Abstract
The cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), an important pest of cotton, is detrimental to cotton production. Light from UV-A ultraviolet lamps is regarded as a form of environmental stress for insects. In order to investigate the response of H. armigera exposed to UV-A, we explored Hap38 MAPK expression and functions. We hope that the findings of this study will lay the foundation for future investigations into the insect’s phototaxis mechanism. A p38 MAPK was cloned and named Hap38 MAPK. A phylogenetic tree showed that Hap38 MAPK was highly conserved. The gene was highly expressed in the thorax and females. Under UV-A stress, the expression of the gene decreased significantly. After silencing Hap38 MAPK, the activity of the antioxidant enzymes SOD, POD, CAT, and GR decreased. This study suggested that Hap38 MAPK responds to UV-A irradiation and plays critical roles in the defense response to environmental stresses.
Similar content being viewed by others
Introduction
The cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), is an omnivorous pest of food crops distributed worldwide, which causes enormous yield losses annually1. It has a wide host range and exhibits rapid reproduction, high adaptability, and high pesticide resistance2. Because H. armigera exhibits typical phototaxis3, research into this pest has been focused upon the photoreactive and phototactic characteristics and microscopic structure of its compound eyes, as well as the effects of the compound eye state on phototactic behaviors4,5,6. Previous studies have demonstrated that the visual physiology and behavioral characteristics of H. armigera are highly sensitive to ultraviolet (UV) light. In recent years, UV-A irradiation has received increasing attention as an important stress factor, causing a series of direct or indirect adverse effects, such as oxidative stress, photoreceptor damage, and apoptosis7, in almost all organisms, including insects. After UV-A irradiation, the metabolism of juvenile hormones of H. armigera was affected, oviposition was increased, and the life span was shortened8,9,10.
As a phenomenon, phototaxis of insects that make UV-A ultraviolet insect trap lamp are widely used. As an environmental stress factor, UV-A causes excessive reactive oxygen species (ROS) in insects, resulting in varying degrees of oxidative damage to some biological macromolecules11,12. These damages will cause a series of biological effects on insects, such as toxin production, mutations, and changes in signal transduction pathways13. MAPK was upregulated following UV light irradiation in insects. Insects respond to UV stress by changing enzyme system activity, juvenile hormone metabolism, and protein expression9,14. Insects can apparently perceive signals using sensors and transmit them to the cellular machinery via signal transduction to regulate gene expression. This study mainly elucidates the function of Hap38 MAPK in response to UVA stress. Mitogen-activated protein kinases (MAPKs) are a large group of protein kinases that perform a wide variety of roles in cellular signal transduction pathways15. There are at least four distinct MAPK signaling modules, which mediate extracellular signals into the nucleus, to activate responsive genes. These include p38 mitogen-activated protein kinase (p38 MAPK), c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK)16,17. Of the above three proteins, p38 MAPK is easily induced by a variety of environmental stresses, such as temperature change, UV radiation, and other external stimuli18. An important member of this family, p38 MAPK, plays a key role in inflammation and stress response, and participates in the regulation of cell survival, differentiation, and apoptosis. The activation of p38 MAPK protein is stimulated by various stresses, such as those induced by extracellular inflammatory factors, UV radiation, and cytotoxic substances19,20. Activated p38 MAPK regulates cell function by regulating the expression of downstream genes encoding various enzymes and transcription factors21. There have been many studies into the stress-induced apoptosis of immune cells, depending on p38 MAPK signaling pathways. For instance, the p38 MAPK signaling pathway is involved in the regulation of heat shock protein 70 (Hsp70), and protects the red blood cells of Sparus aurata exposed to heat stress22. UV-A can selectively activate p38 MAPK in C6 glioma cells of the rat, which in turn enables p38 MAPK to protect the cells from radiation23. In insects, a novel peptide from Vespa ducalis induces apoptosis in osteosarcoma cells by activating p38 MAPK24. This paralytic peptide has been shown to enhance p38 MAPK phosphorylation following induction, initiating immune responses in embryonic cells in Bombyx mori25. In particular, it was shown that Drosophila mutants lacking D-MEKK1 were hypersensitive to environmental stresses, including elevated temperature and increased osmolarity26. Therefore, the p38 MAPK pathway is critical for the response to environmental stresses in insects.
ROS normally exist in cells in a state of equilibrium between production and elimination. Environment stress causes this balance to be disturbed27. To scavenge excess ROS and reduce its toxic effects, insects have evolved an antioxidant system whose activity is upregulated under stressful conditions, and is correlated well with enhanced tolerance. The system involves superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and glutathione reductase (GR)28. The first three enzymes act in coordination to maintain the normal physiological activities of insects. The activity levels of SOD, POD, and CAT in Pieris rapae treated with deltamethrin were higher than those of normal insects, in order to adapt to external toxicity29. GR is the key enzyme of the ascorbic acid glutathione (AsA-GSH) cycle, and can play an indirect antioxidant role30. Injection of Escherichia coli into Ostrinia furnacalis larvae activates GR, leading to the scavenging of excessive ROS, to prevent against their toxic effects31. Under drought stress, salt stress, and cold stress, the activities of SOD, POD, and CAT in maize decrease to varying degrees after the activity of MAPK cascade pathway is inhibited, and the response to stress signals becomes slow. The MAPK pathway participates in the response of maize seedlings to low temperature stress32. The MAPK pathway and enzymes to remove ROS, therefore appear to be involved in regulating the antioxidant enzyme defense response of organisms to stress.
In this study, a gene encoding p38 MAPK in H. armigera was sequenced, characterized, and compared to homologous proteins from other insect species. We also produced tissue- and stage-specific developmental profiles, and investigated the expression levels of p38 MAPK under UV-A irradiation. To explore the role of p38 MAPK in the antioxidant system of H. armigera under UV-A stress, the activities of SOD, POD, CAT, and GR were investigated in H. armigera with silenced Hap38 MAPK. The aims of this work were to reveal the role of the p38 MAPK gene in the response to environmental stress and the regulation of abiotic stress in antioxidant defenses, to explore the molecular mechanisms of the response of H. armigera to environmental stress, and to provide a theoretical framework for formulating scientific control strategies.
Materials and methods
Insect rearing and tissue sampling
Larvae of H. armigera were collected from cotton plants growing in a suburb of Huaxi, Guiyang, Guizhou, China, and subsequently reared in an insectary for several generations at 27 ± 1 °C, 75 ± 5% relative humidity, and a 14 h light/10 h dark photoperiod (light, 06:00–20:00; dark, 20:00–06:00). The larvae were fed with an artificial diet, as described previously33. Within 3 days of the pupae emerging as adults, one male and one female adult were selected from the cages (20 cm × 20 cm × 30 cm), and paired in a plastic container (15 cm height and 9 cm diameter) with 10% honey solution feeding. H. armigera samples were collected at nine developmental stages: egg, 1st–6th instar larvae, 1-, 3-, 5-, and 7-day-old pupae, and 3-day-old adults (male and female). A total of 50 eggs and 8–15 individuals at other developmental stages were collected per biological replicate. Nine different tissues were collected from H. armigera adults, including the head (without antennae or compound eyes), chest, abdomen, antennae, compound eyes, feet, wings, midgut, and ovary (in females). Experiments were performed in triplicate. All samples were immediately frozen in liquid nitrogen and stored at − 80 °C until needed for RNA extraction. Three biological replicates were prepared for each treatment three times for each process.
UV-A irradiation
UV-A light (315–400 nm; Nanjing Huaqiang Electronics Co., Ltd., Nanjing, China) was used to irradiate the adults of H. armigera at an irradiance of 300 mW/cm2. 150 female and 150 male adults were exposed individually to UV-A light 2 h after the start of scotophase, at a temperature of 27 ± 1 °C. Eight adults per treatment were randomly selected at the start of the experiment, at 0 min (control) and subsequently at 30, 60, 90, 120, and 150 min. There were three technical replicates; 144 samples were collected. Samples were immediately frozen in liquid nitrogen and stored at − 80 °C for subsequent RNA extraction.
RNA extraction and cDNA synthesis
Total RNA was extracted from 3-day-old H. armigera adults using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. To remove traces of contaminating genomic DNA, total RNA was treated with DNase I (Takara Bio Inc., Shiga, Japan). To evaluate the purity of the total RNA, the 260/280 ratio was measured using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The integrity of the total RNA was examined using gel electrophoresis with a 1% agarose gel. The first-strand cDNA was synthesized immediately from 1 μg of total RNA using RevertAid First-Strand cDNA Synthesis Kit (Thermo Fisher), according to the manufacturer’s instructions, and stored at − 20 °C until needed for further analysis.
Cloning and sequence analysis of the Hap38 MAPK gene
The Hap38 MAPK gene was amplified from the cDNA template by reverse transcription PCR (RT-PCR). The primers used for the RT-PCR (Table S1) were designed using Primer Premier 6.0 (Premier Biosoft International, Palo Alto, CA, USA) to amplify the conserved regions of the p38 MAPK genes in H. armigera. RT-PCR was carried out using Taq polymerase (Sangon Biotech, Shanghai, China) under the following conditions: initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 54 °C for 30 s, elongation at 72 °C for 1 min, and a final elongation at 72 °C for 10 min. To amplify the 5′ and 3′ ends of the Hap38 MAPK gene, 5′ rapid amplification of cDNA ends (5′ RACE) and 3′ RACE PCRs were carried out, respectively, using SMARTer® RACE 5′/3′ Kits (Clontech, Mountain View, CA, USA), and sequence-specific primers designed using Primer Premier 6.0. The RACE PCRs were performed under the following conditions: 25 cycles of denaturation at 94 °C for 30 s, annealing at 55–65 °C (depending on the primer pair) for 30 s, and extension at 72 °C for 3 min. The PCR products were separated on 1% agarose gel, and the expected bands were gel-purified and cloned. The cloned fragments were then sent to Sangon Biotech for sequencing.
The open reading frame of the gene was identified using ORF finder (http://www.ncbi.nlm.nih.gov/orffinder/), and the amino acid sequence of the encoded protein was determined. The NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi34) database was used to analyze the homology of the Hap38 MAPK gene with its orthologs in other insects. The molecular weight and isoelectric point (pI) of the p38 MAPK proteins were predicted using the ExPaSy proteomics server (http://www.expasy.org). The protein domains of p38 MAPK were predicted using ExPaSy-PROSITE (http://prosite.expasy.org/). O-glycosylation sites were analyzed using NetOGlyc 4.0 Server (http://www.cbs.dtu.dk/services/NetOGlyc/). The N-terminal signal peptide positions were determined using SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/). The family signature sequence of Hap38 MAPK was analyzed using ExPASy ScanProsite (http://prosite.expasy.org/scanprosite/). Phylogenetic analysis of p38 MAPK amino acid sequences was performed using the neighbor-joining method and a bootstrap test with 1000 replicates in MEGA6.
Effect of dsRNA treatment on Hap38 MAPK
dsRNA synthesis primers were designed based in the cDNA sequence of Hap38 MAPK obtained by cloning, and T7 transcription promoters were added to both ends of the target area. Using the recovered high concentration cDNA as a template, dsRNA was prepared according to the instructions from the MEGAscript® kit (Thermo Fisher Scientific). The dsRNA was purified and tested, and stored at − 80 °C.
Six-day pupae were injected with 6 μL PBS solution (control groups) and 1 μg Hap38 MAPK-dsRNA (experimental groups); overall, 60 pupae were tested. After they grew to 3-day-old adults, the tissue samples were collected and the expression levels of Hap38 MAPK were measured. The adults were irradiated with a UV-A lamp, at an intensity of 300 μW/cm2 for 60 min, under temperature and humidity consistent with the normal feeding conditions. After treatment, the samples were frozen in liquid nitrogen. Each treatment had three biological repeats, and each biological repeat contained eight adults.
Antioxidase activity was determined using the SOD, POD, CAT, and GR kits produced by Suzhou Keming Biotechnology Co., Ltd. (Suzhou, China). The H. armigera adults from the different treatment groups were mixed with 150 µL of lysate per 3 mg adult, crushed in a high-speed ball mill for 2 min, centrifuged at 4 °C and centrifuged at 1500 r/min for 10 min. The supernatant was removed, and the protein concentration was measured using the aforementioned quantitative detection kits. The concentration of extracted protein in each group was balanced according to the instruction manual for the SOD, POD, CAT, and GR enzyme activity test kits. The reaction system was added to a 96-well enzyme plate, incubated at 37 °C for color development, the A470 value detected using an enzyme marker, and the protein content calculated.
Quantitative real-time PCR (qRT-PCR) analysis
The extraction of total RNA and synthesis of cDNA from the different tissue types and developmental stages was performed as described above. The relative expression levels of the Hap38 MAPK gene were measured using qRT-PCR with FastStart Essential DNA Green Master Mix (Roche, Indianapolis, IN, USA) on a CFX96™ Real-Time Quantitative PCR system (Bio-Rad, Hercules, CA, USA). The primers used for qRT-PCR are listed in Table 1. qRT-PCR was conducted in a 20 μL reaction volume containing 1 μL of cDNA template, 1 μL of each forward and reverse primer (10 μM), 7 μL of diethyl pyrocarbonate-treated H2O, and 10 μL of FastStart Essential DNA Green Master Mix. The cycling parameters used for qRT-PCR were as follows: 95 °C for 10 min, followed by 40 cycles at 95 °C for 30 s and 60 °C for 30 s. Melting curves were generated at 65–95 °C. β-Actin (GenBank accession no.: HM629441.1) was used as an internal control. To verify reproducibility, each qRT-PCR was performed using three technical replicates and three biological replicates. Relative gene expression was calculated using the 2−ΔΔCT method35. The expression levels of the genes in the adult males, head (antennae and compound eyes were removed), and at 0 min, were normalized.
Statistical analysis
Differences between treatments were analyzed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple comparison tests. All analyses were performed using SPSS version 21.0 (SPSS, Chicago, IL, USA). Data are presented as mean ± standard error (SE) of three biological replicates. P < 0.05 was considered a statistically significant difference, which is clearly illustrated using different letters or * in figure note.
Results
Cloning and sequencing of Hap38 MAPK
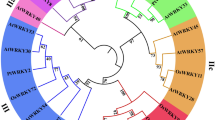
A p38 MAPK gene was cloned from H. armigera and named Hap38 MAPK (GenBank accession no.: MK185422). This gene contains an open reading frame of 1080 bp, encoding 360 amino acids. The putative protein is 41.51 kDa and has a theoretical pH(I) of 5.93. Amino acid sequence analysis using SignalP revealed that the Hap38 MAPK protein contained no signal peptide or transmembrane region, but NetOGlyc identified three O-glycosylation sites and ExPASy ScanProsite identified two domains: a substrate-binding site at positions 25–50, and a threonine-proline-tyrosine motif at 55–158 (Fig. 1A). The sequences of 22 p38 MAPK sequences, including Hap38 MAPK and 21 other p38 MAPK protein sequences from GenBank (Table 1), were aligned and used for homology comparison and the construction of a phylogenetic tree (Fig. 1B). The phylogenetic tree and homology comparison showed that the p38 MAPK proteins were closely related in different Lepidopteran insects. The Hap38 MAPK protein was most closely related to Slitp38 MAPK (99.44%), Tnip38 MAPK (98.33%), Ppolp38 MAPK (95.83%), and Pxutp38 MAPK (95.56%).
Sequence characterization of p38 MAPK from various species. (A) Multiple alignments of the amino acid sequences of Hap38 MAPK with homologs from other insect species. Black represents 100% identity, red represents ≥ 75% identity, green represents ≥ 50% identity, and white represents < 50% identity. Hap38 MAPK (Helicoverpa armigera, MK185422), Slp38 MAPK (Spodoptera litura, XP_022822243.1), Tnp38 MAPK (Trichoplusia ni, XP_026739071.1), Ppp38 MAPK (Papilio polytes, XP_013133297.1), Pxp38 MAPK (Papilio xuthus, XP_013177389.1), and Bap38 MAPK (Bicyclus anynana, XP_023935784.1). (B) Phylogenetic tree of p38 MAPKs from H. armigera (Ha) and other species. The tree was constructed from a multiple alignment using the MEGA6.0 software, and generated with 1000 bootstrap trials using the neighbor-joining method. The numbers show the bootstrap confidence values obtained for each node after 1000 repetitions.
Developmental expression profiles of tissue-specific Hap38 MAPK
The expression profiles of Hap38 MAPK at different developmental stages were quantified. The expression of Hap38 MAPK was significantly higher in the egg and 3-day-old adult females than in other stages (p < 0.05) (Fig. 2). There were significant differences in the expression of Hap38 MAPK in different tissues; specifically, that in the thorax was the highest, followed by those in the abdomen and compound eye (Fig. 3).
Relative expression levels of Hap38 MAPK in different developmental stages of H. armigera. E (Egg), L1–L6 (1st–6th instar larva), P1, P3, P5, P7 (1-, 3-, 5-, and 7-day-old pupa), F (3-day-old adult females) and M (3-day-old adult males). The expression levels of the genes in the adult males are normalized. Data in the figure are expressed as mean ± SE. Different letters above bars indicates significant difference. The values with same superscript letters in the same line are of no significant difference (P > 0.05), those with different letters are of significant or extreme difference (P < 0.05, ANOVA).
Expression of Hap38 MAPK after exposure to UV-A
UV-A treatment induced the expression of Hap38 MAPK in adults; however, significant differences were detected in the expression of Hap38 MAPK between samples exposed to UV-A for different time periods (p < 0.05) (Fig. 4). The expression of Hap38 MAPK first increased in response to UV-A, reaching a peak at 30 min, and then decreased with the increase in treatment time. The expression of Hap38 MAPK was significantly higher at 30, 60, and 90 min of UV-A treatment than in the control. However, at 120 min, the expression level of Hap38 MAPK returned to the level of the control.
dsHap38 MAPK-RNA inhibited the expression of the Hap38 MAPK gene
To further explore the role of Hap38 MAPK in the regulation of H. armigera, the effect of RNAi-based silencing on Hap38 MAPK gene expression was observed by injecting Hap38 MAPK-dsRNA. Compared with the control group, the expression of the Hap38 MAPK gene was significantly decreased in the gene silenced groups (Fig. 5, F = 0.006; df = 4; P = 0.016).
Effect of Hap38 MAPK-dsRNA on the activity of antioxidant enzymes under UV-A
Under UV-A stress, a series of antioxidant enzymes are secreted to facilitate adaptation to the environment. Our previous study confirmed the hypothesis that UV light irradiation increases the level of oxidative stress in H. armigera adults11. As a class of antioxidant enzymes, SOD, POD, CAT, and GR are likely to be transported to their destination via processing involving Hap38 MAPK. Under UV-A stress for 60 min, the activities of SOD, POD, CAT, and GR were significantly lower than those of control after the silencing of Hap38 MAPK. This observation indicated that p38 MAPK signal transduction was blocked in H. armigera injected with Hap38 MAPK-dsRNA. ROS increased in the body, and the peroxidation damage to the plasma membrane and membrane lipids by free radicals increased (Fig. 6).
Discussion
Previous studies on insects have shown that p38 MAPK plays a pivotal role in signal transduction pathways, and is activated by UV light. To explore the biological function, a p38 MAPK gene was cloned and named Hap38 MAPK, which contains an open reading frame of 1080 bp, encoding 360 amino acids, no signal peptide or transmembrane regions. These findings are consistent with the characteristics of p38 MAPK known from other insect species36,37. The p38 MAPK gene of H. armigera had a relatively conservative evolutionary relationship in Lepidoptera and other insects.
To study the expression and physiological functions of H. armigera, the expression level of Hap38 MAPK was detected. The findings were consistent with the mammalian38. The expression level of Hap38 MAPK was the highest in the thorax of adults. The activation of the p38 MAPK signal pathway can regulate skeleton39. Therefore, the high expression level in the chest may be related to regulating muscle differentiation of H. armigera40,41. The expression level of Hap38 MAPK in females were significantly higher, this difference might be determined by the phosphorylation42. It can be inferred that the p38 MAPK signaling pathway plays a critical role in the follicular development of H. armigera43. Hap38 MAPK plays an important regulating role about information transmission and death44.
Insects have strong phototaxis, Entomologists have carried out a considerable amount of research into the effects of UV-A on insects. In this study, the expression level of Hap38 MAPK was significantly increased when UV-A 30 min. Short-term UV-A irradiation induces stress in H. armigera adults, leading to the phosphorylation of Hap38 MAPK and activation of the p38 MAPK pathway, which further leads to the insects to resist UV stress and improve adaptability45,46. Apoptosis in Bombyx mori cells is regulated by p38 MAPK47. The extent of the stress response of H. armigera increased with an increase in the duration of UV-A irradiation. There is an apparent balance between p38 MAPK and JNK signaling in response to the stress. The sustained activation of p38 MAPK resulted in the activation of c-JNK and extracellular signal-regulated protein kinase, which in turn inhibited p38 MAPK expression48,49. The interaction of pathways resulted in a decrease in expression levels below the control level at UV-A 120 min. Our results confirm the hypothesis that UV light irradiation increases the expression level of Hap38 MAPK in H. armigera adults.
ROS is normally in a state of dynamic equilibrium in organisms, this balance is disrupted by UV-A50. As a part of the defense system, SOD, POD, CAT, and GR can maintain the balance of ROS51,52. As an exogenous factor, Meng et al., studied the antioxidant response of H. armigera to UV-A stress, UV light irradiation activated MAPK signal transduction and increased the level of oxidative stress in H. armigera adults11. Meng et al., studied found that exposure to UV light for 30 min resulted in increased total antioxidant capacity, protein carbonyl content and activities of SOD, CAT, POX and GST, and the result confirmed that UV light irradiation increases the level of oxidative stress in H. armigera adults. Now we investigated the expression of the Hap38 MAPK gene in dsRNA-injected group compared with the control under UV-A. After silencing the gene, p38MAPK activity was significantly inhibited, possibly affecting the scavenging of ROS and the improvement of antioxidant capacity. When dsRNA-injected pupae were exposed to UV-A, the activity of SOD, POD, CAT, and GR increased in a short time, indicating that the activity of enzymes was correlated with tolerance to a negative environment11,53. We inferred the different antioxidant enzymes of H. armigera act in a coordinated manner against the stress. Similar effects have been observed in Myzus persicae54. The p38 MAPK signaling pathway was inhibited, it will affect other pathways55. Some drugs have been found to inhibit and improve the level of JNK under stress, as reflected by the levels of SOD and CAT. This process is consistent with our study, which may be related to the enzymes’ regulatory effect on the expression of p38 MAPK. The effects of UV exposures on adult longevity and reproduction in H. armigera were investigated in our previous research8. Exposure to UV-A for longer periods caused a decline in cumulative survival of F1 immature stages. These results suggested that the response mechanisms of insect to UV light irradiation stress is complicate.
Conclusions
In the present study, we investigated the expression patterns of the Hap38 MAPK gene in different tissues, at different stages of development, and under UV-A stress. We characterized the function of Hap38 MAPK, and found that the injection of dsRNA could significantly reduce the expression of Hap38 MAPK and change the activity of antioxidant enzymes. These findings indicate that Hap38 MAPK responds to UV-A stress and has a close relationship with biological antioxidant functions.
Code availability
The datasets generated and/or analysed during the current study are available in the [Genbank] repository, accession number to datasets [https://www.ncbi.nlm.nih.gov/, MK185422, XM_022966475.1, XM_026883270.1, XM_026902837.1, XM_013341701.1, XM_012407911.2, XM_022086633.1, XM_013277843.1, XM_013321935.1, XM_024080016.1, XM_011563257.1, XM_015653904.1, XM_011139608.3, XM_014632703.1, XM_012005168.1, XM_026417006.1, XM_012677991.1, XM_018544516.1, XM_026134947.1, XM_022338737.1, XM_011218520.1].
References
Mapuranga, R., Chapepa, B. & Mudada, N. Strategies for integrated management of cotton bollworm complex in Zimbabwe: A review. Int. J. Agron. Agric. Res. (IJAAR) 7, 23–35 (2015).
Downes, S. et al. A perspective on management of Helicoverpa armigera: Transgenic Bt cotton, IPM, and landscapes. Pest Manag. Sci. 73, 485–492 (2017).
Ding, Y. Q., Gao, W. Z. & Li, D. M. Study on the phototactic behaviour of nocturnal moths the responses of Helicoverpa armigera (Hübner) and Helicoverpa assulta Guenée to different monochromatic light. Acta Entomol. Sin. 17, 307–317 (1974).
Hou, W. W. & He, X. W. Studies on phototactic of nocturnal moths: Change in behavior during the transformation of compound eye. Acta Entomol. Sin. 22, 34–40 (1979).
Gao, W. Z. & Guo, B. Q. The External morphology and fine structure of the compound eye of cotton bollworm moth, Helicoverpa armigera (Hübner). Acta Entomol. Sin. 26, 375–377 (1983).
Wei, G. S., Zhang, Q. W., Zhou, M. Z. & Wu, W. G. Characteristic response of the compound eyes of Helicoverpa armigera to light. Acta Entomol. Sin. 45, 323–328 (2002).
Meyer-Rochow, V. B., Kashiwagi, T. & Eguchi, E. Selective photoreceptor damage in four species of insects induced by experimental exposures to UV-irradiation. Micron 33, 23–31 (2002).
Zhang, C. Y., Meng, J. Y., Wang, X. P., Zhu, F. & Lei, C. L. Effects of UV-A exposures on longevity and reproduction in Helicoverpa armigera, and on the development of its F1 generation. Insect Sci. 18, 697–702 (2011).
Zhang, C. Y., Meng, J. Y., Zhou, L. J., Sang, W. & Lei, C. L. Effects of ultraviolet light stress on juvenile hormone in Helicoverpa armigera adults. Plant Prot. 38, 72–76 (2012).
Wang, Z. J. & Niu, C. Y. Influence of ultraviolet light irradiation on HMG-CoA reductase, vitellogenin mRNA expression and oviposition of Helicoverpa armigera. J. Huazhong Agric. Univ. 33, 46–50 (2014).
Meng, J. Y., Zhang, C. Y., Zhu, F., Wang, X. P. & Lei, C. L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 55, 588–592 (2009).
Sang, W., Ma, W. H., Lin, Q., Zhu, Z. H. & Lei, C. L. The involvement of heat shock protein and cytochrome P450 genes in response to UV-A exposure in the beetle Tribolium castaneum. J. Insect Physiol. 58, 830–836 (2012).
McMillan, T. J. et al. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J. Pharm. Pharmacol. 60, 969–976 (2008).
Meng, J. Y., Zhang, C. Y. & Lei, C. L. A proteomic analysis of Helicoverpa armigera adults after exposure to UV light irradiation. J. Insect Physiol. 56, 405–411 (2010).
Ruiz-Roldán, M. C., Maier, F. J. & Schäfer, W. PTK1, a mitogen-activated-protein kinase gene, is required for conidiation, appressorium formation, and pathogenicity of Pyrenophora teres on barley. Mol. Plant Microbe Interact. 14, 116–125 (2001).
Garrington, T. P. & Johnson, G. L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11, 211–218 (1999).
Morrison, D. K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 4, 843–853 (2012).
Craig, C. R., Fink, J. L., Yagi, Y., Ip, Y. T. & Cagan, R. L. A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 5, 1058–1063 (2004).
Kyriakis, J. M. & Avruch, J. Mammalian mitogen-activated protein kinase signal, transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 (2001).
Sturgill, T. W. MAP kinase: It’s been longer than fifteen minutes. Biochem. Biophys. Res. Commun. 371, 1–4 (2008).
Stoneley, M. et al. C-myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Mol. Cell. Biol. 20, 1162–1169 (2000).
Feidantsis, K., Pörtner, H. O., Markou, T., Lazou, A. & Michaelidis, B. Involvement of p38 MAPK in the induction of Hsp70 during acute thermal stress in red blood cells of the gilthead sea bream, Sparus aurata. J. Exp. Zool. A Ecol. Genet. Physiol. 317, 303–310 (2012).
Zhang, F., Li, Q., Wang, L., Cao, Y. X. & Ling, S. C. Rat glioma C6 cell apoptosis induced by UV radiation via p38-MAPK. J. Fourth Mil. Med. Univ. 24, 20–22 (2003).
Wu, R. et al. A novel peptide from vespa ducalis induces apoptosis in osteosarcoma cells by activating the p38 mapk and jnk signaling pathways. Biol. Pharm. Bull. 41, 458–464 (2018).
Song, L. et al. Paralytic peptide activates innate immunity via PI3K and receptor tyrosine kinase in silkworm (Bombyx mori). Acta Entomol. Sin. 57, 141–149 (2014).
Inoue, H. et al. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J. 20, 5421–5430 (2014).
Lesser, M. P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 68, 253–278 (2006).
Felton, G. W. & Summers, C. B. Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 29, 187–197 (1995).
Li, L. Z., Shen, H. J., Jiang, Q. G. & Ji, B. Z. A study on the activities of endogenous enzymes of protective system in some insects. Acta Entomol. Sin. 37, 399–403 (1994).
Lü, X. M., Yang, Y. F., Lu, X. Y., Jin, J. & Fan, X. M. Effects of NaCl stress on the AsA-GSH cycle in sour jujube seedlings. Plant Physiol. J. 52, 736–744 (2016).
Feng, C. J., Dong, Q. A., Zhai, H. F., Chen, G. B. & Miao, J. L. Immunological and stress response of the hemolymph of Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae) larvae to the injection of Escherichia coli. Acta Entomol. Sin. 54, 117–126 (2011).
Zheng, Y. X. MAPK Cascade Regulates the Antioxidizing Defence Response of Maize Seedlings to Abiotic Stress (Shandong Agricultural University, 2009).
Liang, G. M., Tan, W. J. & Guo, Y. Y. Improvement of artificial breeding of cotton bollworm technology. Plant Prot. 25, 15–17 (1999).
NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 44(1), 7–19. https://doi.org/10.1093/nar/gkv1290 (2016).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Han, Z. S. et al. A conserved p38 mitogen-activated protein kinase pathway regulates Drosophila immunity gene expression. Mol. Cell. Biol. 18, 3527–3539 (1998).
Hu, X. et al. Cloning and bioinformatic analysis of p38 MAPK gene from Plutella xylostella. Shandong Agric Sci 45, 15–18 (2013).
Widmann, C., Gibson, S., Jarpe, M. B. & Johnson, G. L. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180 (1999).
Terzis, G. et al. The degree of p70s6K and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur. J. Appl. Physiol. 110, 835–843 (2010).
Tortorella, L. L., Lin, C. B. & Pilch, P. F. ERK6 is expressed in a developmentally regulated manner in rodent skeletal muscle. Biochem. Biophys. Res. Commun. 306, 163–168 (2003).
Cuenda, A. & Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 1773, 1358–1375 (2007).
Li, X. et al. p38 mitogen-activated protein kinase is crucially involved in osteoclast differentiation but not in cytokine production, phagocytosis, or dendritic cell differentiation of bone marrow macrophages. Endocrinology 144, 4999–5005 (2003).
Hu, S. et al. Expression patterns of p38αmapk during follicular development in the ovaries of neonatal rats. Acta Histochem. 119, 538–542 (2017).
Zhang, P. J., Zhu, L. X. & Geng, X. P. p38 mitogen activated protein kinase pathway and its inhibitor. Anhui Med. Pharm. J. 14, 596–598 (2010).
Johansson, N. et al. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J. Cell Sci. 113, 227–235 (2000).
Chock, K., Allison, J. M. & Elshamy, W. M. BRCA1-IRIS overexpression abrogates UV-induced p38MAPK/p53 and promotes proliferation of damaged cells. Oncogene 29, 5274–5285 (2010).
Fujiwara, Y., Shindome, C., Takeda, M. & Shiomi, K. The roles of ERK and P38 MAPK signaling cascades on embryonic diapause initiation and termination of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 36, 47–53 (2006).
Harper, S. J. & LoGrasso, P. Signalling for survival and death in neurones: The role of stress-activated kinases, JNK and p38. Cell Signal. 13, 299–310 (2001).
Schattauer, S. S. et al. Reactive oxygen species (ROS) generation is stimulated by κ opioid receptor activation through phosphorylated c-Jun N-terminal kinase and inhibited by p38 mitogen-activated protein kinase (MAPK) activation. J. Biol. Chem. 294, 16884–16896 (2019).
Ravanat, J. L., Douki, T. & Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B 63, 88–102 (2001).
Zhou, D. et al. Effects of UV-B radiation in successive generations on the activities of protective enzymes in the grain aphid, Sitobion avenae (Hemiptera: Aphididae). Acta Entomol. Sin. 57, 762–768 (2014).
Qiao, L. et al. Effects of temperature on survival rate and protection enzymes of Empoasca onukii Matsuda. J. Plant Prot. 42, 223–228 (2015).
, Li J, Zhao HY, Zhao XD,. Study on effect of ecological characteristics and enzyme activity of Aphid radiated by different intensities of ultraviolet. J. Northwest Sci.-Tech. Univ. Agric. For. 33, 61–64 (2005).
Liu, X. F., Meng, J. Y., Zhang, Y. & Zhang, C. Y. Effects of UV-B radiation on the antioxidant system of Myzus persicae. J. Environ. Entomol. 40, 1422–1428 (2018).
Hua, X. et al. ROS-induced oxidative injury involved in pathogenesis of fungal keratitis via p38 MAPK activation. Sci. Rep. 7, 10421 (2017).
Funding
This research was funded by the National Key R&D Program of China grant number [2017YFD0200900], and China National Science Foundation Project grant number [31401754, 31460483].
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, M., Liu, X., Meng, J. et al. Molecular characterization and elucidation of the function of Hap38 MAPK in the response of Helicoverpa armigera (Hübner) to UV-A stress. Sci Rep 12, 18489 (2022). https://doi.org/10.1038/s41598-022-23363-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23363-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.