Abstract
Rest pulmonary circulation parameters such as pulmonary transit time (PTT), heart rate corrected PTT (PTTc) and pulmonary transit beats (PTB) can be evaluated using several methods, including the first-pass perfusion from cardiovascular magnetic resonance. As previously published, up to 58% of patients after HTx have diastolic dysfunction detectable only in stress conditions. By using adenosine stress perfusion images, stress analogues of the mentioned parameters can be assessed. By dividing stress to rest biomarkers, potential new ratio parameters (PTT ratio and PTTc ratio) can be obtained. The objectives were to (1) provide more evidence about stress pulmonary circulation biomarkers, (2) present stress to rest ratio parameters, and (3) assess these biomarkers in patients with presumed diastolic dysfunction after heart transplant (HTx) and in childhood cancer survivors (CCS) without any signs of diastolic dysfunction. In this retrospective study, 48 patients after HTx, divided into subgroups based on echocardiographic signs of diastolic dysfunction (41 without, 7 with) and 39 CCS were enrolled. PTT was defined as the difference between the onset time of the signal intensity increase in the left and the right ventricle. PTT in rest conditions were without significant differences when comparing the CCS and HTx subgroup without diastolic dysfunction (4.96 ± 0.93 s vs. 5.51 ± 1.14 s, p = 0.063) or with diastolic dysfunction (4.96 ± 0.93 s vs. 6.04 ± 1.13 s, p = 0.13). However, in stress conditions, both PTT and PTTc were significantly lower in the CCS group than in the HTx subgroups, (PTT: 3.76 ± 0.78 s vs. 4.82 ± 1.03 s, p < 0.001; 5.52 ± 1.56 s, p = 0.002). PTT ratio and PTTc ratio were below 1 in all groups. In conclusion, stress pulmonary circulation parameters obtained from CMR showed prolonged PTT and PTTc in HTx groups compared to CCS, which corresponds with the presumption of underlying diastolic dysfunction. The ratio parameters were less than 1.
Similar content being viewed by others
Introduction
Pulmonary circulation biomarkers obtained with non-invasive methods are not new themselves. Acquired by different modalities, including radionuclide imaging1, contrast-enhanced transthoracic or transesophageal echocardiography2,3, or computed tomography4, they have been studied for decades.
One of the latest techniques to assess pulmonary circulation parameters is magnetic resonance imaging (MRI). Firstly, through MRI angiography5, and later on by the first-pass perfusion from cardiovascular magnetic resonance (CMR)6,7,8. The latter option offers advantages such as using an already employed sequence and opens new opportunities with retrospective studies, although data are still limited.
From the analysis of rest perfusion images, biomarkers such as pulmonary transit time (PTT), pulmonary transit beats (PTB) and pulmonary blood volume index (PBVI), can be obtained. Likewise, by using adenosine stress perfusion images, their analogues, the stress pulmonary transit time (PTT_S) and the stress pulmonary transit beats (PTB_S), can also be determined. To our knowledge, so far, just one article reported stress parameters, but only in hypertrophic cardiomyopathy (HCM)7.
PTT has already shown to be increased in both heart failure with reduced and preserved ejection fraction6,9. As previously published by Meluzin et al.10, by pulmonary capillary wedge pressure acquired by right heart catheterization, 16% of patients after heart transplant (HTx) showed signs of diastolic dysfunction in rest condition, but in stress condition, another 58% showed these signs10. Therefore, we hypothesise that in a population after HTx, the stress biomarkers will be prolonged compared to a group of patients without any signs of systolic or diastolic function impairment.
We further assume that in a healthy population, the stress pulmonary transit biomarkers should be shorter and assessing not only the absolute values of rest and stress parameters, but also their corresponding ratio could be valuable.
As far as we know, these biomarkers have not been studied before on patients after HTx or in the childhood cancer survivors (CCS).
This study aimed to (1) provide more evidence about stress pulmonary circulation biomarkers, (2) present and investigate stress to rest ratio parameters, and (3) assess these biomarkers in patients with presumed diastolic dysfunction after heart transplant (HTx) and in CCS without diastolic dysfunction.
Methods
Study design and population
This retrospective study was performed in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The Ethics Committee of the Faculty of Medicine, Masaryk University, confirmed that this study has been performed on data of patients already participating in other research studies with signed Informed Consent and therefore, according to the Czech legislation, no specific new approval of the ethics committee was required.
In this retrospective study, 48 subjects after HTx and 39 CCS patients were included. Detailed inclusion and exclusion criteria are shown in Table 1.
The HTx group consisted of patients who underwent HTx for different diagnoses and were being monitored at our center. In the first year after transplantation, a detailed follow-up, including myocardial biopsies and other examinations, took place. During the first-year check-up, they underwent more thorough examinations with CMR, including a stress perfusion test.
The CCS group were patients after cardiotoxic chemotherapy in young or adolescent age treated at the paediatric oncology. Patients were treated with anthracyclines in 74.3%, the rest with Cisplatin and high dose Carboplatin. In terms of specific drugs, 93% of patients treated with anthracyclines received Doxorubicin. All subjects were examined and monitored at our department between the years 2016 and 2020. Only patients that after a thorough examination including transthoracic echocardiography (TTE) and CMR methods showed no signs of systolic or diastolic function impairment or another cardiac pathology were enrolled.
Inclusion criteria required a CMR examination with contrast methods and stress perfusion and TTE without signs of pulmonary hypertension (PH) within 30 days of CMR. Stress perfusions were indicated to exclude coronary artery disease, since it was already detected in some patients treated with anthracyclines or alkylating agents11,12 and were performed as part of the study.
Patients in the HTx group were divided into subgroups based on the presence of diastolic dysfunction signs from TTE.
Transthoracic echocardiography
All patients underwent the standard TTE exam, and all examinations were performed by experienced cardiologists in our center and according to the established guidelines.
Signs of PH were defined in accordance with 2015 guidelines13 as estimated pulmonary systolic artery pressure above 40 mmHg, calculated from tricuspid regurgitation jet velocity. The left ventricular diastolic dysfunction was defined as the presence of a grade II or higher diastolic dysfunction assessed by Doppler echocardiography of transmitral velocities of transmitral flow and tissue Doppler imaging of mitral annular velocity.
CMR protocol
The CMR studies were performed similarly as in the case of previous articles from our workplace14—with the standard protocol using 1.5 T scanners (Ingenia, Philips Medical Systems, Best, The Netherlands) equipped with 5- and 32-element phased-array receiver coils allowing for the use of parallel acquisition techniques in the supine position in repeated breath-hold. Functional imaging using balanced steady-state free precession (SSFP, b-TFE) cine sequences included four-chamber, two-chamber and left ventricular outflow tract (LVOT) long-axis views, and a short-axis (SAX) stack from the cardiac base to the apex in the plane perpendicular to the LV long axis. Wall motion abnormalities were visually assessed. LV functional and morphological parameters were calculated from the SAX stack using the summation-of-disc methods following the recommendations on post-processing evaluation from the Society for Cardiovascular Magnetic Resonance15.
Late gadolinium enhancement (LGE) images in all long-axis views and the SAX stack were acquired 10 min after an intravenous bolus of a total 0.2 mmol/kg (stress and rest dual-boluses for perfusion and the bolus of remaining contrast injected after the rest perfusion) of the gadolinium-based contrast agent gadobutrol (Gadovist, Bayer-Schering Pharma, Germany) using inversion-recovery turbo field echo sequence (IR-TFE) and, in case of doubt, also by phase-sensitive inversion recovery (PSIR) TFE. Both 2-dimensional and 3-dimensional data acquisitions were performed. LGE was defined as an area of visually identified contrast enhancement higher than the mean signal intensity of an adjacent area of the reference myocardium.
CMR stress perfusion
CMR first‐pass contrast‐enhanced myocardial perfusion images were acquired by a b-TFE sequence in three SAX sections (basilar, midventricular, and apical) with these parameters: field of view 300 × 300 mm, reconstruction matrix 224, slice thickness 10 mm, acquisition voxel size 2.5 × 2.5 mm, time to repetition (TR) ≈ 2.2 ms, echo time (TE) ≈ 1.1 ms, flip angle 50°, SENSE factor 2.3, number of dynamics = 90, non-shared saturation prepulse. Stress perfusion was acquired after a 4 min adenosine infusion at 140 μg/kg/min. Rest perfusion was acquired 10 min after the stress perfusion with the same parameters. MR perfusion was performed during a dual-bolus administration of gadolinium-based contrast agent gadobutrol. The dual-bolus protocol was adapted from Ishida et al.16. A volume and rate of 0.005 mmol/kg and 4 ml/s for the pre-bolus and 0.05 mmol/kg and 4 ml/s for the main-bolus were used. Stress and rest volumes and rates were matched.
Pulmonary circulation biomarkers analyses
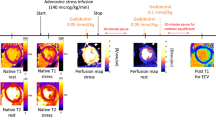
The PTT values were estimated from SAX rest and stress first-pass perfusion images, using an in-house developed algorithm implemented in MATLAB® 9.8 (R2020a) (The Mathworks Inc., Natick, MA, USA). The images were acquired without breath-hold. The analysis starts with applying a motion correction algorithm to optimize imaging registration and avoid potential contamination from pixels in the blood pool. The algorithm initially performs a rigid transformation to improve the misalignment present in the images and ease the following registration steps. Subsequently, an affine transformation, optimized according to the One Plus One Evolutionary strategy17, is applied. The method generates a modification in the image using a current transformation (parent) and a newer one (descendant). Then, it estimates the similarity value, and according to it, keeps the parent or the descendant for a new evolutionary step. Finally, the algorithm uses mutual information as a similarity criterion18. The registration accuracy is visually assessed. Regions of interest (ROIs) in the right ventricle (RV) and the LV are manually traced in both the rest and stress corrected images for the mid-ventricular slice and, in case of repetitive misregistration, in the basal one. The ROIs automatically propagate throughout the stack of images, their average is computed in each one, and signal intensity (SI) curves vs time are obtained. The algorithm recognizes the maximum SI value in each case and determines a threshold (5–10% of the maximum) for establishing the onset SI. The measurement was done considering the main bolus of the dual bolus technique and confirmed with the pre-bolus. PTT was defined as the difference between the onset time in the LV and the RV, and PTB is the number of frames between these times (see Fig. 1). The PBVI, in ml/m2, was calculated as the product of the PTB and the RV stroke volume (RV SV), divided by the body surface area8.
Schematics of the calculation of pulmonary transit time (PTT). (A) Region of interests manually traced in the right ventricle (RV, blue) and the left ventricle (LV, red). (B) Simulated data of the signal intensity (SI) vs time for the RV and LV. The maxima of the SI are marked. The PTT is the difference between the onsets, determined as the signal at 10% of the maximum values. (C) Example of the detection of the onset points in the corrected images. Only a fraction of the images is shown.
The pulmonary transit time ratio (PTT ratio) and pulmonary transit time ratio with heart rate (HR) correction (PTTc ratio) were calculated.
Where applicable, Bazett's formula (19), commonly used to standardize the QT interval across different heart rates, was used to correct the values for HR as previously reported by Ricci et al.7. The formula considers a PTT or PTT_S at a given heart rate and normalizes it by the square root of the corresponding RR interval per second, i.e., ⎷(RR interval/1 s)19. Therefore, the corrected parameters are PTTc (s) = PTT (s)/⎷(RR interval/1 s) and PTTc_S (s) = PTT_S (s)/⎷ (RR interval/1 s). Ratio parameters (PTT ratio and PTTc ratio) were calculated as stress parameters divided by rest parameters; therefore, PTT ratio = PTT_S/ PTT and PTTc ratio = PTTc_S/ PTTc.
Statistical analysis
The categorical data were compared using the χ2 test. In case the null hypothesis was refuted, a series of three Fisher exact tests followed by Bonferroni correction was employed. The continuous data generally followed the Gaussian distribution, as assessed by the Kolmogorov–Smirnov test and visual inspection of histograms. In the case of R-R intervals, square root transformation was applied. One-way ANOVA was used to compare the groups, followed by Tukey Post-hoc test for unequal N. Discrete data (PTB, PTB_S) were assessed using the Kruskal–Wallis test and the Dunn Post-hoc test. In all cases, α = 0.05 was used to define a statistically significant result.
Results
Patient’s population
Basic clinical, TTE and CMR group characteristics are summarized in Table 2.
In the CCS group, no patient showed signs of diastolic dysfunction. The most common indications for treatment were Hodgkin lymphoma (11 patients, 28%) followed by Non-Hodgkin lymphoma (9 patients, 23%) and acute lymphocytic leukaemia (7 patients, 18%).
In the HTx group, seven patients showed grade II or higher diastolic dysfunction. The HTx population was divided based on the presence of diastolic dysfunction as a subgroup without (HTx_A) and another with (HTx_B).
The most common indication for HTx was dilated cardiomyopathy (30 patients, 63%), followed by ischemic heart disease (7 patients, 15%).
Since the number of patients included in HTx_B subgroup was relatively low, we also compared the whole HTx group with the CCS.
Pulmonary circulation biomarkers
The retrospective analysis of biomarkers was successful in all cases, and no adverse effects of adenosine application were observed.The CCS patients showed biomarker values as follows: PTT 4.96 ± 0.93 s, PTB 5.82 ± 0.91, PTT_S 3.76 ± 0.78 s, for the HTx group: PTT 5.59 ± 1.14 s, PTB 7.40 ± 1.65, PTT_S 4.92 ± 1.13 s, for the HTx_A population: PTT 5.51 ± 1.14 s, PTB 7.22 ± 1.62, PTT_S 4.82 ± 1.03 s and lastly for HTx_B population: PTT 6.04 ± 1.13 s, PTB 8.43 ± 1.51 and PTT_S 5.52 ± 1.56 s. Both PTTc and PTB in the CCS group were significantly lower than in the HTx and both HTx_A and the HTx_B subgroups. PTT showed significantly lower values only in the case of HTx and CCS group, when comparing to subgroups lower values without statistical significance were shown. In the case of stress parameters, both PTT_S and PTTc_S were significantly lower in the CCS group than in the HTx groups. The comparison between HTx_A and HTx_B shows trend toward lower values in the first group but without statistical significance. The PTT ratio was significantly lower in the CCS than in the HTx group and HTx_A subgroup.
In addition, PTT-derived parameters did not significantly correlate with cardiac output (CO) at rest, but correlated with the stress CO. There was a difference between the CCS and the HTx group and HTx_A subgroup in the values of stress CO. There was no difference in the resting values.
The complete results are summarized in Table 3. Boxplots for rest and stress transition times and derived ratio parameters are shown in Fig. 2.
Pulmonary transit time parameters including stress and ratio biomarkers. The figure shows the comparison of pulmonary transit time (PTT) (s), stress pulmonary transit time (PTT_S) (s), pulmonary transit time ratio (PTT ratio) and heart rate corrected derived parameters (PTTc (s), PTTc_S (s) and PTTc ratio). Statistical analysis followed the corresponding methods section, α = 0.05 defined a statistically significant result. The average PTT in the childhood cancer survivors (CCS) group was not significantly lower than in the heart transplant without diastolic dysfunction subgroup (HTx_A) or heart transplant with diastolic dysfunction subgroup (HTx_B). PTTc, on the other hand, was significantly lower in both cases. Both PTT_S and PTTc_S were significantly lower in the CCS group than in the HTx subgroups. Stress transition times were shorter than rest times, and therefore ratio parameters were below 1 in all groups.
Rest parameters
The average PTT in the CCS group was not significantly lower neither in the HTx_A (4.96 ± 0.93 s vs. 5.51 ± 1.14 s, p = 0.063) nor the HTx_B group (4.96 ± 0.93 s vs. 6.04 ± 1.13 s, p = 0.13). Although values were lower in the HTx_A subgroup than the HTx_B, the differences did not meet conventional levels of statistical significance.
PTTc, on the other hand, was significantly lower in both cases (5.45 ± 0.87 s (CCS) vs. 6.41 ± 1.3 s, p < 0.001 (HTx_A) and vs. 7.15 ± 1.2 s, p = 0.015 (HTx_B) and the same applies for PTB (5.82 ± 0.91 (CCS) vs. 7.22 ± 1.62, p < 0.001 (HTx_A) and vs. 8.43 ± 1.51, p < 0.001 (HTx_B)). The comparison between HTx_A and HTx_B shows lower values in the first group but without statistical significance. There were no differences in PBVI values.
When comparing CCS and the HTx group as whole, the results copy those of comparing CCS and HTx_A subgroup with 1 exception—PTT was significantly prolonged in the HTx group.
Stress parameters
Both PTT_S and PTTc_S were significantly lower in the CCS group than in the HTx groups: PTT_S 3.76 ± 0.78 s (CCS) vs. 4.82 ± 1.03 s, p < 0.001 (HTx_A) and vs. 5.52 ± 1.56 s, p = 0.003 (HTx_B) and PTTc_S: 4.89 ± 0.89 s (CCS) vs. 5.9 ± 1.14, p < 0.001 (HTx_A) and vs. 6.71 ± 1.63, p = 0.006 (HTx_B).
PTB_S showed lower values in the CCS group, but without statistical significance (6.46 ± 1.07 (CCS) vs. 6.85 ± 1.51, p = 0.78 (HTx_A) and vs. 7.86 ± 2.04, p = 0.19 (HTx_B). Similarly to the rest parameters, the comparison between HTx_A and HTx_B shows lower parameters in the first group, but without statistical significance.
Comparison of CCS and HTx group shows the same results as in the HTx_A group, both PTT_S and PTTc_S were significantly lower in the CCS group, PTB_S showed lower values without statistical significance.
Ratio parameters
The PTT ratio was significantly lower in the CCS than in the HTx_A subgroup (0.77 ± 0.14 vs. 0.88 ± 0.14, p = 0.002) and HTx group as well (0.77 ± 0.14 vs. 0.89 ± 0.14, p < 0.001). Lower parameters without statistical significances were shown when comparing the CCS and the HTx_B groups (0.77 ± 0.14 vs. 0.91 ± 0.14, p = 0.20). Although there were no significant differences between the groups in the case of the PTTc ratio, the PTT ratio and the PTTc ratio were below 1 in all groups, which means PTT_S times were shorter than the rest values.
Regarding the BMI, no statistical significance in any group or parameter was found with only 1 exception—a positive correlation of BMI with PTTc ratio in the CCS group (r = 0.49, p = 0.002). In addition, age did not correlate with PTT-derived parameters in any group (all r values < 0.2, all p values > 0.05).
Discussion
The main goals of this study were to provide more evidence about pulmonary circulation parameters acquired by first-pass perfusion CMR, to investigate stress parameters in the HTx population as a potential marker of diastolic dysfunction and to compare rest and stress parameters and present ratio parameters as potential new biomarkers. The main finding was that stress pulmonary circulation times with and without Bazett’s formula correction were shorter than rest times contrary to the only published article including stress data7. Additionally, more data about pulmonary circulation parameters in new populations and ratio parameters were presented. Since ratio biomarkers are calculated as the ratio of stress and rest values, they describe the heart reaction to adenosine-induced pharmacological stress.
In previously published data7, only HR corrected PTT time was assessed from stress biomarkers and it showed prolongation or no change compared to HR corrected PTT rest time. In the whole study population of 69 HCM patients, the results showed overall prolongation of PTT stress time. When divided into subgroups based on left atrial pressure (LAP), the corrected PTT stress was prolonged in patients with normal LAP, and in increased LAP, the values stayed roughly the same. Therefore, the calculated corrected PTT ratio would have been higher than 1 or close to 1 for this population.
In this study, all presented groups had PTT and PTTc ratios lower than 1, and therefore stress biomarkers were shorter, which is in contrary. One explanation could be based on the diastolic dysfunction in the HCM group. However, this difference is certainly a matter of discussion. Although adenosine indeed induces vasodilation in pulmonary circulation as the authors claim7, its overall effect on the myocardium and circulation itself is still debatable and more complex. The HR increase is well documented20,21, and our data are no exception. However, its effect on contractility is quite controversial. Some studies showed a positive outcome on contractility22 or stated it affects beta-receptors as well23, while others claim it has an indirect effect on contractility but no beta-receptor activity24. Apart from a direct reaction on the myocardium, recent reports focused on its repercussions in the central nervous system (CNS), mainly in the nucleus tractus solitarius but other parts of CNS as well25, and its possible influence on baroreflex via CNS26. However, most of the studies were on rats or mice models or isolated myocardial cells, and for example, the effect on baroreflex could be indirect via hypotension from vasodilatation resulting in increasing HR and contractility of the myocardium. This truly complex problem is quite beyond the scope of this paper to discuss in all the possibilities.
Overall, we presume that in a healthy population, stress biomarkers such as PTT_S should be shorter than their rest analogues, therefore, ratio biomarkers should be less than 1, and they could be potentially used as additional markers of the myocardial reserve and/or diastolic dysfunction. However, further research, including a comprehensive healthy population, is needed.
We hypothesized underlying diastolic function impairment in patients after HTx and the feasibilty of using pulmonary circulation and potentially ratio parameters to assess it. During diastole, the LV receives blood from the left atrium (LA). LV diastolic dysfunction leads to the impairment of its filling function, therefore filling pressure in the LV is increased, which leads to increased pressure in the LA as well. This corresponds with increasing of the pulmonary capillary wedge pressure (PCWP). When PCWP is increased, the whole process of blood circulating through the pulmonary circulation is supposedly impaired as well, resulting in longer PTT times. This mechanism is supposedly more appreciable in stress conditions, which agrees with our results and also possibly explains the difference in stress CO between CCS and HTx_A.
As Meluzin et al. reported10—16% of HTx subjects had signs of diastolic dysfunction in rest conditions (14.6% in this study), but other 58% showed signs of diastolic impairment in stress conditions10. While the difference in PTT was not significant, PTTc, PTT_S and PTTc_S were significantly lower in the CCS group than the HTx_A subgroup, which follows our assumption. In addition, both rest and stress transit times reported by Ricci et al. in HCM patients were higher7. Since LV hypertrophy in HCM patients is most commonly paired with diastolic dysfunction, these results further strengthenour assumptions.
The lower PTT ratio, while constant PTTc ratio between groups, could be influenced by the difference in HR and the results could be affected by the heart denervation in the HTx population, but that would not explain the tendency to higher values in the HTx_B group with diastolic dysfunction as to the HTx_A. Combined with the prolongation of stress parameters, and thus ratio parameters around 1 or more, as reported by Ricci et al. in HCM patients7, this further-more points to diastolic function impairment as the explanation.
However, additional studies with different populations are necessary to confirm these results.
In all cases, no statistically significant differences were found when comparing the HTx_A and the HTx_B groups. However, in cases such as for the PTTc (6.41 ± 1.3 s vs. 7.15 ± 1.2 s, p = 0.44) or PTTc_S (5.9 ± 1.14 s vs. 6.71 ± 1.63 s, p = 0.33), a tendency towards lower values in HTx_A was observed. This is limited by the number of patients included in the HTx_B group. Although this number of patients with diastolic dysfunction included was relatively small, impacting the implications of the results, the proposed method illustrates the feasibility of including pulmonary circulation biomarkers as an additional method to assess diastolic dysfunction.
Since the limited number of subjects in HTx_B subgroup, HTx group as whole was included and compared with CCS group—the results resembled the comparison of CCS and HTx_A group with the excemption of the PTT, which was significantly longer in the HTx group. This result further documents an assumption of the influence of an underlying dysfunction.
It is not completely clear whether to prefer HR-corrected PTT times or not. In Ricci’s study, both rest times and only corrected stress PTT times were reported7, and in Houard et al.6 no HR correction was applied at all. We believe both alternatives are justified. While PTB could be a form of overriding the effect of HR in counting the number of cycles, for the stress parameters and ratios, we think that both possibilities are valid and should be acquired.
It is also possible to evaluate these biomarkers from the peak-to-peak time interval in the signal intensity curve. However, we opted for the onset assessment because the first appearance of the contrast showed good reproducibility.
To our knowledge, only one other study acquiring pulmonary circulation stress biomarkers was published to date, and therefore, with 87 patients included, this is one of the first larger CMR-based studies involving stress circulation parameters. However, more studies have to be made to confirm our results, including different groups with reduced LVEF, other diagnoses, and an aged-match healthy control group, although applying adenosine to a healthy control group could prove ethically problematic. On the other hand, when using the presented or an earlier developed method, a center with a relatively high number of stress perfusions could comfortably conduct other studies retrospectively to gather more data about stress circulation biomarkers. As far as we know, these parameters have never been studied before in CCS or HTx patient groups.
Study limitations
We acknowledge there are limitations to this study. It was a single-center retrospective study, but arguably the principal constrain is the participant’s selection: CCS and HTx. CCS cannot be reffered to as a healthy control group. Although in thorough examinations no signs of systolic or diastolic function impairment, other pathological results or any other cardiac function impairment were observed, patients included underwent potentially cardiotoxic chemotherapy. Their comparability is further limited by basic clinical parameters, mainly age and BMI. However, in neither group the age correlated with pulmonary circulation biomarkers, and the BMI correlated only with the PTTc ratio in the CCS group. Also, selecting patients after HTx could lead to bias with the adenosine effect on a denervated heart. Data on using adenosine or regadenoson on subjects after HTx is limited. In older studies, a hypersensitivity was described27, but it seems safe to administer it28. A newer study from Spain showed its use in the case of stress perfusion as well, although HTx patients presented a lower myocardial perfusion reserve index in comparison to healthy controls29. Furthermore, although the number of participants with diastolic dysfunction (HTx_B group) was low, impacting the implications of the results, the proposed method illustrates the feasibility of including pulmonary circulation biomarkers as a potential additional approach to assess diastolic dysfunction.
Ethics approval and consent to participate
This retrospective study was performed in accordance with the Declaration of Helsinki (2000) of the World Medical Association. The Ethics Committee of the Faculty of Medicine, Masaryk University, confrmed under reference number 02082021, that this study has been performed on data of patients already participating in other research studies with signed Informed Consent about using their data anonymously for research purposes and therefore according to the Czech legislation, no specific new approval of the ethics committee was required. Only participants who signed written Informed Consent about using their data anonymously for research purposes were enrolled in the study. No patient from potentially vulnerable group was enrolled in the study.
Conclusions
The study provided more evidence about pulmonary circulation parameters acquired by first-pass perfusion CMR, investigated stress parameters in an HTx population as a potential marker of diastolic dysfunction and presented ratio parameters as potential new biomarkers.
The results showed lower values of PTTc, PTT_S and PTTc_S in the CCS group, corresponding with the presumption of underlying diastolic dysfunction in HTx patients. As assumed, contrary to the data published for HCM patients7, both PTT_S and PTTc_S times were shorter than their rest analogues in all groups, and therefore the PTT ratio and the PTTc ratio were less than 1.
Data availability
The datasets analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- b-TFE:
-
Balanced turbo field echo
- CCS:
-
Childhood cancer survivors
- CMR:
-
Cardiovascular magnetic resonance
- CNS:
-
Central nervous system
- CO:
-
Cardiac output
- DD:
-
Diastolic diameter
- DD LV:
-
Left ventricular diastolic diameter
- HCM:
-
Hypertrophic cardiomyopathy
- HR:
-
Heart rate
- HTx:
-
Heart transplant
- HTx_A:
-
Heart transplant without diastolic dysfunction subgroup
- HTx_B:
-
Heart transplant with diastolic dysfunction subgroup
- IR-TFE:
-
Inversion-recovery turbo field echo
- LA:
-
Left atrium
- LAP:
-
Left atrial pressure
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- LV LGE:
-
Left ventricular late gadolinium enhancement
- LVOT:
-
Left ventricular outflow tract
- LV SV:
-
Left ventricular stroke volume
- MRI:
-
Magnetic resonance imaging
- PBVI:
-
Pulmonary blood volume index
- PCWP:
-
Pulmonary capillary wedge pressure
- PH:
-
Pulmonary hypertension
- PSIR:
-
Phase-sensitive inversion recovery
- PTB:
-
Pulmonary transit beats
- PTB_S:
-
Stress pulmonary transit beats
- PTT:
-
Pulmonary transit time
- PTTc:
-
Bazett's formula corrected pulmonary transit time
- PTT_S:
-
Stress pulmonary transit time
- PTTc_S:
-
Bazett's formula corrected stress pulmonary transit time
- PTT ratio:
-
Pulmonary transit time ratio
- PTTc ratio:
-
Bazett's formula corrected pulmonary transit time ratio
- ROIs:
-
Regions of interest
- RV:
-
Right ventricle
- RV SV:
-
Right ventricular systolic volume
- SAX:
-
Short-axis
- SI:
-
Signal intensity
- sPAP:
-
Systolic pulmonary artery pressure
- SSFP:
-
Steady-state free precession
- TE:
-
Echo time
- TR:
-
Repetition time
- TTE:
-
Transthoracic echocardiography
References
Slutsky, R. A., Bhargava, V. & Higgins, C. B. Pulmonary circulation time: Comparison of mean, median, peak, and onset (appearance) values using indocyanine green and first-transit radionuclide techniques. Am. Heart J. 106(1 Pt 1), 41–45 (1983).
de Lepper, A. G. W. et al. Noninvasive pulmonary transit time: A new parameter for general cardiac performance. Echocardiography 34(8), 1138–1145 (2017).
Herold, I. H. F. et al. Pulmonary blood volume measured by contrast enhanced ultrasound: A comparison with transpulmonary thermodilution. Br. J. Anaesth. 115(1), 53–60 (2015).
Colin, G. C. et al. Pulmonary hypertension detection by computed tomography pulmonary transit time in heart failure with reduced ejection fraction. Eur. Heart J. Cardiovasc. Imaging 21(11), 1291–1298 (2020).
Shors, S. M. et al. Heart failure: Evaluation of cardiopulmonary transit times with time-resolved MR angiography. Radiology 229(3), 743–748 (2003).
Houard, L. et al. Prognostic value of pulmonary transit time by cardiac magnetic resonance on mortality and heart failure hospitalization in patients with advanced heart failure and reduced ejection fraction. Circ. Cardiovasc. Imaging 14(1), e011680 (2021).
Ricci, F. et al. Pulmonary blood volume index as a quantitative biomarker of haemodynamic congestion in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 20(12), 1368–1376 (2019).
Ricci, F. et al. Prognostic value of pulmonary blood volume by first-pass contrast-enhanced CMR in heart failure outpatients: The PROVE-HF study. Eur. Heart J. Cardiovasc. Imaging 19(8), 896–904 (2018).
Cao, J. J., Li, L., McLaughlin, J. & Passick, M. Prolonged central circulation transit time in patients with HFpEF and HFrEF by magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 19(3), 339–346 (2018).
Meluzin, J. et al. High prevalence of exercise-induced heart failure with normal ejection fraction in post-heart transplant patients. Biomed. Pap. 158(2), 295–302 (2014).
Mulrooney, D. A. et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 339, b4606 (2009).
Yeh, E. T. H. et al. Cardiovascular complications of cancer therapy. Circulation 109(25), 3122–3131 (2004).
2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension | European Heart Journal | Oxford Academic [Internet]. https://academic.oup.com/eurheartj/article/37/1/67/2887599. Accessed 28 Mar 2021.
Panovský, R. et al. Cardiac profile of the Czech population of Duchenne muscular dystrophy patients: A cardiovascular magnetic resonance study with T1 mapping. Orphanet J. Rare Dis. 14(1), 10 (2019).
Schulz-Menger, J. et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J. Cardiovasc. Magn. Reson. 1(15), 35 (2013).
Ishida, M. et al. Development of a universal dual-bolus injection scheme for the quantitative assessment of myocardial perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 13(1), 28 (2011).
Styner, M., Brechbuhler, C., Szckely, G. & Gerig, G. Parametric estimate of intensity inhomogeneities applied to MRI. IEEE Trans. Med. Imaging 19(3), 153–165 (2000).
Pluim, J. P. W., Maintz, J. B. A. & Viergever, M. A. Mutual-information-based registration of medical images: A survey. IEEE Trans. Med. Imaging 22(8), 986–1004 (2003).
Bazett, H. C. An analysis of the time-relations of electrocardiogram. Heart 7, 353 (1920).
Wilson, R. F., Wyche, K., Christensen, B. V., Zimmer, S. & Laxson, D. D. Effects of adenosine on human coronary arterial circulation. Circulation 82(5), 1595–1606 (1990).
Salerno, M. et al. Adenosine stress cardiovascular magnetic resonance with variable-density spiral pulse sequences accurately detects coronary artery disease. Circ. Cardiovasc. Imaging 7(4), 639–646 (2014).
Monahan, T. S., Sawmiller, D. R., Fenton, R. A. & Dobson, J. G. Adenosine A2a-receptor activation increases contractility in isolated perfused hearts. Am. J. Physiol. Heart Circ. Physiol. 279(4), H1472–H1481 (2000).
Hori, M. & Kitakaze, M. Adenosine, the heart, and coronary circulation. Hypertension 18(5), 565–574 (1991).
Chandrasekera, P. C., McIntosh, V. J., Cao, F. X. & Lasley, R. D. Differential effects of adenosine A2a and A2b receptors on cardiac contractility. Am. J. Physiol. Heart Circ. Physiol. 299(6), H2082–H2089 (2010).
Costa, M. A., Matsumoto, J. P. P., Carrettiero, D. C. & Fior-Chadi, D. R. Adenosine A1 and A2a receptors modulate the nitrergic system in cell culture from dorsomedial medulla oblongata. Auton. Neurosci. 229, 102737 (2020).
Tian, L. et al. Blockade of adenosine A1 receptor in nucleus tractus solitarius attenuates baroreflex sensitivity response to dexmedetomidine in rats. Brain Res. 1743, 146949 (2020).
Ellenbogen, K. A., Thames, M. D., DiMarco, J. P., Sheehan, H. & Lerman, B. B. Electrophysiological effects of adenosine in the transplanted human heart. Evidence of supersensitivity. Circulation 81(3), 821–828 (1990).
Flyer, J. N. et al. Prospective study of adenosine on atrioventricular nodal conduction in pediatric and young adult patients after heart transplantation. Circulation 135(25), 2485–2493 (2017).
Jiménez-Jaso, J. M. et al. Valoración del índice de reserva de perfusión miocárdica por resonancia magnética en pacientes con trasplante cardíaco. Radiologia 62(6), 493–501 (2020).
Funding
The work was supported by the European Regional Development Fund—project ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868) and by the project "New trends in diagnostics and therapy of cardiomyopathies" number MUNI/A/1685/2020 with the support of the Specific University Research Grant, as provided by the Ministry of Education, Youth and Sports of the Czech Republic in the year 2021.
Author information
Authors and Affiliations
Contributions
L.O., R.P., J.K. and L.S. were the main designers of this study. L.O. was the major contributor in writing the manuscript. J.M. performed the statistical analysis. L.M., J.G., T.K. and V.K. contributed to patient recruitment, inclusion and revised the article critically. V.F. and T.H. performed CMR examinations and assisted with CMR data analysis. M.M.P. coded the algorithm for the CMR images processing and M.M.P. with G.Z. performed the data analysis. All authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Opatřil, L., Panovsky, R., Mojica-Pisciotti, M. et al. Stress pulmonary circulation parameters assessed by a cardiovascular magnetic resonance in patients after a heart transplant. Sci Rep 12, 6130 (2022). https://doi.org/10.1038/s41598-022-09739-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09739-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.