Abstract
Fusarium circinatum, a fungal pathogen deadly to many Pinus species, can cause significant economic and ecological losses, especially if it were to become more widely established in Europe. Early detection tools with high-throughput capacity can increase our readiness to implement mitigation actions against new incursions. This study sought to develop a disease detection method based on volatile organic compound (VOC) emissions to detect F. circinatum on different Pinus species. The complete pipeline applied here, entailing gas chromatography—mass spectrometry of VOCs, automated data analysis and machine learning, distinguished diseased from healthy seedlings of Pinus sylvestris and Pinus radiata. In P. radiata, this distinction was possible even before the seedlings became visibly symptomatic, suggesting the possibility for this method to identify latently infected, yet healthy looking plants. Pinus pinea, which is known to be relatively resistant to F. circinatum, remained asymptomatic and showed no changes in VOCs over 28 days. In a separate analysis of in vitro VOCs collected from different species of Fusarium, we showed that even closely related Fusarium spp. can be readily distinguished based on their VOC profiles. The results further substantiate the potential for volatilomics to be used for early disease detection and diagnostic recognition.
Similar content being viewed by others
Introduction
Forests globally are increasingly threatened by alien invasive pathogens and pests. Globalization is primarily responsible for the increasing rate of establishment of invasive alien species (IAS) and no saturation point is yet predictable1. Climate change also compounds the spread of IAS through the elimination of environmental barriers, allowing IAS to establish and survive in new geographic locations. There are many potential pathways of introduction of alien pests and pathogens affecting trees in urban and forested landscapes, e.g., trade of plant-derived commodities2,3,4,5 including seeds6, potting substrates and other plant products valuable for other human activities. Preventing new introductions of IAS is achievable through better biosecurity measures at, for example, border entry locations. However, biosecurity in the plant trade is often curtailed by a lack of resources and necessary skills to recognize problems during plant inspections and a lack of modernized tools with high throughput capacity for detection of alien species in plant shipments7,8,9,10. Countries with stricter border control have fewer established quarantine alien insects11 and fungal plant pathogens12. According to Santini et al.2 approximately 50 invasive forest pathogens currently found in Europe are accidentally introduced alien species, of those approximately 26% attack gymnosperms mainly causing dieback, death and/or reduced growth. Of all invasive forest pathogens in Europe, only 1% have been successfully eradicated by sanitary measures2, a likely result of missing the critical window where early detection and rapid response could lead to effective eradication of the founding population.
To combine recent technological advances with knowledge about specific metabolic responses in pests, pathogens and the trees that they infect is a challenge that calls for interdisciplinary competence. Traditional approaches of disease detection are unsatisfactory for largescale plant screening; usually shipments are only spot-checked if at all, and apart from visual scouting for symptoms, testing is generally targeted and uses tedious and expensive DNA-based or serological detection assays13. Innovative methods that are better suited for early and rapid detection are needed13. Detecting volatile organic compounds (VOCs) released by pathogens and during disease is one such method that could be utilized as an early warning system, facilitating the choice of plant material to be processed for more specific DNA-based diagnosis. As stated by Materić et al.14, VOCs are secondary metabolites produced by all living organisms, the composition of which is unique to every species and presumably also all specific plant-pathogen interactions, comparable to a chemical fingerprint. Emission rates and composition of VOCs are highly dynamic, influenced by biotic and abiotic stresses, and can serve as an indicator of plant health status14. Sampling of VOCs can be done in non-destructive ways from many plants simultaneously, and could serve as a high-throughput detection tool for plant diseases13,15,16,17.
Detection of plant pathogens by analysis of VOCs emitted during infection has been reported in multiple studies, for example, for the early detection of spoilage diseases in crops and grains15,18,19,20,21,22 and the general understanding is that VOC emissions reflect the specific plant-pathogen combination23. Similar methods have also been developed for woody plants with importance for the food industry, like palm24 and lemon trees25. An example of a VOCs application currently near commercial use is the in-field detection of Candidatus Liberibacter asiaticus, a bacterium that is the causal agent of citrus greening disease, commonly known as Huanglongbing, that has devastated the global citrus fruit industry26,27. Pathogen detection methods based on VOCs in a forestry context is far less developed. However, Vuorinen et al.28 could distinguish birch trees exposed to pathogens or herbivores on the basis of VOC profiles, and similarly Johne et al.29 could differentiate between two pathogenic fungi in horse chestnut trees (Aesculus spp.). It has been shown that VOCs may serve as an indicator of fungal infection in asymptomatic spruce30 and recently, Brilli et al.31 found a few VOCs to be uniquely emitted from Ceratocystis platani-infected asymptomatic Platanus trees, highlighting the potential for targeted VOCs analysis for disease detection. Further method development is needed for VOCs applications in the forestry field.
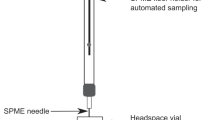
A method utilizing VOCs requires strategies for collection, separation, detection and analysis of the VOCs. Plant VOC collection is most extensively performed by headspace (HS) sampling, a non-destructive approach offering a more realistic picture of the plant VOC profile as compared to alternatives such as extractions of VOCs from plant tissues in organic solvents. Sampling of HS can be achieved using dynamic methods or by static solid phase micro-extraction (SPME)16. SPME fibers are inert and reusable sampling devices having absorbant or adsorbent coatings to which the targeted compounds are sorbed. The chemical properties of the coating determines what type of compounds can be sampled successfully. SPME is easy to use, and once equilibrium with the surrounding HS is reached, the SPME fiber can be thermally desorbed in a gas chromatograph (GC) for subsequent separation of the components in the sample16. Analytes separated by GC are most commonly analysed by a flame ionization detector or a mass spectrometer (MS)15. The final challenge to complete a detection method pipeline lies in the analysis of the big data sets generated from GC–MS analysis, which can be done utilizing for example MZMine 2, an open-source software for MS data processing32. This makes the pipeline fully machine based and, correctly implemented, this approach has potential to be as easily applied as the ion mobility mass spectrometry routinely used in airport security.
Fusarium is a large genus of (mostly) plant-associated filamentous fungi, consisting of 23 defined species complexes and almost 300 distinct species33,34. Fusarium circinatum, the causal agent of pine pitch canker (PPC) disease35, poses a serious threat to pine forests across the globe36. The Fusarium fujikuroi species complex, to which F. circinatum belongs, includes several clades of species with a wide plant host range and varying host specificity37. The American-clade species F. circinatum causes a serious disease on a variety of pine (Pinus) species and on Douglas fir (Pseudotsuga menziesii)38. Early symptoms of F. circinatum infection on pine include resinous cankers, chlorosis and/or wilting of needles while late symptoms appear as shoot dieback, reddening and dead foliage39. This pathogen originates from the south-eastern USA but has now been recorded in 14 countries across Africa, Asia, South America, and south-western Europe35,38. In countries with significant coniferous timber production, preventing the introduction of F. circinatum is crucial. Models of the potential spread and damage caused by F. circinatum suggest that currently, the pathogen may cause limited damage in pine forests and plantations in Northern Europe, but the potential distribution is expected to expand northward in all climate change scenarios40. Even in currently unfavorable geographic regions, F. circinatum can thrive in nurseries where it acts as a damping off disease and causes considerable financial consequences also in regions where field conditions are generally not considered suitable for PPC38. Plants infected in nurseries will be the origin of future outbreaks in the forest when planted. Once established, F. circinatum spreads readily by rain splash, wind and vectoring insects10,41 but is also soil-borne39. Asymptomatic infection has been reported42,43 even in non-pine44, grass45 and herb species46, making visual detection impossible, emphasizing the need for reliable high-throughput diagnostic protocols. Furthermore, Fusarium spp. are morphologically very similar, can sometimes be difficult to distinguish by culture morphology, and therefore require more detailed molecular analysis to identify to a species level37.
Host susceptibility to PPC varies among pine species. Pinus radiata is the most planted conifer globally47 and has a large economic and societal value. The species is known to be highly susceptible to PPC and is the main host in northern Spain where PPC is established and causing significant damage42. Pinus sylvestris, a dominant tree species in northern European forests, and the most widely distributed pine species in the world48, is also shown to be susceptible to PPC based on greenhouse and field inoculation trials on young trees of Spanish, Scottish and Czech origin38,44,49. Pinus pinea is distributed all around the Mediterranean basin including northern Spain, which could enable rapid spread of this plant disease. However, P. pinea has remarkable phenotypic plasticity in functional traits that may explain its relatively higher resistance to F. circinatum-infection50 compared to other pine species.
The aim of this study was to develop a disease detection method based on VOC emissions from pine seedlings. By establishing a library of chemical fingerprints characterizing specific emission profiles, it should prove possible to non-destructively scan plant consignments in ports of entry or plant nurseries to detect the presence of disease, and rapidly respond with further measures to limit its establishment and potential losses. The study sought to: (1) test whether in vitro VOC signatures can distinguish between different Fusarium spp. (2) examine whether in vitro F. circinatum VOCs are present in in vivo, and (3) test whether infection of F. circinatum on pine seedlings can be detected on the basis of VOCs prior to expression of visible symptoms of disease.
Results and discussion
Fusarium spp. cultured on defined media are readily distinguished by VOC profiles
As an initial proof-of-concept pilot study, VOC profiles of four Fusarium spp. (F. circinatum, F. oxysporum f.sp. pini, F. bulbicola and F. graminearum) grown in vitro were compared to test whether analysis of VOCs alone could distinguish closely related species. The selection of the three Fusarium spp. included here in addition to F. circinatum was based on their genetic proximity to F. circinatum51,52. The VOCs were collected using SPME, analyzed by GC–MS before the output data was processed through an objective pipeline. Several combinations consisting of 3–6 VOCs fulfilled the criteria to distinguish the four species with a significant accuracy (p ≤ 0.05) for every pairwise interspecific comparison, explained further below. An example of a VOC combination utilizable to distinguish the Fusarium spp. regardless of timepoint, i.e. 7–21 days post inoculation (dpi), with a 0% confusion matrix error rate and p = 0.006, is shown in Table 1. A total of 211 different VOCs were detected from the four Fusarium spp. A visualized principal component analysis (PCA) of the Fusarium spp. separation based on the 11 VOCs identified by ten repeated Randomforest runs demonstrated the unambiguous groupings irrespective of time point (Fig. 1). A larger study with more replicates would be required to draw confident conclusions regarding the VOC emission characteristics by each species. The results presented here do point to the potential for VOCs analysis as a novel fungus identification method to replace current inadequate or challenging morphological or time-consuming DNA-based approaches that often fall short due to the high morphological and genetic similarity of these species.
The Randomforest selected VOCs observed in Table 1 could not be identified further than to chemical classes, as none of their respective MS data matched any compound in the MS databases, see methods. All three of the compounds were, however, sesquiterpenes, a chemical class previously reported to be emitted from species in the Fusarium fujikuroi species complex53. It is known that plant emitted monoterpenes such as limonene and linalool can inhibit germination of fungal spores54,55, which makes it interesting to find that hyphae of plant pathogenic fungi emit similar compounds, such as the sesquiterpenes found here, emitted by Fusarium spp. There were a number of compounds found to be exclusively detected in just one of the four Fusarium spp. despite the close genetic proximity of the species, for example oxygenated sesquiterpene 2 exclusively emitted by F. circinatum (Table 1). This finding demonstrated the ease with which a VOCs-based detection method could distinguish between morphologically and genetically similar Fusarium spp.
VOC profiles can distinguish between F. circinatum-inoculated and mock-inoculated seedlings
VOCs were sampled from stem-inoculated seedlings of P. sylvestris, P. radiata and P. pinea. Fusarium circinatum-inoculated seedlings were compared to control (mock-inoculated) seedlings, hereafter referred to as “inoculation types”, at 7, 14 and 28 dpi. The same pipeline used for the in vitro studied Fusarium spp. was applied to these in vivo samples, including MZMine 2, Randomforest and PERMANOVA for GC–MS data analysis, which resulted in a number of significant distinctions (Table 2). There were significant (p ≤ 0.05) differences in VOC profiles between the two different inoculation types of P. radiata at all time points, including the earliest time point at 7 dpi when no symptoms were yet visible. In terms of detection tool development for biosecurity, the ability to detect disease earlier than the point of symptom appearance is an important detail. This enables identification of infected, yet apparently healthy seedlings that could otherwise slip through ports of entry and plant nurseries unnoticed, an introduction pathway that remains difficult to address. For P. sylvestris, significant differences were seen at 14 and 28 dpi, and for P. pinea, considered to have very low susceptibility to F. circinatum, no symptoms developed and no significant differences in VOCs emissions were observed between the inoculation types at any time point. These results are visualized by a principal component analysis (Fig. 2). None of the VOCs detected were exclusively detected in the F. circinatum inoculated seedlings, therefore the analysis was based on relative quantitative comparisons between samples.
Principal component analysis of VOC subsets from Pinus spp. seedlings inoculated with F. circinatum or mock inoculated. n = 5–6 seedlings per inoculation type, sampled at three time points: 7, 14 and 28 days post inoculation (dpi). Percentages given on each axis of the plots show the total variance explained by that principal component.
Randomforest produced a subset of eight compounds for P. sylvestris, three for P. radiata and five for P. pinea, which were subsequently utilized in the statistical models (Table 3). These compounds were detected through machine learning, within the complete VOC profiles, as important because of their low error rate as indicators of the seedling inoculation type irrespective of time point (see methods). A total of 307 unique VOCs were found between the three Pinus spp., most of which were present in all three species. It is possible that VOCs other than the subset found here by Randomforest could strengthen the outcomes, for example the distinction between P. sylvestris inoculation types specifically at 7 dpi, if Randomforest had been set to examine each timepoint separately. However, the objective here was to find VOCs that allow for a robust distinction irrespective of time post infection, as a detection method must be applicable regardless of the (often unknown) infection age.
Observations of symptom development were carefully documented throughout the experiment. All Pinus spp. had resinous wounds at the inoculation site at 7 dpi, but were otherwise asymptomatic at this time, regardless of inoculation type. Fusarium circinatum-inoculated P. sylvestris and P. radiata were consistently symptomatic at 14 dpi, with light chlorosis and/or slight wilting of needles, which was described as grade 1 symptoms in the scale used by Martín-Rodrigues et al.39. At 28 dpi, symptoms on P. sylvestris and P. radiata had progressed to grade 3, with severe wilting (Fig. 3). Pinus pinea seedlings remained asymptomatic at all time points. Symptom development on P. sylvestris and P. radiata were consistent with previous reports of inoculations on 2-year-old P. radiata59. The P. sylvestris used in this study had similar susceptibility as P. radiata to F. circinatum, underlining the potentially serious threat posed by PPC to forests of northern Europe dominated by P. sylvestris.
Symptom development in P. sylvestris shoots following stem inoculations. (a) Mock inoculated P. sylvestris seedling at 28 dpi, a healthy shoot with no signs of disease; (b) Fusarium circinatum inoculated P. sylvestris seedling at 28 dpi, displaying characteristic symptoms of shoot wilting and needle chlorosis.
No VOCs detected were uniquely, and consistently, emitted from F. circinatum-inoculated seedlings. Therefore, no single VOC detected here can independently be used as a reliable indicator of disease, which also rules out the idea of identifying a F. circinatum-specific VOC emitted regardless of growth medium, as could be done for example in the study by Brilli et al.31. This means that multivariate data analysis, preferably using a machine learning-based pipeline as presented in this study, is required.
The use of a fully automatic pipeline entailing automated data analysis and machine learning instead of manual processing, beyond being immensely time saving, eliminates the risks of introducing human errors, arbitrariness and need for GC–MS expertise. Advanced competence is needed to manually process GC–MS data, identifying a few hundred VOCs per run sample, and peaks (corresponding to VOCs in the samples) often coincide, making manual peak integration impossible. By elimination of manual processing, the detection method can be performed by nonexperts, overcoming barriers of entry to use this kind of detection method. Machine learning can, in addition, allow for detection of multivariate patterns that are difficult or impossible to detect manually, increasing the detection accuracy. Its accuracy can be further improved by calibrating the models using much bigger data sets than for example the ones available in this study.
The VOCs identified as predictors of the Pinus seedling inoculation type were tentatively structurally identified based on retention indices and comparison with mass spectral data from libraries and previous literature (Table 3). These VOCs were predominantly terpenoids, chemicals strongly associated with VOC emission from pine trees. One example is verbenone, a monoterpene found important in the distinction of mock- and F. circinatum-inoculated P. sylvestris as well as P. radiata, as emission levels were higher in infected trees. Verbenone is known to be emitted from a variety of plants, and also functions as an insect pheromone with important roles, for example, as a repellent to mountain pine beetles60. A list of the Randomforest-selected VOCs and some of their known functions is found in the supplementary information (Table S1).
Our study showed that VOCs analysis can distinguish F. circinatum-infected P. radiata seedlings before visible symptom development, suggesting the potential to scale up this detection tool for in-field use. In this study, a benchtop GC–MS instrument was used, but other options include the electronic nose (E-nose) and portable GC–MS instruments. In contrast to the E-nose that only detects specifically targeted VOCs classes, GC–MS theoretically can detect all VOCs present in a sample. Additionally, the E-nose detection limits are typically in the μg L−1 range, as compared to pg-ng for FID and MS15, suggesting that the portable GC–MS may be a better option for use in an up-scaled and in-field scenario. When combined with SPME HS sampling, it has potential as an application for high-throughput detection of problems in large plant shipments. Portable GC–MS instruments are commercially available, and for example are currently employed by Homeland Security in the U.S., with similar sensitivity to a basic benchtop instrument61. Portable GC–MS has been used to distinguish between healthy and pest-infested milkweed (Asclepias spp.)62 as well as to readily identify potential fungal biomarkers when coupled with SPME63. This would make an interesting alternative for testing in future work with forest pathogens, especially F. circinatum based on our results, for detecting the pathogen in asymptomatic seedlings in nursery consignments, but also in soil, another known pathway of introduction for this pathogen.
Comparing the VOCs profiles of pine seedlings inoculated with different pine pathogens would be an important next step to this work. Such a comparison could determine whether VOCs profiles of pine seedlings inoculated with different pathogens can be distinguished from one another (as described in horse chestnut trees by Johne et al.29), or whether pine seedlings’ VOCs responses to fungal pathogens are non-specific, yet further investigations are warranted.
Methods
Fusarium spp. cultured on defined media
For examination and comparison of VOCs produced by Fusarium, four Fusarium spp. were grown on defined Elliott’s medium agar (EMA) without sterol64: Fusarium circinatum, the closely related F. bulbicola, the intermediately related F. oxysporum f.sp. pini51 and the more distantly related F. graminearum52. For strain information, see Table S2. EMA was dispensed in slanted 20 mL clear glass vials (Merck KGaA, Darmstadt, Germany), capped with permeable magnetic screw caps with polytetrafluoreten/silicone 1.33 mm septa (Merck KGaA, Darmstadt, Germany). The capped vials were incubated at room temperature under natural light conditions and sampled for 24 h at days 7, 14 and 21 after sub-culture by inserting divinylbenzene/carboxen/polydimethylsiloxane SPME fibers through the septa. The SPME needle size was 24 ga, 2 cm long and coated with 30 μm (CAR/PDMS layer), 50 μm (DVB layer) (Merck KGaA, Darmstadt, Germany).
Fusarium circinatum inoculated Pinus spp
Fusarium circinatum strain FcCa6 (obtained from the laboratory of Prof. Julio Javier Díez) was stem-inoculated on 1 year-old P. sylvestris, P. radiata and P. pinea (for information on sources, see Table S3). The seedlings were obtained from Viveros y Servicios Forestales Caselas, S.L., a nursery in Mondoñedo Lugo, Spain, and transported by express courier in December 2020 to the forest pathology laboratory of the Universidad de Valladolid, Palencia, Spain. The plant material used in this study complies to relevant guidelines and all necessary permissions were in place. Seedlings were transplanted to 0.77 L pots into black peat moss previously autoclaved twice at 121 °C for 20 min. Plants were acclimated for 3 months in a climate chamber at 21.5 °C under a 16/8 h day/night regime and approximately 68% relative humidity. Throughout the acclimation and experimental period, seedlings were watered twice a week.
Stem inoculations were performed, using a method described elsewhere59,65, by cutting a small wound on the stem, approximately 7 cm above the root collar and applying 20 µL of a potato dextrose broth (PDB) (Sharlau Microbiology, Barcelona, Spain) based spore suspension containing 106 F. circinatum spores mL-1, directly to the surface of the wound. Wounds were covered with Parafilm (Bemis Company Inc., Neenah, USA) until the start of the SPME sampling. Mock inoculations were identical but without spores in the PDB. During the experiment, symptom development was observed and documented on the seedlings. To confirm that the mycelial growth seen on stems was F. circinatum, the mycelia were harvested and sub-cultured to EMA64 before examination under the microscope, where coiled sterile hyphae characteristic of F. circinatum were seen. VOCs sampling was performed using SPME fibers for static HS sampling: each seedling, including pot, and SPME fiber was wrapped in 38 L high-density polyethylene bags (Labbox labware, Barcelona, Spain), maintained at room temperature for 24 h and thereafter the SPME samples were immediately analyzed using GC–MS.
GC–MS and data analysis
Immediately after sampling, the SPME fibers were manually injected through an ultra-inert, splitless, straight, 2 mm liner (Agilent, Santa Clara, USA) on a 6890 N GC (Agilent Technologies, Santa Clara, USA) coupled with a 5973 MS (Agilent Technologies, Santa Clara, USA). The column was a HP-5 ms ultra inert 60 m GC column, 0.25 mm, 0.25 µm, 7 inch cage (Agilent, Santa Clara, USA). A C8-C20 hexane mix (Merck KGaA, Darmstadt, Germany) was used as an assurance that there was no shift in retention time over the project time span. GC–MS was performed through MSD ChemStation version E.02.02.1431 (Agilent Technologies, Santa Clara, USA) with an initial oven temperature of 50 °C, followed by an 8 °C/min increase to 100 °C, subsequently increasing by 4 °C/min to 160 °C, a final ramp of 16 °C/min to 280 °C and hold for 2.5 min (Table S4). GC–MS data were transformed to .cdf files and processed (ADAP chromatogram builder, chromatogram deconvolution, multivariate curve resolution) and aligned (ADAP aligner) with MZMine 2 (v 2.53)32. The Randomforest compound selection (see below) for distinguishing between mock- or F. circinatum-inoculated seedlings (in vivo), or Fusarium species (in vitro), were tentatively identified by matching mass spectrometry data and back-calculated retention indices66 with literature values from authentic standards found in Nist20 and Wiley12 MS databases.
Programming, machine learning and statistical tests
Randomforest and VarSelRF67 are two packages in R68 that were used to select a reduced model to a set of VOCs that were predictive of Fusarium spp. in the in vitro and inoculation type in the in vivo experiments69. Randomforest is used to tune and reduce the model error and VarSelRF chooses a model of VOCs with the lowest error rate. VarSelRF uses the confusion matrix testing parameter “out-of-bag” error as a criterion to remove variables (i.e. individual VOCs) in a backward elimination starting with the least important VOCs. The least important variables are those defined by Randomforest from the mean decrease in accuracy70. The model selection stops when current out-of-bag error rate becomes larger than the previous iteration. The selected subsets of Fusarium spp. and Pinus spp. VOCs were then run through Permutational Multivariate Analysis of Variance (PERMANOVA)71, to determine relative differences in VOCs between Fusarium spp., or inoculation type in Pinus spp. Posthoc Holm tests72 were thereafter applied. Data for PERMANOVA were Hellinger transformed. The Stats package, prcomp function was used to generate the PCA analysis and plot, scaling the input data to visually display differences among the compared groups (RStudio, version 1.1.456).
Additional information
All necessary permissions were obtained to complete this study, no ethics considerations are applicable. Supplementary data is publicly available through the Swedish National Data Service (SND), doi: https://doi.org/10.5878/hc9w-7694. The voucher specimens of the three Pinus species included in this study were provided by Viveros y Servicios Forestales Caselas, S.L., a nursery in Mondoñedo Lugo, Spain, but have not been deposited to any publicly available herbarium. The Fusarium circinatum isolate FcCa6 used in this study was identified in previous work by Martínez-Álvarez73, provided and maintained by the laboratory of Prof. Díez, available in lab collections in several countries but yet no public herbarium.
Data availability
The data that support the findings of this study are openly available in doi: https://doi.org/10.5878/hc9w-7694.
References
Seebens, H. et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 8(1), 14435 (2017).
Santini, A. et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 197(1), 238–250 (2013).
Rabitsch, W. Pathways and vectors of alien arthropods in Europe: Chapter 3. BioRisk 4, 1 (2010).
Pysek, P. et al. Scientists’ warning on invasive alien species. Biol. Rev. Camb. Philos. Soc. 1, 1 (2020).
Roques, A. et al. Temporal and interspecific variation in rates of spread for insect species invading Europe during the last 200 years. Biol. Invas. 18(4), 907–920 (2016).
Cleary, M. et al. Cryptic risks to forest biosecurity associated with the global movement of commercial seed. Forests 10(5), 1 (2019).
Avtzis, D. N. & Wegensteiner, R. Forest insects and pathogens in a changing environment—Ecology, monitoring. Genetics. Forests 1, 1 (2019).
Early, R. et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 7(1), 12485 (2016).
Roques, A. Alien forest insects in a warmer world and a globalised economy: Mpacts of changes in trade, tourism and climate on forest biosecurity. NZ J. Forest. Sci. 40, 77–94 (2010).
Zamora-Ballesteros, C. et al. Pine pitch canker (PPC): Pathways of pathogen spread and preventive measures. Forests 10(12), 1 (2019).
Bacon, S. J., Bacher, S. & Aebi, A. Gaps in border controls are related to quarantine alien insect invasions in Europe. PLoS ONE 7(10), e47689 (2012).
Sikes, B. A. et al. Import volumes and biosecurity interventions shape the arrival rate of fungal pathogens. PLoS Biol. 16(5), e2006025 (2018).
Martinelli, F. et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 35(1), 1–25 (2015).
Materić, D. et al. Methods in plant foliar volatile organic compounds research. Appl. Plant Sci. 3(12), 1500044 (2015).
Jansen, R. M. et al. Detection of diseased plants by analysis of volatile organic compound emission. Annu. Rev. Phytopathol. 49, 157–174 (2011).
Tholl, D. et al. Practical approaches to plant volatile analysis. Plant J. 45(4), 540–560 (2006).
Morath, S. U., Hung, R. & Bennett, J. W. Fungal volatile organic compounds: A review with emphasis on their biotechnological potential. Fungal Biol. Rev. 26(2–3), 73–83 (2012).
Blasioli, S. et al. Electronic nose as an innovative tool for the diagnosis of grapevine crown gall. Anal. Chim. Acta 672(1–2), 20–24 (2010).
Laothawornkitkul, J. et al. Volatile organic compounds as a diagnostic marker of late blight infected potato plants: A pilot study. Crop. Prot. 29(8), 872–878 (2010).
Rutolo, M. F., Clarkson, J. P. & Covington, J. A. The use of an electronic nose to detect early signs of soft-rot infection in potatoes. Biosyst. Eng. 167, 137–143 (2018).
Cui, S. et al. Development of fast E-nose system for early-stage diagnosis of aphid-stressed tomato plants. Sensors 19(16), 3480 (2019).
De Lacy Costello, B. P. J. et al. Gas chromatography–mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol. 50(4), 489–496 (2001).
Ponzio, C. et al. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct. Ecol. 27(3), 587–598 (2013).
Zainol Hilmi, N. H., Idris, A. S. & Mohd Azmil, M. N. Headspace solid-phase microextraction gas chromatography–mass spectrometry for the detection of volatile organic compounds released from Ganoderma boninense and oil palm wood. For. Pathol. 49(4), 12531 (2019).
Aksenov, A. A. et al. Detection of huanglongbing disease using differential mobility spectrometry. Anal. Chem. 86(5), 2481–2488 (2014).
Graham, J. H., Gottwald, T. R. & M. SÈtamou,. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 45, 265–278 (2020).
Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus [Asia; South Africa; Brazil; Florida]. 2006.
Vuorinen, T. et al. Epirrita autumnata induced VOC emission of silver birch differ from emission induced by leaf fungal pathogen. Arthropod-Plant Interact. 1(3), 159–165 (2007).
Johne, A. B., Weissbecker, B. & Schütz, S. Approaching risk assessment of complex disease development in horse chestnut trees: a chemical ecologist’s perspective. J. Appl. Entomol. 132(5), 349–359 (2008).
Vezzola, L. C. et al. Tree-ring volatile terpenes show potential to indicate fungal infection in asymptomatic mature Norway spruce trees in the Alps. For. Int. J. For. Res. 92(2), 149–156 (2018).
Brilli, F., et al. Volatile Organic Compounds (VOC) as Biomarkers for Detection of Ceratocystis Platani (2020)
Pluskal, T. et al. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinf. 11, 395 (2010).
Geiser, D. M. et al. One fungus, one name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 103(5), 400–408 (2013).
Summerell, B. A. Resolving Fusarium: Current Status of the Genus. Annu. Rev. Phytopathol. 57(1), 323–339 (2019).
Hepting, G. H. & Roth, E. R. Pitch canker, a new disease of southern pines. J. For. 44, 742–744 (1946).
EPPO. EPPO A2 List of Pests Recommended for Regulation as Quarantine Pests (2021)
Niehaus, E.-M. et al. Comparative “Omics” of the Fusarium fujikuroi species complex highlights differences in genetic potential and metabolite synthesis. Genome Biol. Evol. 8(11), 3574–3599 (2016).
Drenkhan, R. et al. Global geographic distribution and host range of fusarium circinatum, the causal agent of pine pitch canker. Forests 11(7), 724 (2020).
Martín-Rodrigues, N. et al. New insights into radiata pine seedling root infection by Fusarium circinatum. Plant. Pathol. 64(6), 1336–1348 (2015).
Watt, M. S. et al. Dothistroma needle blight and pitch canker: the current and future potential distribution of two important diseases of Pinus species. Can. J. For. Res. 41(2), 412–424 (2011).
Fernández, F. et al. Pine pitch canker and insects: Relationships and implications for disease spread in Europe. Forests 10(8), 1 (2019).
European and Mediterranean Plant Protection Organization. EPPO reporting service. 2021(No. 8; 169)
Hernandez-Escribano, L. et al. Root infection of canker pathogens, Fusarium circinatum and Diplodia sapinea, in asymptomatic trees in Pinus radiata and Pinus pinaster plantations. Forests 9(3), 1 (2018).
Martínez-Álvarez, P., Pando, V. & Diez, J. J. Alternative species to replace Monterey pine plantations affected by pitch canker caused by Fusarium circinatum in northern Spain. Plant. Pathol. 63(5), 1086–1094 (2014).
Swett, C. L. & Gordon, T. R. First Report of Grass Species (Poaceae) as Naturally Occurring Hosts of the Pine Pathogen Gibberella circinata. Plant Dis. 96(6), 908–908 (2012).
CABI. Gibberella circinata (Pitch Canker). 2022. Accessed 20 October 2022]. https://www.cabi.org/isc/datasheet/25153.
Farjon, A. Pinus radiata, from the website: ‘Threatened Conifers of The World’. 2019 [cited 2022 17 Januray]. https://threatenedconifers.rbge.org.uk/conifers/pinus-radiata.
Durrant, T., de Rigo, D., & Caudullo, G. Pinus sylvestris in Europe: Distribution, habitat, usage and threats (2016)
Martín-García, J. et al. Evaluation of the susceptibility of several Czech Conifer Provenances to Fusarium circinatum. Forests 9(2), 1 (2018).
Zamora-Ballesteros, C. et al. Dual RNA-Sequencing Analysis of Resistant (Pinus pinea) and Susceptible (Pinus radiata) Hosts during Fusarium circinatum Challenge. Int. J. Mol. Sci. 22(10), 5231 (2021).
O’Donnell, K., Cigelnik, E. & Nirenberg, H. I. Molecular Systematics and Phylogeography of the Gibberella fujikuroi Species Complex. Mycologia 90(3), 465–493 (1998).
Watanabe, M. et al. Molecular phylogeny of the higher and lower taxonomy of the Fusarium genus and differences in the evolutionary histories of multiple genes. BMC Evol. Biol. 11, 322–322 (2011).
Dickschat, J. S. Fungal volatiles—A survey from edible mushrooms to moulds. Nat. Prod. Rep. 34(3), 310–328 (2017).
Quintana-Rodriguez, E. et al. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J. Ecol. 103(1), 250–260 (2015).
Slinski, S. L., Zakharov, F. & Gordon, T. R. The effect of resin and monoterpenes on spore germination and growth in Fusarium circinatum. Phytopathology 105(1), 119–125 (2015).
Angioni, A. et al. chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food Chem. 52(11), 3530–3535 (2004).
Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4 ed. (Allured publishing corp., Carol Stream, IL, 2007)
Saroglou, V. et al. Analysis of the essential oil composition of eight Anthemis species from Greece. J. Chromatogr. A 1104(1), 313–322 (2006).
Martín-Rodrigues, N. et al. Spatial and temporal dynamics of the colonization of Pinus radiata by Fusarium circinatum, of conidiophora development in the pith and of traumatic resin duct formation. New Phytol. 198(4), 1215–1227 (2013).
Fettig, C. J. & Munson, A. S. Efficacy of verbenone and a blend of verbenone and nonhost volatiles for protecting lodgepole pine from mountain pine beetle (Coleoptera: Curculionidae). Agric. For. Entomol. 22(4), 373–378 (2020).
National Urban Security Technology Laboratory, Field Portable Gas Chromatograph Mass Spectrometer (GC/MS) Assessment Report. 2020, U.S. Department of Homeland Security, Science and Technology Directorate.
Sharma, R. et al. Rapid in situ analysis of plant emission for disease diagnosis using a portable gas chromatography device. J. Agric. Food Chem. 67(26), 7530–7537 (2019).
Beck, J. J. et al. Differentiation of volatile profiles from stockpiled almonds at varying relative humidity levels using benchtop and portable GC-MS. J. Agric. Food Chem. 64(49), 9286–9292 (2016).
Elliott, C. G., Hendrie, M. R. & Knights, B. A. The sterol requirement of Phytophthora cactorum. J Gen Microbiol 42(3), 425–435 (1966).
Amaral, J. et al. Pinus susceptibility to pitch canker triggers specific physiological responses in symptomatic plants: An integrated approach. Front. Plant Sci. 10(509), 1 (2019).
Boswell, P. G. et al. Easy and accurate calculation of programmed temperature gas chromatographic retention times by back-calculation of temperature and hold-up time profiles. J. Chromatogr. A 1263, 179–188 (2012).
Liaw, A.W. M. Classification and regression by Randomforest. R News. p. 18–22 (2002).
RStudio Team. RStudio: Integrated Development for R. In RStudio, PBC, Boston MA. (2022).
Jaeger, D. M., Runyon, J. B. & Richardson, B. A. Signals of speciation: volatile organic compounds resolve closely related sagebrush taxa, suggesting their importance in evolution. New Phytol. 211(4), 1393–1401 (2016).
Diaz-Uriarte, R. Package ‘varSelRF’. (2017).
Anderson, M. J. & Walsh, D. C. I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing?. Ecol. Monogr. 83(4), 557–574 (2013).
Martinez Arbizu, P. pairwiseAdonis: Pairwise multilevel comparison using adonis. In R package version 0.4. (2017).
Martínez-Álvarez, P., Alves-Santos, F. M. & Diez, J. J. In vitro and in vivo interactions between Trichoderma viride and Fusarium circinatum. Silva Fennica 46(3), 1 (2012).
Acknowledgements
This study was financially supported by The Swedish Research Council Formas, Grant #2018-00966, Crafoordska stiftelsen Grant #20200631, Carl Tryggers Stiftelse för Vetenskaplig Forskning Grant 18:67, The Royal Swedish Academy of Agriculture and Forestry, Stiftelsen fonden för skogsvetenskaplig forskning, Erasmus+ Staff mobility grant, Anna-Britta & Vadim Söderströms resestipendium and NordGen Forest SNS scholarships. J.N.S. was supported by The European Union’s Horizon Europe research and innovation programme under the MSCA agreement No 101068728. Thanks to dr. R.R. Vetukuri for providing F. graminearum, to the staff of Laboratorio de Técnicas Instrumentales, Universidad de Valladolid, for providing access to lab facilities and to J-E. Englund for assistance in making the experimental design.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
I.N., P.S., M.C., J.J.D., B.B. and S.W. conceived, designed and supervised the project. I.N. conducted experiments and wrote the manuscript. B.B. analyzed the GC–MS data. D.L.P. performed statistical analyses. J.N.S. and T.S.G. performed the post-hoc PCR and culturomic assays. All authors commented on the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nordström, I., Sherwood, P., Bohman, B. et al. Utilizing volatile organic compounds for early detection of Fusarium circinatum. Sci Rep 12, 21661 (2022). https://doi.org/10.1038/s41598-022-26078-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26078-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.