatiles. The non-metric multidimensional scaling approach based on the similarity percentages (SIMPER) analysis was used to quantify and illustrate the relative contribution of different compounds to the dissimilarity between volatiles from different cookies. Tabulated results were expressed as mean ± standard deviation.
Data availability
All relevant data are presented in the paper.
References
Zielińska, E. & Pankiewicz, U. Nutritional, physiochemical, and antioxidative characteristics of shortcake biscuits enriched with Tenebrio molitor flour. Molecules 25, 5629 (2020).
Zielińska, E., Baraniak, B., Karaś, M., Rybczyńska, K. & Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 77, 460–466 (2015).
Jensen, N. H. & Lieberoth, A. We will eat disgusting foods together—Evidence of the normative basis of Western entomophagy-disgust from an insect tasting. Food Qual. Prefer. 72, 109–115 (2019).
La Barbera, F., Verneau, F., Amato, M. & Grunert, K. Understanding Westerners’ disgust for the eating of insects: The role of food neophobia and implicit associations. Food Qual. Prefer. 64, 120–125 (2018).
Traksele, L. et al. Investigation of in vitro and in vivo digestibility of black soldier fly (Hermetia illucens L.) larvae protein. J. Funct. Foods 79, 104402–104408 (2021).
Yang, Q. et al. Nutritional composition and protein quality of the edible beetle Holotrichia parallela. J. Insect Sci. Insect Sci. 14, 1–4 (2014).
Manditsera, F. A., Luning, P. A., Fogliano, V. & Lakemond, C. M. M. Effect of domestic cooking methods on protein digestibility and mineral bioaccessibility of wild harvested adult edible insects. Food Res. Int. 121, 404–411 (2019).
Kinyuru, J. N., Kenji, G. M., Njoroge, S. M. & Ayieko, M. Effect of processing methods on the In Vitro protein digestibility and vitamin content of edible winged termite (Macrotermes subhylanus) and grasshopper (Ruspolia differens). Food Bioprocess Technol. 3, 778–782 (2009).
Megido, R. C. et al. Effect of household cooking techniques on the microbiological load and the nutritional quality of mealworms (Tenebrio molitor L.). Food Res. Int. 106, 503–508 (2018).
Madibela, O. R., Seitiso, T. K., Thema, T. F. & Letso, M. Effect of traditional processing methods on chemical composition and in vitro true dry matter digestibility of the Mophane worm (Imbrasia belina). J. Arid Environ. 68, 492–500 (2007).
Hamerman, E. J. Cooking and disgust sensitivity influence preference for attending insect-based food events. Appetite 96, 319–326 (2016).
Gmuer, A., Nuessli Guth, J., Hartmann, C. & Siegrist, M. Effects of the degree of processing of insect ingredients in snacks on expected emotional experiences and willingness to eat. Food Qual. Prefer. 54, 117–127 (2016).
Wendin, K. M. E. & Nyberg, M. E. Factors influencing consumer perception and acceptability of insect-based foods. Curr. Opin. Food Sci. 40, 67–71 (2021).
Caparros Megido, R. et al. Consumer acceptance of insect-based alternative meat products in Western countries. Food Qual. Prefer. 52, 237–243 (2016).
Ogunlakin, G. O., Oni, V. T. & Olaniyan, S. A. Quality evaluation of biscuit fortified with edible termite (Macrotermes nigeriensis). Asian J. Biotechnol. Bioresour. Technol. 4, 1–7 (2018).
Schouteten, J. J. et al. Emotional and sensory profiling of insect-, plant- and meat-based burgers under blind, expected and informed conditions. Food Qual. Prefer. 52, 27–31 (2016).
House, J. Consumer acceptance of insect-based foods in the Netherlands : Academic and commercial implications. Appetite 107, 47–58 (2016).
Ghosh, S., Jung, C. & Meyer-rochow, V. B. What governs selection and acceptance of edible insect species. In Edible Insects in Sustainable Food Systems (eds Halloran, A. et al.) 331–351 (Springer, Cham, 2018).
Tzompa-Sosa, D. A., Yi, L., Van Valenberg, H. J. F. & Lakemond, C. M. M. Four insect oils as food ingredient: Physical and chemical characterisation of insect oils obtained by an aqueous oil extraction. J. Insects as Food Feed 4, 279–292 (2019).
Kouřimská, L. & Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 4, 22–26 (2016).
Roncolini, A. et al. Protein fortification with mealworm (Tenebrio molitor L.) powder : Effect on textural, microbiological, nutritional and sensory features of bread. PLoS One 14, 1–29 (2019).
Ssepuuya, G. et al. Effect of heat processing on the nutrient composition, colour, and volatile odour compounds of the long-horned grasshopper Ruspolia differens Serville. Food Res. Int. 129, 108831–108841 (2020).
Cheseto, X., Baleba, S., Tanga, C. M., Kelemu, S. & Torto, B. Chemistry and sensory characterization of a bakery product prepared with oils from African edible insects. Foods 9, 800–826 (2020).
Melgar-Lalanne, G., Hernández-Álvarez, A. J. & Salinas-Castro, A. Edible insects processing: Traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 18, 1166–1191 (2019).
Guclu, G., Sevindik, O., Kelebek, H. & Selli, S. Determination of volatiles by odor activity value and phenolics of CV. Ayvalik Early-Harvest Olive Oil. Foods 5, 46–58 (2016).
Starowicz, M. Analysis of volatiles in food products. Separations 8, 157–170 (2021).
Yang, D. S., Shewfelt, R. L., Lee, K. S. & Kays, S. J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 56, 2780–2787 (2008).
Stull, V. J. Impacts of insect consumption on human health. J. Insects Food Feed 7, 695–713 (2021).
Akullo, J., Nakimbugwe, D., Obaa, B. B., Okwee-Acai, J. & Agea, J. G. Development and quality evaluation of crackers enriched with edible insects. Int. Food Res. J. 25, 1592–1599 (2018).
Ochieng, B. O. et al. Dynamics in nutrients, sterols and total flavonoid content during processing of the edible Long-Horned grasshopper (Ruspolia differens Serville) for food. Food Chem. 383, 132397 (2022).
Das, M. & Mandal, S. Assessment of nutritional quality and anti-nutrient composition of two edible grasshoppers (Orthoptera: Acrididae)—A search for new food alternative. Int. J. Med. Pharm. Sci. 3, 31–48 (2013).
Kiin-kabari, D. B. & Giami, S. Y. Physico chemical properties and in-vitro protein digestibility of non-wheat cookies prepared from plantain flour and bambara groundnut protein concentrate. J. Food Res. 4, 78–86 (2015).
Abdel-Aal, E. S. Effects of baking on protein digestibility of organic spelt products determined by two in vitro digestion methods. LWT-Food Sci. Technol. 41, 1282–1288 (2008).
Adeboye, A., Bolaji, T. & Fatola, O. Nutritional composition and sensory evaluation of cookies made from wheat and palm weevil larvae flour blends. Ann. Food Sci. Technol. 17, 543–547 (2016).
Awobusuyi, T. D., Pillay, K. & Siwela, M. Consumer acceptance of biscuits supplemented with a sorghum–insect meal. Nutrients 12, 895–907 (2020).
González, C. M., Garzón, R. & Rosell, C. M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. Technol. 51, 1–26 (2019).
Ojinnaka, M. C., Ofoelo, M. U. & Ezenwa, L. I. Nutritional evaluation of wheat cakes enriched with edible African termites (Macrotermes nigeriensis). Agro-Science 12, 35–42 (2015).
Osimani, A. et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 48, 150–163 (2018).
Bawa, M., Songsermpong, S., Kaewtapee, C. & Chanput, W. Nutritional, sensory, and texture quality of bread and cookie enriched with house cricket (Acheta domesticus) powder. J. Food Process. Preserv. 44, 14601–14609 (2020).
Sogari, G., Mora, C. & Menozzi, D. Edible Insects in the Food Sector: Methods, Current Applications and Perspectives (Springer Nature, Cham, 2019).
Evans, J. et al. A descriptive sensory analysis of honeybee drone brood from Denmark and Norway. J. Insects as Food Feed 2, 277–283 (2016).
Mainley, D. Technology of Biscuits, Crackers and Cookies (Woodhead Publication, Sawston, 2011).
Mishyna, M., Chen, J. & Benjamin, O. Sensory attributes of edible insects and insect-based foods—Future outlooks for enhancing consumer appeal. Trends Food Sci. Technol. 95, 141–148 (2019).
Liu, M. et al. Characterization of the key aroma constituents in fried tilapia through the sensorics concept. Foods 11, 494–507 (2022).
Gasior, R. & Wojtycza, K. Sense of smell and volatile aroma compounds and their role in the evaluation of the quality of products of animal origin—A review*. Ann. Anim. Sci. 16, 3–31 (2016).
Domínguez, R., Gómez, M., Fonseca, S. & Lorenzo, J. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 97, 223–230 (2014).
Kim, J. et al. Development of a post-processing method to reduce the unique off-flavor of Allomyrina dichotoma : Yeast fermentation. LWT 150, 111940–111946 (2021).
Noor Aziah, A. A., Mohamad Noor, A. Y. & Ho, L. H. Physicochemical and organoleptic properties of cookies incorporated with legume flour. Int. Food Res. J. 19, 1539–1543 (2012).
Chavan, U. D., Mckenzie, D. B. & Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 8146, 177–187 (2001).
Wang, X. et al. Subunit, amino acid composition and in vitro digestibility of protein isolates from Chinese kabuli and desi chickpea (Cicer arietinum L.) cultivars. Food Res. Int. 43, 567–572 (2010).
Lawless, H. T. & Heymann, H. Sensory evaluation of food principles and practices. J. Wine Res. 24, 80–80 (2013).
ISO. ISO 5492 Sensory Analysis. General Guidance for the Selection, Training and Monitoring of Assessors. (2008).
Mekonnen, B. et al. Re-analysis of abdominal gland volatilome secretions of the african weaver ant, Oecophylla longinoda (Hymenoptera formicidae). Molecules 26, 871–882 (2021).
R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2020).
Acknowledgements
The authors wish to thank Jackton Ooko Ongere, Joshua Wambua, Shem Ondiaka, Rachami Isaiah E. and Faith Nyamu Wamurango for their substantial contribution in providing technical support during data collection.
Funding
Financial support for this research was provided by the BioInnovate Africa Programme (INSBIZ—Contribution ID No. 51050076); Australian Centre for International Agricultural Research (ACIAR) (ProteinAfrica – Grant no: LS/2020/154), the Curt Bergfors Foundation Food Planet Prize Award, Bill & Melinda Gates Foundation (INV-032416), Norwegian Agency for Development Cooperation, the Section for research, innovation, and higher education (RAF–3058 KEN–18/0005); the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); Australian Centre for International Agricultural Research (ACIAR), the Federal Democratic Republic of Ethiopia and the Government of the Republic of Kenya. The first author, Brian O. Ochieng, was financially supported by the Center of Excellence in Sustainable Agriculture and Agribusiness Management (CESAAM) of Egerton University, Kenya. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. Therefore, the views expressed herein do not necessarily reflect the official opinion of the donors.
Author information
Authors and Affiliations
Contributions
B.O.O.: Conceptualization; methodology; software; validation; formal analysis; investigation; data curation; visualization, writing—original draft preparation; writing—review and editing; J.A.O: Conceptualization; methodology; writing—review and editing; supervision; project administration. J.M.N.: Conceptualization; methodology; writing—review and editing; supervision; project administration. X.C.: Investigation; formal analysis; data curation; writing—review and editing. C.M.M.: Investigation; formal analysis; data curation; writing-review and editing. C.M.T: Conceptualization; methodology; resources; visualization; supervision; project administration; funding acquisition; writing—original draft preparation; writing—review and editing. All the authors critically reviewed and approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ochieng, B.O., Anyango, J.O., Nduko, J.M. et al. Aroma characterization and consumer acceptance of four cookie products enriched with insect (Ruspolia differens) meal. Sci Rep 13, 11145 (2023). https://doi.org/10.1038/s41598-023-38166-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38166-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.