Abstract
Blocking the abrupt increase in systolic blood pressure associated with autonomic response during bladder hydrodistention in patients with interstitial cystitis/bladder pain syndrome (IC/BPS) is essential for patient safety. We conducted this study to compare autonomic responses during bladder hydrodistention in patients with IC/BPS under general and spinal anaesthesia. Thirty-six patients were randomly allocated to a general anaesthesia (GA, n = 18) or a spinal anaesthesia (SA, n = 18) group. Blood pressure and heart rate were measured continuously and ΔSBP, defined as maximum increases in SBP during bladder hydrodistention from baseline, was compared between groups. Heart rate variability was analysed using electrocardiograms. The post-anaesthesia care unit assessed postoperative pain using a numeric (0–10) rating scale. Our analyses yield a significantly greater ΔSBP (73.0 [26.0–86.1] vs. 2.0 [− 4.0 to 6.0] mmHg), a significantly lower root-mean-square of successive differences in heart rate variability after bladder hydrodistention (10.8 [7.7–19.8] vs. 20.6 [15.1–44.7] ms), and significantly higher postoperative pain scores (3.5 [0.0–5.5] vs. 0.0 [0.0–0.0]) in the GA compared to the SA group. These findings suggest that SA has advantages over GA for bladder hydrodistention in preventing an abrupt increase in SBP and postoperative pain in IC/BPS patients.
Similar content being viewed by others
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic pain syndrome that causes bladder pain associated with bladder filling and is commonly accompanied by urinary symptoms in the absence of infection and other aetiology1. According to a community survey study, 2.7–6.5% of women in the United States have symptoms consistent with IC/BPS; however, the condition is often underdiagnosed and undertreated2. While the pathophysiology of IC/BPS has not been fully elucidated, a deficiency of glycosaminoglycan covering the urothelium surface, immunological reactions, activated mast cells, neural changes, and inflammation have been suggested3.
Bladder hydrodistention is not only a diagnostic tool, but also a treatment option for patients with IC/BPS. Although it is non-specific, diagnostic information on, for example, Hunner’s lesions or mucosal rainy bleeding can be obtained through cystoscopy with bladder hydrodistention4. Additionally, bladder hydrodistention can improve the symptoms in patients refractory to conservative treatments such as medication and behavioural therapy5. Approximately half of the patients with IC/BPS who undergo bladder hydrodistention show improvements in long-term outcomes6, 7. However, since marked autonomic responses, such as increased blood pressure, are observed during bladder hydrodistention in IC/BPS patients8. adequate anaesthesia that can block the autonomic response is essential for patient safety. According to the Japanese guideline, spinal anaesthesia is a recommended anaesthetic method for bladder hydrodistention; however, the supporting evidence is contradictory9.
In a retrospective study with a limited number of patients, autonomic responses during bladder hydrodistention were greater in patients under general anaesthesia than in those under spinal anaesthesia10. Spinal anaesthesia can block all sensory, motor, and autonomic nerve transmission and11 lower serum concentration of catecholamines12. In contrast, some sensory responses and autonomic reflexes are preserved during general anaesthesia, even with the loss of consciousness13. Therefore, general anaesthesia may not be sufficient to block autonomic responses to bladder hydrodistention in patients with IC/BPS14,15,16. However, no prospective study has compared anaesthetic techniques in terms of autonomic responses during bladder hydrodistention in patients with IC/BPS.
In this study, we aimed to compare changes in systolic blood pressure (SBP) during bladder hydrodistention in patients with IC/BPS under general and spinal anaesthesia. We hypothesized that the change would be less prominent in patients under spinal anaesthesia.
Materials and methods
Ethical approval
This study was approved by the Institutional Review Board of Seoul National University Hospital (approval number: 1806-039-949, date: July 10, 2018) and registered in the Korean national registry of clinical trials before patient recruitment (number: KCT0003225, registration date: 28/09/2018), All patients provided written informed consent. We conducted this study in accordance with the Helsinki Declaration and the Good Clinical Practice guidelines and reported the findings based on the applicable Consolidated Standard of Reporting Trials guidelines. A reporting guideline checklist is included in the Supplementary Information file.
Population
All adult patients who were diagnosed with IC/BPS and scheduled to undergo bladder hydrodistention for either diagnostic or therapeutic purposes at Seoul National University Hospital since October 2018 to December 2021 were eligible for this study. Among these patients, those aged 20 or older who had an American Society of Anesthesiologists (ASA) physical status of I–III were included. The exclusion criteria were contraindications for general anaesthesia or spinal anaesthesia (e.g., severe cardiopulmonary dysfunction, coagulopathy, taking anticoagulants, septicaemia, skin infection near the lumbar puncture site, spinal cord lesion, increased intracranial pressure, neurologic disorders, or spinal deformities).
Randomization
Before patient recruitment, an anaesthesiologist who was not involved in this study prepared a random allocation sequence with a one-to-one ratio using a computer-generated randomized table. Patients were allocated to two groups: the general anaesthesia (GA) group and the spinal anaesthesia (SA) group. The clinical research coordinator who was blinded to this study managed the randomization table and notified the attending anaesthesiologist of the group allocation on the day of surgery.
Protocol
After entering the operation room, patients underwent standard monitoring procedures, including assessments for percutaneous oxygen saturation and non-invasive blood pressure, as well as electrocardiography.
In the GA group, a bolus injection of 1.5–2.0 mg/kg propofol and the target-controlled infusion of remifentanil with an effect-site concentration of 4.0 ng/mL were used for anaesthesia induction. After confirming the loss of consciousness, 0.6 mg/kg rocuronium was administered to facilitate insertion of a supraglottic airway device. After appropriate placement of the supraglottic airway device, a 20-gauge catheter was inserted into the radial artery to monitor invasive blood pressure continuously. Anaesthesia was maintained using sevoflurane and remifentanil. The depth of anaesthesia was monitored using the bispectral index (Medtronic, Ireland), with a target of 40–60 during the procedure.
The effect-site concentration of remifentanil was increased up to 4–6 ng/mL to block autonomic nervous system responses 90 s before initiating bladder hydrodistention. If the mean blood pressure (MBP) dropped below 65 mmHg, the target effect-site concentration of remifentanil was lowered by 1 ng/mL.
In the SA group, spinal anaesthesia was performed at the lateral decubitus position, using a 25-gauge Quincke needle under aseptic drapes. Considering the height of the patient, 12–14 mg of 0.5% bupivacaine was injected intrathecally. The accepted sensory block level was T10 or higher. Subsequently, radial artery catheterization was performed to monitor invasive blood pressure.
In both groups, a rescue drug (ephedrine 5–10 mg, phenylephrine 20–50 µg) was administered if the MBP dropped below 65 mmHg, based on the decision of the attending anaesthesiologist. If the patient’s heart rate (HR) fell below 45 beats/min, 0.5 mg of atropine was injected intravenously. Midazolam or dexmedetomidine was administered intravenously if patients in the SA group required sedation.
Operational procedure
After the induction of anaesthesia, patients were placed in the lithotomy position. A 30°-angled cystoscope was inserted through the urethra to drain the bladder of all urine. Hunner’s lesions were identified and thoroughly inspected under the cystoscope, and their location, number, and area were noted. Bladder hydrodistention was then performed by filling the bladder with normal saline by gravity from a height of 80 cm above the pubic symphysis. Maximum filling in the bladder was maintained for 8 min, and the maximum bladder volume was recorded. Changes in Hunner’s lesions and glomerulation were observed while the saline was drained. Bladder biopsy was performed and the bleeding area, including the mucosal crack, was cauterized to control bleeding. The hyperaemic and congestion areas with Hunner’s lesions were also coagulated. After confirming that there was no bleeding, the surgeon inserted a 6.6-mm three-way catheter for postoperative continuous irrigation. A bimanual examination was performed to check whether any tumour was present in the pelvic cavity, and the surgery was terminated. The entire procedure was performed by a single surgeon (SJO) with the assistance of an attending urologist (YJK).
Measurements
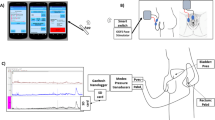
The vital signs and electrocardiogram were recorded during the patient’s stay in the operating room using the Vital Recorder (version 1.8.19.5; https://vitaldb.net/vital-recorder, accessed Feb 21, 2018) with a resolution of 0.5 and 500 samples per second, respectively17.
The primary outcome measure was ΔSBP, defined as the maximum increase in SBP during bladder hydrodistention from baseline (measured 5 min after the induction of anaesthesia). The secondary outcome measures were the maximum increase in MBP, diastolic blood pressure (DBP), and HR during bladder hydrodistention from baseline. Standard heart rate variability (HRV) measures18 such as root-mean-square of successive differences (RMSSD), the standard deviation of the normal-to-normal interval (SDNN), and the high-frequency/low-frequency ratio, were calculated using the Vital Recorder on 5-min electrocardiograms recorded before and after bladder hydrodistention, respectively.
The recorded cystoscopic descriptions included Hunner’s lesions, bleeding patterns, and the maximal bladder volume. Postoperative pain scores were evaluated in the post-anaesthesia care unit (PACU) based on a numeric rating scale (NRS), with 0 reflecting “no pain” and 10 “the worst pain imaginable.” If the patient complained of pain in the PACU, 25 µg of fentanyl was administered intravenously. The number of patients who required analgesics in the PACU was also recorded.
Statistical analysis
The data are presented as numbers (proportions) for categorical variables and means (standard deviations) or medians (interquartile ranges) for continuous variables, depending on the normality of their distributions evaluated with the Shapiro–Wilk test. We used the Pearson’s chi-square test or Fisher’s exact test to compare categorical variables and Student’s t-tests and the Mann–Whitney U test to compare continuous variables with normal and skewed distributions, respectively.
Since rescue vasopressor administered before bladder hydrodistention can cause an elevation of blood pressure, we performed a post hoc subgroup analysis on the patients who were not administered rescue vasopressors.
All statistical analyses were conducted using SPSS statistical software for Windows, version 25.0 (IBM, Armonk, NY, USA) and R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). We considered a P value less than 0.05 as statistically significant.
Sample size calculation
A previous retrospective study reported a ΔSBP in patients with IC/BPS of 56.26 ± 30.38 mmHg under general anaesthesia10. We considered a mean difference in ΔSBP between the two anaesthesia groups of 30 mmHg or more statistically significant. We estimated a required minimum sample size of 16 patients in each group, based on α = 0.05 and power (1 − β) = 0.8. Considering a 10% dropout rate, we recruited 18 patients for each group.
Results
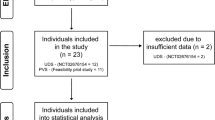
The data of 36 patients who underwent bladder hydrodistention between October 2018 and December 2021 at our institution were entered in our analyses (see Fig. 1).
The patients had been randomly allocated to the GA and the SA group. Table 1 lists the patients’ characteristics, 72-h voiding diaries, and intraoperative findings.
Hemodynamic and autonomic responses are described in Table 2.
ΔSBP was significantly greater in the GA than in the SA group (73.0 [26.0–86.1] vs. 2.0 [− 4.0 to 6.0] mmHg; median difference [95% CI], − 71.0 [− 84.0, − 30.5] mmHg; P < 0.001). ΔDBP, ΔMBP, and ΔHR were also greater in the GA group (ΔDBP: 42.5 [24.0–56.0] vs. 3.0 [− 3.0 to 6.0] mmHg; P < 0.001, ΔMBP: 42.9 ± 28.3 vs. − 1.4 ± 29.0 mmHg; P < 0.001, ΔHR: 18.6 ± 19.6 vs 3.5 ± 14.9 beats per min; P = 0.014). No patient showed an SBP elevation of more than 20% from baseline in the SA group.
The HRV analysis did not yield significant differences in HRV-related measures between the two groups (Table 2); however, RMSSD after bladder hydrodistention was significantly lower in the GA group (10.8 [7.7–19.8] vs. 20.6 [15.1–44.7] ms; P = 0.045).
Anaesthetic and operational findings are listed in Table 3. No patients in the SA group were administered rescue drugs, whereas 77.8% of patient in the GA group received rescue vasopressors during surgery (P < 0.001). There were no differences in surgery, anaesthesia, or PACU stay time.
Pain scores in the PACU were significantly higher in the GA group (3.5 [0.0–5.5] vs. 0.0 [0.0–0.0]; P < 0.001). No patient required rescue analgesics in the SA group, whereas two patients were administered analgesics in the GA group (2 [11.1%] vs. 0 [0.0%]; P = 0.486).
In terms of cystoscopic findings, mucosal rainy bleeding was less frequently observed in the SA group (3 [16.7%] vs. 10 [55.6%]; P = 0.037).
The results of the subgroup analysis with respect to hemodynamic and autonomic responses are presented in Table 4. Six patients treated with rescue vasopressors before bladder hydrodistention, such as during anaesthesia induction, were excluded from the GA group. ΔSBP, the proportion of patients with an SBP increase of more than 20%, ΔDBP, ΔMBP, and ΔHR were all significantly greater and the RMSSD after bladder hydrodistention was still significantly lower in the GA than in the SA group.
Discussion
In this study, we demonstrated that SA has some advantages over GA in preventing abrupt hemodynamic responses during bladder hydrodistention in patients with IC/BPS. In particular, an SBP increase of more than 20% from baseline was only observed in the GA group. Moreover, the number of patients requiring rescue vasopressors was smaller and postoperative pain scores in PACU were lower in the SA group.
In healthy individuals, as the hydrostatic pressure rises during bladder filling, afferent Aδ fibres in the hypogastric and pelvic nerves increase their activity to stimulate the sympathetic nervous system, resulting in relaxation of the bladder smooth muscles19. However, in previous studies using the IC/BPS animal model, C-fibre sensitized by chronic inflammation of the bladder showed hyperexcitability with a reduced threshold, resulting in increased sympathetic tone20. This mechanism explains the hike in blood pressure accompanied by increased urine norepinephrine and decreased vagal activity in patients with IC/BPS21, 22. The results of the present study demonstrated that spinal anaesthesia, which produces intense blockade of neuronal transmission, could have advantages compared to general anaesthesia for blocking autonomic responses. These findings are also consistent with the results of the previous retrospective study10.
HRV is a widely used physiologic test to evaluate the imbalances within the autonomic nervous system. According to the results of the present study, measures of HRV did not differ between the groups before bladder hydrodistention. However, RMSSD after bladder hydrodistention was significantly lower in the GA group. RMSSD reflects the vagal-mediated changes in HR23. These results may thus be due to the increased sympathetic tone after bladder hydrodistention in the GA group. A previous study has also reported lower RMSSD in patients with chronic pain24.
Postoperative pain increases recovery times and the length of hospital stays, and lowers patient satisfaction25, 26. Previous studies conducted in patients undergoing other urologic procedures have also reported that spinal anaesthesia is superior to general anaesthesia in terms of higher patient satisfaction, shorter recovery times, and lower levels of postoperative pain27, 28. In the present study, patients in the SA group neither experienced postoperative pain nor required rescue analgesics in the PACU. However, our follow-up period was short, not reflecting the full extent of postoperative pain. Therefore, although SA was superior for short-term postoperative pain after hydrodistention in this study, further research based on long-term follow-up is needed.
Although spinal anaesthesia has several advantages over general anaesthesia during bladder hydrodistention, there are possible caveats to spinal anaesthesia; particularly, bladder rupture may occur due to adductor muscle spasms triggered by the stimulation of the obturator nerve during electrocautery for coagulation. A previous study observed an obturator reflex in 20% of patients under GA; such a case was, however, not observed in this study29. Obturator nerve block might be considered in patients at high risk of bladder injury due to adductor muscle spasm. In addition, in this study, mucosal waterfall bleeding was more frequent in the SA group, which may have been the result of spinal anaesthesia-induced vasodilation in the lower body.
This study has several limitations. First, we determined the remifentanil concentration (4–6 ng/mL) prior to bladder hydrodistention in the GA group based on the remifentanil concentration required for preventing hemodynamic responses to tracheal intubation in a previous study30. However, this remifentanil concentration was not sufficient to block autonomic responses during bladder hydrodistention under GA in our IC/BPS patients. Nevertheless, a further increase in remifentanil dose can cause more decrease in systolic blood pressure before hydrodistension in the GA group. Future studies on effective strategies (e.g., short acting vasodilator administration) for preventing abrupt increases in SBP during bladder hydrodistention under GA are needed. Second, we did not evaluate postoperative pain in the general ward. There is, however, a possibility of rebound pain arising after spinal anaesthesia. Third, we did not compare long-term outcomes, such as recurrence and symptom improvement, for the two anaesthetic techniques.
In conclusion, we found that spinal anaesthesia has some advantages over general anaesthesia for bladder hydrodistention in terms of preventing an abrupt increase in SBP and controlling pain in the PACU.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abrams, P. et al. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 61, 37–49. https://doi.org/10.1016/s0090-4295(02)02243-4 (2003).
Konkle, K. S. et al. Comparison of an interstitial cystitis/bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology study. J. Urol. 187, 508–512. https://doi.org/10.1016/j.juro.2011.10.040 (2012).
Karamali, M. et al. Molecular pathogenesis of interstitial cystitis/bladder pain syndrome based on gene expression. J. Cell Physiol. 234, 12301–12308. https://doi.org/10.1002/jcp.28009 (2019).
Homma, Y. et al. Clinical guidelines for interstitial cystitis and hypersensitive bladder updated in 2015. Int. J. Urol. 23, 542–549. https://doi.org/10.1111/iju.13118 (2016).
Kuo, H. C. & Chancellor, M. B. Comparison of intravesical botulinum toxin type A injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int. 104, 657–661. https://doi.org/10.1111/j.1464-410X.2009.08495.x (2009).
Lee, S. W. et al. Long-term outcomes of ulcerative interstitial cystitis after complete transurethral resection with therapeutic hydrodistention. Int. Urol. Nephrol. 53, 219–227. https://doi.org/10.1007/s11255-020-02637-1 (2021).
Ens, G. & Garrido, G. L. Role of cystoscopy and hydrodistention in the diagnosis of interstitial cystitis/bladder pain syndrome. Transl. Androl. Urol. 4, 624–628. https://doi.org/10.3978/j.issn.2223-4683.2015.09.04 (2015).
Kobi, S. et al. Autonomic response during bladder hydrodistention in patients with bladder pain syndrome. J. Urol. 188, 117–121. https://doi.org/10.1016/j.juro.2012.02.2561 (2012).
Homma, Y. et al. Japanese guideline for diagnosis and treatment of interstitial cystitis. Int. J. Urol. 16, 4–16. https://doi.org/10.1111/j.1442-2042.2008.02208.x (2009).
Kim, S. W. et al. Autonomic response during bladder hydrodistention reflects the severity of symptoms in patients with bladder pain syndrome/interstitial cystitis. Neurourol. Urodyn. 36, 677–682. https://doi.org/10.1002/nau.22994 (2017).
Hambly, P. R. & Martin, B. Anaesthesia for chronic spinal cord lesions. Anaesthesia 53, 273–289. https://doi.org/10.1046/j.1365-2044.1998.00337.x (1998).
Stevens, R. A. et al. Sympathetic block during spinal anesthesia in volunteers using lidocaine, tetracaine, and bupivacaine. Reg. Anesth. 22, 325–331. https://doi.org/10.1016/s1098-7339(97)80006-5 (1997).
Sanders, R. D. et al. Unresponsiveness ≠ unconsciousness. Anesthesiology 116, 946–959. https://doi.org/10.1097/ALN.0b013e318249d0a7 (2012).
Gori, M. C. et al. Central sensitization in the bladder pain syndrome. JSM Pain Manag. 1, 1004 (2016).
Seybold, V. S. The role of peptides in central sensitization BT—sensory nerves. In (eds Canning, B. J. & Spina D) 451–91 (2009) https://doi.org/10.1007/978-3-540-79090-7_13.
Bennett, G. J. Update on the neurophysiology of pain transmission and modulation: Focus on the NMDA-receptor. J. Pain Symptom Manag. 19, 2–6. https://doi.org/10.1016/S0885-3924(99)00120-7 (2000).
Lee, H.-C. & Jung, C.-W. Vital Recorder-a free research tool for automatic recording of high-resolution time-synchronised physiological data from multiple anaesthesia devices. Sci. Rep. 8, 1527. https://doi.org/10.1038/s41598-018-20062-4 (2018).
Electrophysiology TF of the ES of C the NAS of P. Heart rate variability. Circulation 93, 1043–1065. https://doi.org/10.1161/01.CIR.93.5.1043 (1996).
Merrill, L. et al. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat. Rev. Urol. 13, 193–204. https://doi.org/10.1038/nrurol.2016.13 (2016).
Yoshimura, N. et al. Bladder afferent hyperexcitability in bladder pain syndrome/interstitial cystitis. Int. J. Urol. 21(Suppl 1), 18–25. https://doi.org/10.1111/iju.12308 (2014).
Stein, P. C., Torri, A. & Parsons, C. L. Elevated urinary norepinephrine in interstitial cystitis. Urology 53, 1140–1143. https://doi.org/10.1016/s0090-4295(98)00663-3 (1999).
Williams, D. P. et al. Effects of chronic pelvic pain on heart rate variability in women. J. Urol. 194, 1289–1294. https://doi.org/10.1016/j.juro.2015.04.101 (2015).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front Public Health 5, 258. https://doi.org/10.3389/fpubh.2017.00258 (2017).
Koenig, J. et al. Chronic pain and heart rate variability in a cross-sectional occupational sample: Evidence for impaired vagal control. Clin. J. Pain 32, 218–225 (2016).
Carr, D. B. & Goudas, L. C. Acute pain. Lancet 353, 2051–2058. https://doi.org/10.1016/S0140-6736(99)03313-9 (1999).
Apfelbaum, J. L. et al. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth. Analg. 97, 534–540. https://doi.org/10.1213/01.ANE.0000068822.10113.9E (2003).
Karacalar, S. et al. Spinal-epidural anesthesia versus general anesthesia in the management of percutaneous nephrolithotripsy. J. Endourol. 23, 1591–1597. https://doi.org/10.1089/end.2009.0224 (2009).
Tyritzis, S. I. et al. Spinal versus general anaesthesia in postoperative pain management during transurethral procedures. ISRN Urol. 2011, 895874. https://doi.org/10.5402/2011/895874 (2011).
Soberón, J. R. Jr. et al. Obturator nerve blockade vs. neuromuscular blockade for the prevention of adductor spasm in patients undergoing transurethral resection of bladder tumors: A randomized controlled trial. Pain Med. 22, 1253–1260. https://doi.org/10.1093/pm/pnaa448 (2021).
Albertin, A. et al. The effect-site concentration of remifentanil blunting cardiovascular responses to tracheal intubation and skin incision during bispectral index-guided propofol anesthesia. Anesth. Analg. 101, 125–130. https://doi.org/10.1213/01.ANE.0000153012.35120.FE (2005).
Funding
This work was supported solely by institutional and departmental sources.
Author information
Authors and Affiliations
Contributions
Y.J.K. wrote the main manuscript text. contributed to data curation, formal analysis, investigation, and writing the original draft. H-K.Y., Y.J.K., S.-J.O., M.H., and H.-P.P. contributed to conceptualization, data collection. H-C.L. contributed to conceptualization, supervision, data curation, and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.J., Yoon, HK., Kang, Y.J. et al. Autonomic responses during bladder hydrodistention under general versus spinal anaesthesia in patients with interstitial cystitis/bladder pain syndrome: a randomized clinical trial. Sci Rep 13, 9248 (2023). https://doi.org/10.1038/s41598-023-36537-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36537-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.