Abstract
Climate change, food scarcity, salt stress, and a rapidly growing population are just a few of the major global challenges. The current study examined into whether Moringa oleifera (L.) leaf extract and green algae (Ulva intestinalis) could help improve salt tolerance in Mentha species (Mentha piperita; Mentha longifolia). Moringa leaf extract (MLE) and green algae (GA) were applied to Mentha seedlings under three different salt treatments: 0 mM, 20 mM, 40 mM, 60 mM, and 90 mM, respectively. For each treatment, three biological replicates were conducted, with each replicate containing at least three plants. Mentha species were negatively affected by salt stress in terms of shoot length, fresh and dry weight, photosynthetic pigments, and antioxidant enzyme activities. However, the use of MLE and GA significantly improved the development and physiology of Mentha species under salt stress conditions. The MLE and GA treatments dramatically (p ≤ 0.001) increased SOD activity by 7% and 10%, CAT activity by 16% and 30%, APX activity by 34% and 56%, GPX activity by 12% and 47%, respectively, in Mentha piperita seedlings, which in turn strikingly increased superoxide dismutase (SOD) activity by 6% and 9%, catalase (CAT) activity by 15%, 28% and 44%, 27%, ascorbate peroxidase (APX) activity by 39% and 60%, glutathione peroxidase (GPX) activity by 23% and 58%, respectively, in Mentha longifolia seedlings, relative to the control. Aiming to answer questions about the relationship between plant extraction and traditional agricultural methods, this research greatly advances the goal of sustainable development for improving plant productivity by providing a much safer and more environmentally friendly adaptability.
Similar content being viewed by others
Introduction
The effects of climate change are having a negative impact on agricultural production all around the world1,2,3. Salinization of the soil due to climate change is causing yield loss worldwide. Salt stress is one of the most important abiotic stresses limiting plant development and productivity, especially in arid and semi-arid regions around the world4,5. It has become a significant concern in areas covering around 1125 million hectares worldwide, of which 76 million hectares are directly affected by human activities, resulting in a 1.5-million-hectare annual loss of arable land due to salinization6. Exposure to salt stress has dramatically influenced physiological responses such as altered plasma membrane integrity, increased reactive oxygen species (ROS) generation, decreased photosynthetic efficiency, decreased stomatal aperture size, and insufficient accessibility to antioxidant enzymes7,8,9. Furthermore, ROS buildup generates oxidative bursts in cellular compartments, causing proteins, DNA, and lipids to change10,11,12.
Different techniques have been proposed to mitigate the negative effects of salt stress. These approaches include the use of salt-tolerant varieties, stress signaling molecules, osmoprotectants, green algae and plant extracts. Plant and green algae extract, which are both physiologically safe and economically sustainable, have demonstrated a great deal of promise for crop enhancement in moderate-stress conditions in recent years13. Water extracts from a variety of cultivated plants have been noted that enhance plant growth and yield in both normal and stressful conditions by altering phytohormone metabolism, photosynthetic activity, and antioxidant defense system14. Moringa oliefera (L.) has received much interest from researchers because its leaves contain more minerals, growth hormones, vitamins, and antioxidants15,16,17,18,19. MLE applied to plant leaves has been demonstrated to promote seedling establishment, seedling growth, and eventually production in abiotically stressed field crops20,21,22,23,24,25. Ulva intestinalis L. is a marine green alga in the Ulvaceae family with a tubular frond and unbranched thalli26. It is a rich source of physiologically active molecules such as essential fatty acids, vitamins, amino acids, minerals, and growth stimulating substances; they have also been found to boost plant growth performance, antioxidant activities, and tolerance to abiotic stress27,28.
Mentha species are members of the Lamiaceae family, which possesses medicinal and fragrant properties. Since this particular species displays significant biological activities, it has been utilized as a treatment for a variety of respiratory conditions, including bronchitis, sinusitis, and even the common cold29. Moreover, it has the potential to be employed in the pharmaceutical and food industries as an efficient and cost-effective source of natural commercial antioxidants29. However, no research has been undertaken to our knowledge on the influence of MLE and GA extracts on the growth and physiology of Mentha species under salt stress conditions. Thus, the primary goal of this study is to investigate into the potential effects of MLE and GA on the growth and physiological attributes of Mentha species grown under salt stress conditions. The findings of this study will aid in improving Mentha species productivity in salt-stressed conditions.
Materials and methods
Experimental particulars
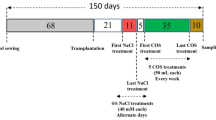
The Department of Biology, College of Science, Imam Abdulrahman Bin Faisal University (26.3928° N, 50.1926° E) undertook this study to investigate the effect of MLE and GA on the growth and physiology of Mentha species (Mentha piperita L. and Mentha longifolia L.) identified by Šarić-Kundalić et al.30, and growing under salt stress. Cultivated (Ulva intestinalis L.) identified based on Budd31 techniques, and collected from Az Zakhnuniyah is an island located on the western coast of the Arabian Gulf (N 25° 54′ 72.94″, E 50° 32′ 53.31″) and Moringa (Moringa oliefera L.) leaves were collected from Al-Ahsa city market (Voucher number-IAU:104598). On the other hand, Mentha seeds were collected from the local market in Dammam, Saudi Arabia. The experiment used a completely randomised design with split plot layouts. Pots (40 cm in height and 25 cm diameter) were filled with compost, sand (45.29%), silt (36.22%), and clay (21.14%), with pH and EC of 7.6 and 2.52 dS m−1, respectively. Soil pH was measured by pH meter (Divinext 3), whereas the EC was measured by EC meter (HI98331). In each of the pots, three seeds of each Mentha species were sowed. This study was performed with the local (Saudi Arabia) regulations implemented for studying towards the plants.
Salt stress treatments and preparation of extracts
Treatments were prepared based on the methods of Gholamnia et al.8. During the experiment, different doses of NaCl (0, 20, 40, 60, and 90 mM) were added to the experimental pots to produce salt stress. Moringa leaves that were mature and healthy were harvested and cleaned with tap water before being stored in the refrigerator overnight. An assembled machine was used for the extraction procedure. Distilled water was used to dilute the extracts to a concentration of 3%. To eliminate pollutants, tap water and distilled water were used to rinse Ulva intestinalis. It was homogenized in distilled water (1:1 by volume) at room temperature and stored until further use was needed. 100% of the liquid extract was consumed. The final extract yielded a 2% solution in distilled water.
Determination of growth parameters
Plant lengths determined by using a metric scale and expressed in centimeter (cm). The plant materials were split into shoots and roots after being cleaned with double distilled water to eliminate sand particles. The fresh weights (FW) and dry weights (DW) were measured with an analytical balance (HR-200) and expressed in grams (g).
Photosynthetic pigment determination
Arnon32 approach was used to extract photosynthetic pigments. At room temperature, a 0.25 g leaf sample was taken and ground with 5 ml of 80% acetone. After that, the extract was centrifuged at 3000 rpm for 10 min at 40 °C. The absorbance of the supernatant at 663 and 645 nm was used to determine the chlorophyll a and b concentrations.
Proline determination
The Bates et al.33 method was used to estimate proline concentration. 10 mL of aqueous sulfosalicylic acid and 0.5 g of newly plucked leaves (3%). After that, the mixture was filtered through a Whatman No. 40 filter paper. The mixture was placed in test tubes, and 2 mL of ninhydrin solution and 2 mL of glacial acetic acid were added. The mixture was then heated at 95 °C for over an hour before being placed in an ice bath to cool. The mixture was then extracted with 10 mL of toluene as a chromophore, and the reaction mixture was constantly circulated via an air stream for 1–2 min to separate the aqueous phase from the chromophore, which contained toluene. Finally, the separated colored phase was allowed to dry at room temperature for 2–3 min before its absorbance was measured with a spectrophotometer to be 520 nm.
Total sugar content determination
The method described by Du Bois et al.34 was used to calculate the total soluble sugar content (1956). To extract 0.5 g of fresh leaves, 10 mL of ethanol (80%) was employed. After centrifugation, the supernatant was combined with 2.5 mL of 5% phenol solution (v/v) and 0.5 mL of sulfuric acid. To heat the combination, it was immersed in a water bath for 20 min. A standard curve was used to calculate the total soluble sugar concentration, and the absorbance at 490 nm was calculated.
Extraction and measurement of antioxidant enzyme activity
The antioxidant enzymes were extracted using the Mukherjee and Choudhuri35 approach. In 10 mL of phosphate buffer, 0.5 g of fresh leaves were extracted (pH 7). After that, the homogenate was centrifuged at 15,000 rpm for 10 min at 4 °C. The supernatant was then maintained at 20 °C to assess antioxidant enzyme activity.
Superoxide dismutase (SOD) activity determination
The nitro-blue-tetrazolium (NBT) reduction procedure was used to measure SOD activity36. The reaction mixture (3 mL) includes 50 enzyme extract, 150 riboflavin (13 M), 2.5 mL methionine (13 M), 250 NBT (63 M), and 50 phosphate buffers (50 mM, pH 7.8). The absorbance at 560 nm was measured using a spectrophotometer (LKB-Biochrom 4050).
Catalase (CAT) activity determination
The Aebi37 approach was used to measure CAT activity. The enzyme extract (40 mL) was combined with 0.016 mL of H2O2 (30%) and a 10 mM phosphate buffer solution (pH 7.0). Finally, the absorbance at 240 nm was evaluated using a spectrophotometer (LKB-Biochrom 4050).
Ascorbate peroxidase (APX) activity determination
The APX activity was evaluated using the Nakano and Asada38 approach. The reaction mixture contained 0.1 M potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM EDTA, 1.0 mM H2O2, and 20 µL enzyme extract (2.22 mL). The enzyme coefficient of 2.8 mM−1 cm−1 was used to calculate APX activity.
Guaiacol peroxidase (GPX) activity determination
The GPX activity was measured at 25 °C using the Elia et al.39 technique. The reaction mixture includes 0.2 mL enzyme extract, 10 mM sodium phosphate buffer (pH 7.0), 1 mL H2O2 (30%), 1 mL guaiacol (0.05 M), and 2 mL distilled water. Guaiacol oxidation was determined by measuring the rise in absorbance at 470 nm over a one-minute period. One unit of POD is the amount of enzyme required to catalyse the reduction of 1 M of H2O2, using guaiacol as the hydrogen donor, per minute under certain conditions, and was calculated using the enzyme coefficient 26.6 mM−1 cm−1.
Statistical evaluation
The MINITAB-17 statistical software was used to perform analysis of variance (ANOVA) on the data, and the results were displayed as treatment mean ± SE (n = 3). The LSD test reveals that bars with the same letter are not statistically different at the p < 0.05 level.
Results
Growth conditions
To analyze the beneficial effects of MLE and GA on Mentha species, we looked at a variety of morphological traits, including shoot length, shoot fresh and dry weight, and root fresh and dry weight. Shoot length, fresh and dry weight, and root fresh and dry weight were all reduced significantly (p ≤ 0.001) when the Mentha species were subjected to varied doses of NaCl (20, 40, 60, and 90 mM) compared to control (Table 1). The salt treatments (20, 40, 60, 90 mM) dramatically (p ≤ 0.001) decreased shoot length by 5%, 20%, 29%, 39%, root length by 8%, 20%, 29%, 39%, shoot fresh weight by 32%, 35%, 45%, 70%, shoot dry weight by 7%, 27%, 49%, 64%, root fresh weight by 20%, 37%, 61%, 69%, respectively, which in turn strikingly decreased the root dry weight by 33%, 63%, 73%, 92% in Mentha piperita seedlings, relative to the control. Conversely, salt treatments reduced shoot length, fresh and dry weight, and root fresh and dry weight of Mentha longifolia. Nonetheless, exogenous MLE and GA treatment significantly improved these parameters in both Mentha species when exposed to salt.
Photosynthetic pigments
In comparison to control seedlings, salt treatments (20, 40, 60, 90 mM) resulted in significant (p ≤ 0.001) decreases in total chlorophyll a and chlorophyll b content in Mentha piperita of 8%, 15%, 37%, 67% and 5%, 14%, 24%, 64% and in Mentha longifolia of 10%, 16%, 38%, 72% and 9%, 18%, 46%, 71%, respectively. Nonetheless, Mentha species treated with MLE and GA showed significantly greater chlorophyll content (p < 0.001) (Fig. 1).
Proline content
In Mentha piperita and Mentha longifolia, the salt treatments (20, 40, 60, 90 mM) resulted to substantial (p < 0.001) increases in proline content of 9%, 19%, 30%, 42% and 9%, 21%, 32%, 40%, respectively, compared to those in control seedlings. When compared to the salt-stressed Mentha seedlings, the exogenous administration of MLE and GA considerably (p < 0.001) lowered proline content.
Soluble sugar content
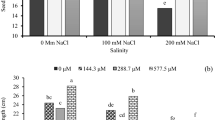
Salt treatments of 20, 40, 60, and 90 mg/L raised the soluble sugar content in Mentha piperita by 6%; Mentha longifolia by 4%; Mentha longifolia by 18%; and Mentha longifolia by 32% compared to those in control seedlings that did not receive MLE and GE, respectively (Fig. 2). In spite of this, the exogenous infusion of MLE and GA considerably (p < 0.001) reduced the soluble sugar content.
Effect of MLE (Moringa oleifera) and GA (Ulva intestinalis) on proline (A) and total soluble sugar (B) content in the Mentha species under salt stress (0 mM, 20 mM, 40 mM, 60 mM, 90 mM). The data displayed are the means (± SE) of three replicates, and bars of dissimilar letters differ significantly at the p ≤ 0.05 level.
Antioxidant enzyme activity
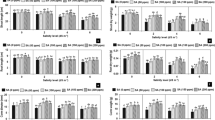
20, 40, 60, and 90 mM salt treatments significantly (p ≤ 0.001) increased SOD activity by 10%, 19%,42%, and 49%, CAT activity by 11%, 15%, 33%, and 34%, APX activity by 27%, 60%, 73%, and 74%, GPX activity by 31%, 44%, 66% and 72%, respectively, relative to with respect to those in untreated Mentha piperita plants (Fig. 3). Correspondingly, the salt treatments (20, 40, 60, and 90 mM) led to significant (p ≤ 0.001) increases in SOD activity by 10%, 25%, 41%, and 53%, CAT activity by 6%, 9%, 26%, and 27%, APX activity by 28%, 62%, 70%, and 71%, GPX activity by 27%, 44%, 63%, and 66%, respectively, with respect to those in control Mentha longifolia seedlings. However, the application of MLE and GA resulted in a considerable improvement in these antioxidant enzyme parameters (Fig. 4). Furthermore, when exposed to high salt concentrations, GA treatment was more effective than MLE treatment in regulating these antioxidant enzymes (SOD, CAT, APX, and GPX).
Effect of MLE (Moringa oleifera) and GA (Ulva intestinalis) on the antioxidant enzymes (A SOD; B CAT; C APX and D GPX) in the Mentha species under salt stress (0 mM, 20 mM, 40 mM, 60 mM, 90 mM). The data displayed are the means (± SE) of three replicates, and bars of dissimilar letters differ significantly at the p ≤ 0.05 level.
Discussion
According to the current report results, salt stress significantly reduced the shoot and root biomass of both Mentha seedlings. The decrease in growth caused by salinity could be attributed to decreased nutrient uptake by plants or increased sodium redistribution from roots to shoots40. However, the current study found that applying MLE and GA to Mentha species increased their growth and physiological greatly. Similar outcome was observed in rice where MLE increased the growth and biomass under drought stress41. These findings suggest that MLE and GA promote Mentha species growth by altering physiological processes.
In order to determine the level of salt stress, photosynthetic systems can be employed as indicators42,43,44. Reduced photosynthetic pigments are caused by salt stress and chlorophyll content was reported to be greater in stress-free conditions than in salt-stressed environments. In this present study, salt stress lowered the photosynthetic pigments of Mentha species. These findings back up the findings of Ahanger et al.45, who found that salt stress reduced chlorophyll concentration in wheat. In the current study, exogenous administration of MLE and GA significantly boosted the amount of photosynthetic pigments during salt stress. Moringa leaves are abundant in chlorophyll and carotenoids (xanthin, beta-carotene, alpha-carotene, and lutein), which have antioxidant effects15. MLE has also been shown to accelerate the synthesis of cytokinin’s, preventing early leaf senescence and resulting in a bigger leaf area with higher chlorophyll content46. The current study findings are consistent with Khan et al.41 discovery that MLE application significantly boosted photosynthetic pigments in wheat cultivated under favorable conditions. According to Yasmeen et al.46, foliar application of MLE during the tillering and heading phases increases chlorophyll a and b levels in wheat. The aqueous extract of Ulva intestinalis also increased the levels of chlorophyll a and b in parsley seedlings47.
The total soluble sugars and proline content were determined to understand more about MLE and GA effects on salt stressed seedlings. Total soluble sugars are well-known as one of the essential organic solutes that maintain cell homeostasis48,49,50, and proline aids in cell osmotic adjustment in the presence of salt stress49,50. According to our findings, total soluble sugars and proline levels increased in the Mentha species under salt stress when compared to the control condition. A similar study in chickpea found that salt stress boosted the synthesis of total soluble sugars and proline levels in wheat49,51. MLE and GA combined application reduced total soluble sugars and proline levels under salt stress. Seedlings of Mentha may be able to tolerate salt stress by lowering endogenous proline production. Similarly, when exposed to salt stress alone, MLE reduced the proline concentration in Brassica napus leaves52. Ibrahim et al.53 reported that ascorbic acid, betaine, glutathione, and proline are some of the bioactive components found in Ulva lactuca extract. These components, along with others, have the potential to alleviate the negative effects of salt stress.
Antioxidant defenses are essential in determining a plant's tolerance for stressful conditions54,55,56,57,58. With the beginning of salt stress, the activities of enzymatic antioxidants were found to be increased in the Mentha seedlings. Hanafy59 found a significant increase in the activities of enzyme antioxidants (GR, SOD, APX, and GPX) in rice that had been exposed to salt stress. The use of MLE and GA increased the antioxidant activity of enzymatic antioxidants in Mentha species, which was especially noticeable under salt stress. Increased SOD, CAT, APX, and GPX activity may be related with the activation of antioxidant responses that protect the plant from oxidative damage, according to our findings. According to Foyer and Noctor60, the initiation of enzymatic antioxidant activities in plants is a natural response for resisting oxidative stress. Similarly, MLE administration resulted in a significant increase in SOD activity in soybean, which was followed by the application of glutathione reductase (GR) and APX, respectively. Zaki and Rady61 found that seed soaking or foliar spray treatment of MLE increased the antioxidant enzyme activities such as SOD, and APX in common bean (Phaseolus vulgaris L.) plants. Microalgae, on the other hand, were found to boost SOD, CAT, APX, and peroxidase (POD) activities in wheat seedlings under salt stress62. Furthermore, similar studies were conducted on several plants and showed that using Ulva lactuca and marine algae extracts increased the antioxidant enzyme activities. The increase in enzyme activity could be indicative of the presence of antioxidant and osmoprotectant substances.
Conclusion
Salt stress has a deleterious impact on the growth and physiology of the Mentha species. MLE and GA demonstrated the best biostimulant potential in terms of improved growth and physiology of Mentha seedlings grown under normal and salt stress. Foliar application of MLE and GA significantly improved photosynthetic pigments, osmolytes, and antioxidant enzyme activity under normal and salt stress conditions. Overall, these findings suggest that MLE and GA can be used to promote field plant development in both normal and salt-stressed environments. More research is required, however, to determine the effectiveness of MLE and GA in reducing the harmful effects of soil salinization on plants, as well as the optimal dose. Furthermore, the molecular processes underlying MLE and GA-mediated salt tolerance in plants must be understood.
References
Francini, A. & Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulture 67, 5 (2019).
Qamar, et al. Mitigating water stress on wheat through foliar application of silicon. Asian J. Agric. Biol. 8(1), 1–10. https://doi.org/10.35495/ajab.2019.04.174 (2020).
Batool, et al. Impact of natural and synthetic plant stimulants on Moringa seedlings grown under low-temperature conditions. Int. Lett. Nat. Sci. 76, 51 (2019).
Rehman, M. Z. et al. Comparative effects of different soil conditioners on wheat growth and yield grown in saline-sodic soils. Sains Malays. 45, 339–346 (2016).
Shareef, H. J. Organic fertilizer modulates IAA and ABA levels and biochemical reactions of date palm Phoenix dactylifera L. Hillawi cultivar under salinity conditions. Asian J. Agric. Biol. 8(1), 24–30. https://doi.org/10.35495/ajab.2019.02.062 (2020).
Hossain, M. S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 1, 1–3 (2019).
Huang, P. et al. Seed priming with sorghum water extract improves the performance of Camelina (Camelina sativa (L.) Crantz) under salt stress. Plants 10(4), 749. https://doi.org/10.3390/plants10040749 (2022).
Gholamnia, A. et al. Expression profiling of rosmarinic acid biosynthetic genes and some physiological responses from Mentha piperita L. under salinity and heat stress. Physiol. Mol. Biol. Plants 28, 545–557 (2022).
Li, Z., Yang, H., Wu, X., Guo, K. & Li, J. Some aspects of salinity responses in peppermint (Mentha× piperita L.) to NaCl treatment. Protoplasma 252(3), 885–899 (2015).
Tanveer, M. & Ahmed, H. A. I. ROS signalling in modulating salinity stress tolerance in plants. In Salt and Drought Stress Tolerance in Plants (eds Hasanuzzaman, M. & Tanveer, M.) 299–314 (Springer, 2020).
Jahan, M. S. et al. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA-and GA-mediated pathways. Front. Plant Sci. 12, 381 (2021).
Khan, S. et al. Moringa leaf extract improves biochemical attributes, yield and grain quality of rice (Oryza sativa L.) under drought stress. PLoS One 16, e0254452 (2021).
Khan, S., Basra, M. A., Nawaz, M., Hussain, I. & Foidl, N. Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). South Afr. J. Bot. 129, 74–81 (2020).
Cheng, F. & Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 6, 1020 (2015).
Hasan, M. M. et al. Effects of magnetized water on phenolic compounds, lipid peroxidation and antioxidant activity of Moringa species under drought stress. J. Anim. Plant Sci. 28, 803–810 (2018).
Hasan, M. M. et al. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Pol. J. Environ. Stud. 539, 1145–1155 (2019).
Hasan, M. M. et al. Evidence-based assessment of Moringa oleifera used for the treatment of human ailments. In Plant and Human Health (eds Ozturk, M. & Hakeem, K. R.) 121–137 (Springer, 2019).
Batool, et al. Foliar application of moringa leaf extract improves the growth of moringa seedlings in winter. South Afr. J. Bot. 129, 347–353 (2020).
Iqbal, et al. Comparative study of water extracts of Moringa leaves and roots to improve the growth and yield of sunflower. South Afr. J. Bot. 129, 221–224 (2020).
Khan, et al. Application of Moringa leaf extract as a seed priming agent enhances growth and physiological attributes of rice seedlings cultivated under water deficit regime. Plants 261, 11. https://doi.org/10.3390/plants11030261 (2022).
Basra, S. M. A., Iftikhar, M. N. & Afzal, I. Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int. J. Agric. Biol. 13, 1006–1010 (2011).
Hasan, M. M. et al. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol. J. Environ. Stud. 1, 29 (2020).
Zahra, N. et al. Plant growth promoters mediated quality and yield attributes of milk thistle (Silybum marianum L.) ecotypes under salinity stress. Sci. Rep. 11(23200), 4 (2021).
Irshad, S. et al. Foliar application of potassium and moringa leaf extract improves growth, physiology and productivity of kabuli chickpea grown under varying sowing regimes. PLoS One 17(2), e0263323. https://doi.org/10.1371/journal.pone.0263323 (2022).
Khan et al. (2021). Impact of natural and synthetic growth enhancers on the productivity and yield of quinoa (Chenopodium quinoa willd.) cultivated under normal and late sown circumstances.
Sun, S. et al. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Mar. Drugs 17, 179 (2020).
Kim, D. H. et al. Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresour. Technol. 161, 348–353 (2014).
Peasura, N., Laohakunjit, N., Kerdchoechuen, O. & Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 81, 912–919 (2015).
Mahboubi, M. & Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 119, 325–327 (2008).
Šarić-Kundalić, B. et al. Multivariate numerical taxonomy of Mentha species, hybrids, varieties and cultivars. Sci. Pharm. 77(4), 851–876 (2009).
Budd, G. C. & Pizzola, P. Ulva intestinalis. Gut Weed 20, 25 (2008).
Arnon, D. I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1 (1949).
Bates, L. S., Waldren, R. P. & Teari, D. Rapid determination of free proline for water stress studies. Plant Soil 39, 205–207 (1973).
DuBois, M. et al. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Mukherjee, S. P. & Choudhuri, M. A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 58, 166–170 (1983).
Hasanuzzaman, M. & Fujita, M. Exogenous sodium nitroprusside alleviates arsenic induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22, 584–596 (2013).
Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126 (1984).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22(867–880), 1981 (1981).
Elia, A. C. et al. Antioxidant responses and bioaccumulation in Ictalurus melasunder mercury exposure. Ecotoxicol. Environ. Saf. 55, 162–167 (2003).
Saddiq, M. S. et al. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 11(6), 1193 (2021).
Khan, S. et al. Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ. Sci. Pollut. Res. 24, 27601–27612 (2017).
Alharbi, B. M. et al. Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ. 25, 67 (2021).
Zahra, N. et al. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 178, 55–69 (2022).
Rehman, et al. Application of plant growth promoters on sugarcane (Saccharum officinarum L.) budchip under subtropical conditions. Asian J. Agric. Biol. https://doi.org/10.35495/ajab.2020.03.202 (2021).
Ahanger, M. A. et al. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 19, 479 (2019).
Yasmeen, A. Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul. 69, 225–233 (2013).
Paulert, R. et al. Ulva intestinalis Extract acts as biostimulant and modulates metabolites and hormone balance in Basil (Ocimum basilicum L.) and Parsley (Petroselinum crispum L.). Plants 10, 1391 (2021).
Jahan, M. S. et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 167, 309–320 (2021).
Khan, A. et al. Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 6, 20 (2017).
Ahmad, P. et al. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 7, 347 (2016).
Latef, A. A. Changes of antioxidative enzymes in salinity tolerance among different wheat cultivars. Cereal Res. Commun. 38, 43–55 (2010).
Rossi, et al. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L.. Environ. Pollut. 219, 2836 (2016).
Ibrahim, W. M., Ali, R. M., Hemida, K. A. & Sayed, M. A. Role of Ulva lactuca extract in alleviation of salinity stress on wheat seedlings. Sci. World J. 25, 25 (2014).
Alabdallah, et al. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 10, 1730 (2021).
Alabdallah, N. M. A. & Hasan, M. M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. https://doi.org/10.1016/j.sjbs.2021.05.081 (2021).
Hasan, et al. Insights into 28-homobrassinolide (HBR)- mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Int. 15, 371–385 (2020).
Hasan, et al. Spermine: Its emerging role in regulating drought stress responses in plants. Cells 261, 1–15 (2021).
Hasan, M. M. et al. ABA induced stomatal movements in vascular plants during dehydration versus rehydration. Environ. Exp. Bot. 186, 1–8 (2021).
Hanafy, R. Using Moringa oliefera leaf extract as a bio-fertilizer for drought stress mitigation of Glycine max L. plants. Egypt. J. Bot. 57, 281–292 (2007).
Foyer, C. H. & Noctor, G. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 119, 355–364 (2003).
Zaki, S. S. & Rady, M. M. Moringa oleifera leaf extract improves growth, physiochemical attributes, antioxidant defense system and yields of salt-stressed Phaseolus vulgaris L. plants. Int. J. Chem. Tech. Res. 8, 120–134 (2015).
Baky, A. E. H., Hussein, M. M. & El Baroty, G. Induces of antioxidant compounds and salt tolerance in wheat plant, irrigated with seawater as response to application of microalgae spray. Am. J. Agric. Biol. Sci. 9, 127–137 (2014).
Acknowledgements
The authors extend their sincere appreciation to the College of Science, Imam Abdulrahman Bin Faisal University, Saudi Arabia. Ulva intestinalis was collected from Az Zakhnuniyah and Moringa (Moringa oliefera L.) leaves were collected from Al-Ahsa city under permit No.1-11-1-2590.
Author information
Authors and Affiliations
Contributions
All Authors contributed equally towards this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Taisan, W.A., Alabdallah, N.M. & Almuqadam, L. Moringa leaf extract and green algae improve the growth and physiological attributes of Mentha species under salt stress. Sci Rep 12, 14205 (2022). https://doi.org/10.1038/s41598-022-18481-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18481-5
This article is cited by
-
Enhancing sweet potato (Ipomoea batatas) resilience grown in cadmium-contaminated saline soil: a synergistic approach using Moringa leaf extract and effective microorganisms application
Environmental Science and Pollution Research (2024)
-
Insight into the physiological and biochemical mechanisms of biostimulating effect of Ascophyllum nodosum and Moringa oleifera extracts to minimize cadmium-induced oxidative stress in rice
Environmental Science and Pollution Research (2023)
-
Mitigation of Salt Stress in Soybean (Glycine max (L.) Merrill) Using Exogenous Application of Onion Extract
Journal of Soil Science and Plant Nutrition (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.