Abstract
In our search for novel small molecules activating procaspase-3, we have designed and synthesized two series of novel (E)-N'-arylidene-2-(2-oxoindolin-1-yl)acetohydrazides (4) and (Z)-2-(5-substituted-2-oxoindolin-1-yl)-N'-(2-oxoindolin-3-ylidene)acetohydrazides (5). Cytotoxic evaluation revealed that the compounds showed notable cytotoxicity toward three human cancer cell lines: colon cancer SW620, prostate cancer PC-3, and lung cancer NCI-H23. Especially, six compounds, including 4f–h and 4n–p, exhibited cytotoxicity equal or superior to positive control PAC-1, the first procaspase-3 activating compound. The most potent compound 4o was three- to five-fold more cytotoxic than PAC-1 in three cancer cell lines tested. Analysis of compounds effects on cell cycle and apoptosis demonstrated that the representative compounds 4f, 4h, 4n, 4o and 4p (especially 4o) accumulated U937 cells in S phase and substantially induced late cellular apoptosis. The results show that compound 4o would serve as a template for further design and development of novel anticancer agents.
Similar content being viewed by others
Introduction
Normal cells in human body divide and die in a tightly regulated manner. Cell cycle and apoptosis are two processes linked to normal cellular growth and death. In case abnormality occurs, the cells keep dividing and are able to escape apoptosis, leading to the formation of extra mass tissue in the body, known as tumors. Malignant tumors, or cancer, remains one of the deadliest diseases nowadays since the cancer cells are able to spread throughout the body and metastasize to other organs, making the treatment extremely difficult1. Over decades, targeting cell cycle and apoptosis, especially apoptosis or programmed cell death process, are among the most common and effective approaches for anticancer drug development2,3.
With advances in molecular cell biology, many proteins involved in cellular apoptotic pathways, e.g. BIM, BAX, Bcl-2, p53, RIP, DED, Apo2L, and XIAP, to name a few, have been identified and employed as molecular targets for anticancer therapy3. As a result, a number of small molecules targeting these proteins have been discovered. For example, GDC-0152 (a XIAP’s inhibitor), tenovin-1 (a p53 activator), or ABT-199 (an inhibitor of Bcl-2) have been demonstrated to effectively induce apoptosis and ultimately caused the death of cancer cells4,5,6.
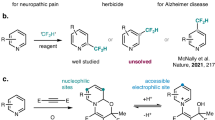
Also played important roles in regulation of apoptotic pathways are caspases7,8. Currently, caspases, with at least fourteen members, are a large group of of cysteine proteases enzymes7,8. These enzymes are involved in both extrinsic and intrinsic pathways of the apoptotic machine7,8. Among these, caspase-3, known as the executioner caspase, is one of the key enzymes regulating apoptosis responses7,8. Caspase-3 exists as a low activity zymogen in cells, known as procaspase-37,8, which has been found to be overexpressed in many types of human cancers9 (e.g. neuroblastoma10, breast cancer11, lung carcinoma12, hepatocellular carcinoma13, lymphoma and Hodgkin's Disease14). Due to their overexpression in cancer cells, it is well established that targeting caspases would be more advantageous over inhibiting other apoptotic proteins9. Great efforts of medicinal chemists have therefore placed on the development of novel caspase activators. Consequently, several small molecules as caspase activators have been reported15,16,17,18. In 2016, PAC-1, the first procaspase activating compound (Fig. 1), was granted as Orphan Drug designation for treatment of blioblastoma by the U.S. FDA. Analysis of structure–activity relationships of PAC-1 clearly indicates the importance of the ortho-hydroxy-N-acylhydrazone moiety (B-region, Fig. 1) in the interaction with zinc ion in the active binding site of caspases to form a strong complex structure17,18. Based on that feature, we recently reported several series of 4-oxoquinazoline-based acetohydrazides (I, II) which incorporated the N-acylhydrazone functionality and found many compounds with potent procaspase-3 activating activity as well as strong antitumor cytotoxicity19,20. Encouraged by these results, in this investigation we expand our design to compounds series III and IV bearing 2-oxoindoline ring. The 2-oxoindoline is an important scaffold with diverse biological potentials21. This paper describes the results obtained from synthesis, bioevaluation of these novel compounds.
Materials and methods
Chemistry
The reagents, solvents used in this work were purchased from commercially available vendors (mainly, Aldrich, Fluka Chemical Corp. (Milwaukee, WI, USA), or Merck) and used directly unless otherwise indicated. Thin layer chromatography (TLC) was performed in Whatman Silica Gel GF250. The TLC plate was visualized using 254 nm UV light. Gallenkamp (LabMerchant, UK) melting Point Apparatus was used for recording melting points of the compounds and are uncorrected. Re-crystallization in solvents or column chromatography on silica gel was used for purification of final compounds. Merck (silica gel 240 to 400 mesh) was used as stationary phase in column flash chromatography. 1H NMR were analyzed on a 500 MHz spectrometer (Bruker). DMSO-d6 was used as NMR solvent unless otherwise indicated. Chemical shifts are reported ppm. Mass spectra of the compounds were performed in PE Biosystems API2000 (electron ionization (EI), Perkin Elmer-USA) and Mariner (Electrospray ionization (ESI), Azco Biotech-USA) mass spectrometers, respectively. The elemental analyses (C, H, N) of the final compounds were recored on a Perkin Elmer elemental analyzer (model 2400).
Cytotoxicity assay
Three human cancer cell lines: colon cancer (SW620), prostate cancer (PC3), and lung cancer (NCI-H23) were used for sceening the cytotoxicity of the compounds. The cancer cells were purchased from American Type Culture Collection (Manassas, VA, USA). Other reagents/media for cell culture were obtained from GIBCO (Grand Island, New York, USA). The testing cancer cells were culture in Dulbecco’s Modified Eagle Medium until confluence. Then, they were trypsinized and suspended at the level of 3 × 104 cells/mL of cell culture medium. On day 0, cancer cells were seeded at a volume of 180 µL/well of 96-well plates and incubated for 24 h at 37 °C in a 5% CO2 incubator. On day 1, 20 µL of various concentrations of testing compounds were added to each well of the 96-well plates. Chemicals were dissolved in dimethyl sulfoxide (DMSO, stock) and diluted in culture medium (1% DMSO) before adding to the culture. After 48 h incubation, the sulforhodamine B assay was used for cell density determination with slight modifications22,23,24,25,26. The Probits were used for the calculation of IC50 values. The reported IC50 were the averages of three independent screening (SD ≤ 10%)27.
Cell cycle analysis
U937 human lymphoma cells (5 × 105/mL per well) were plated in 6-well culture plates and allowed to grow for 24 h. In our first experiment, we examined the effects of 4f, 4h, 4n, 4o, 4p, and PAC-1 on cell cycles at 50 µM. In the second experiment, we examined the dose-dependent effect of 4o at 5, 10, and 30 µM and PAC-1 at 30 µM on cell cycles. The cells were treated with compounds for 24 h, and then harvested. The harvested cells were washed twice with ice-cold PBS, fixed in 75% ice-cold ethanol, and stained with propidium iodide (PI) in the presence of RNase at room temperature for 30 min. The stained cells were analyzed for DNA content using a FACScalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and the data were processed using Cell Quest Pro software (BD Biosciences).
Apoptosis assay
The Annexin V-FITC/PI dual staining assay was used to determine the percentage of apoptotic cells. U937 cells (5 × 105/mL per well) were plated in 6-well culture plates and allowed to grow for 24 h. In our first experiment, we examined the effects of 4f, 4h, 4n, 4o, 4p, and PAC-1 on apoptosis at 50 µM. In the second experiment, we examined the dose-dependent effect of 4o at 5, 10, and 30 µM and PAC-1 at 30 µM on apoptosis. The cells were treated with compounds for 24 h, and then harvested. The harvested cells were washed twice with ice-cold PBS and incubated in the dark at room temperature in 100 mL of 1 × binding buffer containing 1 µL Annexin V-FITC and 12.5 mL PI. After 15 min incubation, cells were analyzed for percentage undergoing apoptosis using a FACScalibur flow cytometer (BD Biosciences). The data were processed using Cell Quest Pro software (BD Biosciences).
Caspase-3 activation assay
Caspase activity was measured by using caspase 3 assay kit according to the manufacturer’s instructions (abcam, MA, USA). U937 human lymphoma cells (5 × 105/mL per well) were plated in 6-well culture plates and allowed to grow for 24 h. The cells were treated with compounds for 24 h, and then harvested. The harvested cells were washed twice with ice-cold PBS and treated with lysis buffer included in the kit. Cell lysate (100 µg/50 µL) was mixed with 50 µL of 2 × reaction buffer and 5 µL of DEVD-p-NA substrate as the instruction of caspase-3 assay kit (Abcam, cat. N. ab39401). Fluorescence was measured after one-hour incubation.
Results and discussion
Chemistry
Figure 2 illustrates the synthesis of the target 2-oxoindoline-based acetohydrazides (4, 5). The synthesis of final compounds proceeded via three steps. The first step was a nucleophilic substitution between 2-oxoindoline derivatives and ethyl chloroacetate with the presence of potassium carbonate with a catalytic amount of KI in acetone to afford the selectively N-alkylated intermediate esters (2). The yields of this step were generally excellent (90–93%). The second step was acyl transfer reaction of the esters 2a–b with hydrazine monohydrate afforded the hydrazides 3a–b. This reaction occurred under refluxing conditions, and ethanol was found as an effective solvent for this reaction. With hydrazids 3a–b in hand, the desired products 4a–p, 5a–f were obtanined in moderate overall yields via aldol condensation of 3a–b with benzaldehydes or isatins (Fig. 2).
The identification of the structures of the 4, 5 were performed using the analysis of IR, MS, 1H NMR and 13C NMR. The most important peak was the singlet at around 4.4–5.3 ppm of 1H NMR spectra which attributable for two protons occurred of the methylene protons of N-alkylated compounds. Please see Supporting Information for copy of 1H NMR and 13C NMR spectra. The configuration of the compound was well established previously28,29,30,31.
The preparation of the acetohydrazides incorporating 2-oxoindoline (4, 5) was described as follow: K2CO3 (206.9 mg, 1.5 mmol) were added to a solution of 5-substituted-2-oxoindoline (1 mmol) in 50 mL acetone. The mixtures were then refluxed for 30 min. A catalytic amount of KI (16.6 mg, 0.1 mmol) was then added to the mixture. The whole mixture was stirred for additional 15 min followed by dropwise of 0.13 mL of ethyl chloroacetate (1.2 mmol). Finally, the mixture was heated to 60 °C for additional of 3 h. After reaction completion showed by TLC, the reaction solvents were evaporated. The residues were then re-dissolved in DCM (50 mL), filtered and evaporatred the solvent under reduced pressure to afford the intermediate ester derivatives 2. The compounds were used for the next step without additional purification.
Each of the intermediate esters 2 (0.5 mmol) was dissolved in ethanol (10 mL). Then, hydrazin hydrate 80% (0.12 mL) was added dropwise. The reaction mixture was stirred at 25 °C until all starting material consumed. The obtained white precipitates solid were filtered, washed with 3 × 20 mL of cold-ethanol. The solid turned yellow as pure 3a–b were dried and used to the next step without additional purification.
Absolute ethanol (20 mL) was added to dissolve the acetohydrazides 3a–b (0.5 mmol), followed by 2 drops of glacial acetic acid, benzaldehyde or isatine derivatives (1.0 mmol) were added. The mixtures were refluxed until the reaction completed by TLC (4–6 h). The resulting precipitate was filtered and washed with ethanol (3 times). The obtained yellow solid residuces were dried under reduced pressure. The residuces were purified using either re-crystalised in ethanol, or column chromatography (MeOH:DCM) to obtain the desired product 4a–p, 5a–f.
(E)-N'-Benzylidene-2-(2-oxoindolin-1-yl)acetohydrazide (4a)
Yellow solid; Yield: 55%. mp: 167–168 °C. Rf = 0.51 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3186 (NH); 3059 (CH aren); 2968, 2851 (CH, CH2); 1721, 1678 (C=O); 1614 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.72, 11.70 tautomeric NH (keto-enol), exchangeable by D2O (s, 1H, CONH); 8.25, 8.06 (s, 1H, CH=N); 7.75, 7.71 (dd, J = 7.75 Hz, J′ = 1.75 Hz, 2H, H-2′, H-6′); 7.48–7.44 (m, 3H, H-3′, H-4′, H-5′); 7.29 (d, J = 7.00 Hz, 1H, H-7); 7.23 (t, J = 7.50 Hz, 1H, H-5); 7.02 (t, J = 7.50 Hz, 1H, H-6); 6.95 (d, J = 8.00 Hz, 1H, H-4); 4.89, 4.47 (s, 1.50H, 0.50H, CH2-CONH); 3.64 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.20, 168.50, 163.78, 147.76, 145.18, 144.49, 134.54, 134.47, 130.63, 130.74, 129.30, 127.91, 127.59, 127.43, 125.02, 124.90, 124.73, 124.64, 122.41, 122.23, 109.23, 109.12, 41.29, 35.49. HR-MS (ESI) m/z: 294.1225 [M + H]+. HR-MS (ESI) m/z calculated for C17H16N3O2 [M + H]+ 294.1243. Found 294.1225.
(E)-N'-(2-Chlorobenzylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (4b)
Yellow solid; Yield: 60%. mp: 179–181 °C. Rf = 0.53 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3179 (NH); 3069 (aromatic CH, & CH2 aliphatic); 2984, 2945 (CH, CH2); 1717, 1680 (C=O); 1613 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.95, 11.85 (s, 1H, CONH); 8.64, 8.44 (s, 1H, CH=N); 8.07, 7.95 (dd, J = 7.50 Hz, J′ = 2.00 Hz, 1H, H-6′); 7.56–7.42 (m, 3H, H-3′, H-4′, H-5′); 7.30 (d, J = 7.00 Hz, 1H, H-7); 7.23 (t, J = 7.25 Hz, 1H, H-5); 7.02 (t, J = 7.75 Hz, 1H, H-6); 6.96 (d, J = 8.00 Hz, 1H, H-4); 4.91, 4.48 (s, 2H, CH2–CONH); 3.64 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.20, 175.12, 168.66, 163.00, 145.12, 144.85, 143.65, 140.53, 133.65, 133.47, 132.09, 131.89, 131.74, 130.39, 128.14, 128.09, 127.95, 127.91, 127.53, 127.38, 125.02, 124.90, 124.76, 124.65, 122.44, 122.25, 109.24, 109.12, 42.08, 41.33, 40.61, 35.49. HR-MS (ESI) m/z: 328.0846 {[M + H]+, 35Cl}; 330.0815 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C17H15ClN3O2 [M + H]+ 328.0853. Found 328.0846.
(E)-N'-(3-Chlorobenzylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (4c)
Yellow solid; Yield: 58%. mp: 181–182 °C. Rf = 0.53 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3065 (CH aren); 2957 (CH, CH2); 1717, 1676 (C=O); 1614 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 12.50, 12.20 (s, 1H, CONH); 8.45 (s, 1H, CH=N); 8.27, 8.20 (s, 1H, H-2′); 8.13–8.10 (m, 1H, H-6′); 7.93–7.89 (m, 2H, H-4′, H-5′); 7.71 (d, J = 7.00 Hz, 1H, H-7); 7.65 (t, J = 7.50 Hz, 1H, H-5); 7.44 (t, J = 7.50 Hz, 1H, H-6), 7.37 (d, J = 8.00 Hz, 1H, H-4), 5.32, 4.90 (s, 1.50H, 0.50H, CH2–CONH); 4.06 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.65, 175.56, 169.14, 164.46, 146.55, 145.58, 143.33, 137.15, 134.61, 131.61, 130.70, 130.55, 128.34, 127.39, 127.03, 126.77, 126.62, 125.33, 125.08, 122.87, 122.68, 109.66, 109.55, 41.81, 35.92. HR-MS (ESI) m/z: 328.0851 {[M + H]+, 35Cl}; 330.0821 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C17H15ClN3O2 [M + H]+ 328.0853. Found 328.0851.
(E)-N'-(4-Chlorobenzylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (4d)
Yellow solid; Yield: 62%. mp: 185–187 °C. Rf = 0.53 (DCM:MeOH=14:1). IR (KBr, cm−1): 3183 (NH); 3061 (CH aren); 2959, 2922, 2851 (CH, CH2); 1711, 1674 (C=O); 1616 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.76 (s, 1H, CONH); 8.24, 8.04 (s, 1H, CH=N); 7.78, 7.73 (d, J = 8.50 Hz, 2H, H-2′, H-6′); 7.53, 7.51 (d, J = 8.50 Hz, 2H, H-3′, H-5′); 7.29 (d, J = 7.00 Hz, 1H, H-7); 7.23 (t, J = 7.50 Hz, 1H, H-5); 7.01 (t, J = 7.25 Hz, 1H, H-6); 6.95 (d, J = 7.50 Hz, 1H, H-4); 4.89, 4.45 (s, 1.50H, 0.50H, CH2–CONH); 3.64 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.19, 175.09, 168.57, 163.88, 146.46, 145.15, 144.87, 143.20, 134.89, 133.51, 133.44, 129.37, 129.23, 129.09, 127.90, 125.01, 124.89, 124.73, 124.64, 122.42, 122.23, 109.23, 109.11, 42.02, 41.31, 35.49. HR-MS (ESI) m/z: 328.0844 {[M + H]+, 35Cl}; 330.0813 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C17H15ClN3O2 [M + H]+ 328.0853. Found 328.0844.
(E)-N'-(4-Methoxybenzylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (4e)
Yellow solid; Yield: 51%. mp: 168–169 °C. Rf = 0.47 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3188 (NH); 3092, 3065 (CH aren); 2970, 2839 (CH, CH2); 1703, 1674 (C=O); 1605 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.57 (s, 1H, CONH); 8.19, 7.99 (s, 1H, CH=N); 7.69, 7.65 (d, J = 9.00 Hz, 2H, H-2′, H-6′); 7.29 (d, J = 7.00 Hz, 1H, H-7); 7.22 (t, J = 7.25 Hz, 1H, H-5); 7.04–7.00 (m, 2H, H-4, H-6); 6.94 (d, J = 7.50 Hz, 2H, H-3′, H-5′); 4.86, 4.44 (s, 1.50H, 0.50H, CH2–CONH); 3.81 (s, 3H, CH3); 3.63 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.18, 175.08, 168.22, 163.48, 161.38, 161.23, 147.65, 145.20, 144.91, 144.36, 129.20, 129.02, 127.90, 127.07, 125.01, 124.89, 124.72, 124.62, 122.39, 122.20, 144.79, 109.23, 109.11, 55.78, 41.00, 41.27, 35.50. HR-MS (ESI) m/z: 324.1342 [M + H]+. HR-MS (ESI) m/z calculated for C18H18N3O3 [M + H]+ 324.1348. Found 324.1342.
(E)-N'-(2-Hydroxybenzylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (4f)
Yellow solid; Yield: 45%. mp: 173–174 °C. Rf = 0.45 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3183 (NH); 3061 (CH aren); 2974, 2914, 2839 (CH, CH2); 1713, 1670 (C=O); 1611 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.90, 11.65 (s, 0.50H, 0.50H, CONH); 10.95, 10.08 (s, 0.50H, 0.50H, OH); 8.46, 8.36 (s, 0.50H, 0.50H, CH=N); 7.77, 7.55 (dd, J = 7.75 Hz, J′ = 1.75 Hz, 0.50H, 0.50H, H-6′); 7.31–7.21 (m, 3H, H-5, H-7, H-4′); 7.05–6.94 (m, 4H, H-4, H-6, H-3′, H-5′); 4.86, 4.49 (s, 1.00H, 1.00H, CH2–CONH); 3.63 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.19, 175.14, 168.13, 163.70, 157.76, 156.90, 147.92, 145.18, 144.84, 141.93, 131.97, 131.72, 129.61, 127.95, 127.89, 126.84, 125.04, 124.89, 124.75, 124.62, 122.45, 122.21, 120.57, 119.89, 119.83, 119.10, 116.83, 116.63, 109.26, 109.13, 41.94, 41.24, 40.60, 40.43, 35.50. HR-MS (ESI) m/z: 310.1183 [M + H]+. HR-MS (ESI) m/z calculated for C17H16N3O3 [M + H]+ 310.1192. Found 310.1183.
(E)-N'-(2-Hydroxy-4-methoxybenzylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (4g)
Yellow solid; Yield: 52%. mp: 177–178 °C. Rf = 0.43 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3184 (NH); 3063 (CH aren); 2972, 2922, 2851 (CH, CH2); 1709 1670 (C=O); 1628, 1609 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.50 (s, 1H, CONH); 8.37, 8.26 (s, 0.50H, 0.50H, CH=N); 7.66, 7.44 (d, J = 8.50 Hz, 0.50H, 0.50H, H-6′); 7.29 (t, J = 7.50 Hz, 1H, H-5); 7.23 (dd, J = 7.88 Hz, J′ = 3.88 Hz, 1H, H-7); 7.05–7.00 (m, 1H, H-4); 6.95 (t, J = 7.50 Hz, 1H, H-6); 6.53–6.47 (m, 2H, H-3′, H-5′); 4.83, 4.47 (s, 1.00H, 1.00H, CH2–CONH); 3.77 (s, 3H, CH3); 3.63 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.18, 175.13, 167.78, 163.40, 162.62, 162.41, 159.77, 158.50, 148.55, 145.20, 144.85, 142.47, 131.34, 128.39, 127.94, 127.88, 125.03, 124.88, 124.73, 124.60, 122.43, 122.19, 113.52, 112.11, 109.27, 109.13, 106.93, 106.86, 101.60, 101.40, 55.77, 55.65, 41.90, 41.20, 35.50. HR-MS (ESI) m/z: 340.1289 [M + H]+. HR-MS (ESI) m/z calculated for C18H18N3O4 [M + H]+ 340.1297. Found 340.1289.
(E)-N'-(3-Allyl-2-hydroxybenzylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (4h)
Yellow solid; Yield: 64%. mp: 185–186 °C. Rf = 0.55 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3190 (NH); 3057 (CH aren); 2974, 2913, 2853 (CH, CH2); 1715, 1668 (C=O); 1614 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.95 (s, 1H, CONH); 11.65 (s, 1H, OH); 8.40, 8.28 (s, 0.70H, 0.30H, CH = N); 7.42–7.19 (m, 4H, H-5, H-7, H-6′, H-4′); 7.05–6.88 (m, 3H, H-4, H-6, H-5′); 6.01–5.97 (m, 1H, CH=CH2); 5.09–5.01 (m, 2H, CH=CH2); 4.87, 4.52 (s, 0.60H, 1.40H, CH2–CONH); 3.64 (s, 2H, H-3a, H-3b); 3.41, 3.36 (d, J = 6.50 Hz, 2H, CH2–CH). 13C-NMR (125 MHz, DMSO-d6): δ 175.15, 67.60, 163.79, 155.93, 154.85, 150.22, 146.59, 145.10, 144.81, 136.98, 132.22, 129.75, 128.65, 127.95, 127.85, 127.81, 127.61, 125.04, 124.88, 124.77, 124.61, 122.48, 122.25, 120.22, 119.59, 119.12, 117.75, 116.30, 116.18, 109.41, 109.14, 41.91, 41.09, 35.50, 35.75. HR-MS (ESI) m/z: 350.1500 [M + H]+. HR-MS (ESI) m/z calculated for C20H20N3O3 [M + H]+ 350.1505. Found 350.1500.
(E)-N'-Benzylidene-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4i)
Yellow solid; Yield: 62%. mp: 173–174 °C. Rf = 0.55 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3184 (NH); 3090 (CH aren); 2968, 2918, 2849 (CH, CH2); 1717, 1674 (C=O); 1607 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.71 (s, 1H, CONH); 8.24, 8.05 (s, 1H, CH=N); 7.75, 7.71 (dd, J = 7.25 Hz, J′ = 1.75 Hz, 2H, H-2′, H-6′); 7.48–7.44 (m, 3H, H-3′, H-4′, H-5′); 7.20 (dd, J = 8.50 Hz, J′ = 2.50 Hz, 1H, H-7); 7.07 (td, J = 9.13 Hz, J′ = 2.33 Hz, 1H, H-6); 6.97 (dd, J = 8.50 Hz, J′ = 4.00 Hz, 1H, H-4); 4.89, 4.47 (s, 1.50H, 0.50H, CH2–CONH); 3.68 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 174.98, 174.88, 168.44, 163.70, 159.57, 157.69, 147.78, 144.50, 141.46, 141.20, 134.52, 134.45, 130.65, 130.48, 129.30, 127.59, 127.42, 126.88, 126.80, 126.73, 114.07, 113.89, 112.80, 112.67, 112.60, 112.47, 109.97, 109.91, 109.78, 42.14, 41.47, 40.61, 40.44, 35.88. HR-MS (ESI) m/z: 312.1139 [M + H]+. HR-MS (ESI) m/z calculated for C17H15FN3O2 [M + H]+ 312.1148. Found 312.1139.
(E)-N'-(2-Chlorobenzylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4j)
Yellow solid; Yield: 65%. mp: 185–186 °C. Rf = 0.57 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3183 (NH); 3096 (CH aren); 2953, 2918, 2851 (CH, CH2); 1680 (C=O); 1607 (C=C). 1H-NMR(500 MHz, DMSO-d6): δ 11.95, 11.89 (s, 1H, CONH); 8.63, 8.44 (s, 1H, CH=N); 8.07, 7.94 (dd, 1H, J = 7.50 Hz, J′ = 2.00 Hz, H-6′); 7.56–7.42 (m, 3H, H-3′, H-4′, H-5′), 7.20 (dd, J = 8.00 Hz, J′ = 2.50 Hz, 1H, H-7); 7.07 (td, J = 9.25 Hz, J′ = 2.50 Hz, 1H, H-6); 6.98 (dd, J = 8.50 Hz, J′ = 4.50 Hz, 1H, H-4); 4.91, 4.81 (s, 1H, CH2–CONH); 3.68 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 174.99, 174.90, 168.61, 163.91, 159.58, 157.70, 143.66, 141.41, 140.54, 133.65, 133.47, 132.11, 131.91, 131.72, 130.39, 128.15, 128.08, 127.51, 127.38, 126.80, 126.73, 114.08, 113.90, 112.69, 112.63, 112.49, 109.98, 109.92, 55.38, 42.20, 41.51, 35.87. HR-MS (ESI) m/z: 346.0753 {[M + H]+, 35Cl}; 348.0723 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C17H14ClFN3O2 [M + H]+ 346.0759. Found 346.0753.
(E)-N'-(3-Chlorobenzylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4k)
Yellow solid; Yield: 58%. mp: 187–188 °C. Rf = 0.57 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3183 (NH); 3088 (CH aren); 2918, 2851 (CH, CH2); 1719, 1676 (C=O); 1607 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.82 (s, 1H, CONH); 8.23, 8.03 (s, 1H, CH=N); 7.84, 7.76 (s, 1H, H-2′); 7.70–7.68 (m, 1H, H-6′); 7.51–7.47 (m, 2H, H-4′, H-5′); 7.20 (dd, J = 8.00 Hz, J′ = 2.00 Hz, 1H, H-7); 7.07 (td, J = 9.13 Hz, J′ = 2.17 Hz, 1H, H-6); 6.97 (dd, J = 8.50 Hz, J′ = 4.50 Hz, 1H, H-4); 4.91, 4.48 (s, 2H, CH2–CONH); 3.68 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 174.99, 174.89, 168.65, 163.94, 159.57, 157.69, 146.11, 142.88, 141.44, 136.80, 136.71, 134.18, 134.09, 131.21, 131.17, 130.27, 130.12, 126.97, 126.80, 126.73, 126.58, 126.35, 126.17, 114.07, 113.89, 112.69, 112.61, 112.49, 109.97, 109.90, 109.78, 42.14, 41.57, 35.87. HR-MS (ESI) m/z: 346.0751 {[M + H]+, 35Cl}; 348.0720 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C17H14ClFN3O2 [M + H]+ 346.0759. Found 346.0751.
(E)-N'-(4-Chlorobenzylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4l)
Yellow solid; Yield: 63%. mp: 191–192 °C. Rf = 0.57 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3183 (NH); 3094 (CH aren); 2968, 2853 (CH, CH2); 1714, 1672 (C=O); 1611 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.77 (s, 1H, CONH); 8.24, 8.04 (s, 1H, CH=N); 7.78, 7.73 (d, J = 8.75 Hz, 1.50H, 0.50H, H-2′, H-6′); 7.52 (d, J = 8.50 Hz, 2H, H-3′, H-5′); 7.20 (dd, J = 8.00 Hz, J′ = 2.00 Hz, 1H, H-7); 7.06 (td, J = 9.13 Hz, J′ = 2.17 Hz, 1H, H-6); 6.97 (dd, J = 9.00 Hz, J′ = 4.50 Hz, 1H, H-4); 4.89, 4.47 (s, 1.50H, 0.50H, CH2–CONH); 3.68 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 174.98, 174.88, 168.52, 157.69, 146.47, 143.21, 141.45, 135.09, 134.90, 133.42, 129.37, 129.23, 129.09, 126.80, 126.72, 114.07, 113.89, 112.68, 112.48, 109.97, 109.90, 43.14, 41.48, 35.87. HR-MS (ESI) m/z: 346.0751 {[M + H]+, 35Cl}; 348.0721 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C17H14ClFN3O2 [M + H]+ 346.0759. Found 346.0751.
(E)-N'-(4-Methoxybenzylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4m)
Yellow solid; Yield: 60%. mp: 175–176 °C. Rf = 0.53 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3184 (NH); 3088 (CH aren); 2936, 2837 (CH, CH2); 1709, 1670 (C=O); 1607 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.57 (s, 1H, CONH), 8.18, 7.99 (s, 1H, CH=N); 7.68, 7.65 (d, J = 9.00 Hz, 2H, H-2′, H-6′); 7.20 (d, J = 7.00 Hz, 1H, H-7); 7.06 (t, J = 9.25 Hz, 1H, H-6); 7.01 (d, J = 9.00 Hz, 2H, H-3′, H-5′); 6.96 (dd, J = 7.75 Hz, J′ = 4.25 Hz, 1H, H-4); 4.90, 4.44 (s, 1.50H, 0.50H, CH2-CONH); 3.81 (s, 3H, CH3); 3.67 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 174.97, 174.86, 168.16, 163.40, 161.38, 161.23, 159.55, 157.67, 147.67, 144.38, 141.50, 141.22, 129.21, 129.01, 127.05, 126.79, 126.71, 1114.79, 114.06, 113.88, 112.78, 112.66, 112.59, 112.46, 109.96, 109.90, 109.82, 55.79, 42.12, 41.45, 35.88. HR-MS (ESI) m/z: 342.1247 [M + H]+. HR-MS (ESI) m/z calculated for C18H17FN3O3 [M + H]+ 342.1254. Found 342.1247.
(E)-N'-(2-Hydroxybenzylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4n)
Yellow solid; Yield: 55%. mp: 173–174 °C. Rf = 0.51 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3188 (NH); 3063 (CH aren); 2922, 2853 (CH, CH2); 1719, 1670 (C=O); 1622 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.90, 11.75 (0.50H, 0.50H, CONH); 10.95, 10.05 (s, 0.50H, 0.50H, OH); 8.46, 8.35 (s, 0.50H, 0.50H, CH=N); 7.76, 7.56 (dd, 0.50H, 0.50H, H-6′); 7.31–7.25 (m, 1H, H-4′); 7.21 (td, J = 9.38 Hz, J′ = 3.00 Hz, 1H, H-5′); 7.11–7.05 (m, 1H, H-3′); 6.99–6.86 (m, 3H, H-4, H-6, H-7); 4.87, 4.49 (s, 1.00H, 1.00H, CH2–CONH); 3.67 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 159.56, 157.78, 157.75, 157.68, 156.90, 147.91, 141.95, 141.48, 141.13, 131.98, 131.73, 129.59, 126.98, 126.90, 126.83, 126.79, 126.71, 120.55, 119.89, 119.84, 119.10, 116.82, 116.62, 114.13, 114.06, 113.94, 113.88, 112.81, 112.65, 112.61, 112.45, 109.99, 104.93, 109.86, 109.80, 42.06, 41.43, 35.88. HR-MS (ESI) m/z: 328.1087 [M + H]+. HR-MS (ESI) m/z calculated for C17H15FN3O3 [M + H]+ 328.1097. Found 328.1087.
(E)-N'-(2-Hydroxy-4-methoxybenzylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4o)
Yellow solid; Yield: 52%. mp: 186–187 °C. Rf = 0.53 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3177 (NH); 3065 (CH aren); 2926 (CH, CH2); 1715, 1668 (C=O); 1607 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 11.80, 11.51 (s, 0.50H, 0.50H, CONH); 11.27, 10.17 (s, 0.50H, 0.50H, OH); 8.36, 8.25 (s, 0.50H, 0.50H, CH=N); 7.66, 7.44 (d, J = 9.00 Hz, 0.50H, 0.50H, H-6′); 7.20 (td, J = 9.50 Hz, J′ = 2.50 Hz, 1H, H-6); 7.12–7.04 (m, 1H, H-7); 6.98–6.95 (m, 1H, H-4); 6.53–6.47 (m, 2H, H-3′, H-5′); 4.83, 4.47 (s, 1.00H, 1.00H, CH2-CONH); 3.77 (s, 3H, –OCH3); 3.63 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.19, 163.07, 140.82, 132.73, 132.24, 127.96, 124.96, 124.75, 122.47, 121.72, 120.09, 11.46, 109.29, 35.44. HR-MS (ESI) m/z: 358.1195 [M + H]+. HR-MS (ESI) m/z calculated for C18H17FN3O4 [M + H]+ 358.1203. Found 358.1195.
(E)-N'-(3-Allyl-2-hydroxybenzylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (4p)
Yellow solid; Yield: 65%. mp: 194–195 °C. Rf = 0.57 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3061 (CH aren); 2974, 2913 (CH, CH2); 1715, 1668 (C=O); 1607 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 12.10, 11.85 (s, 1H, 0.25H, CONH); 11.67, 10.15 (s, 1H, OH); 8.39, 8.27 (s, 1H, CH=N); 7.40, 7.34 (dd, J = 8.00 Hz, J′ = 1.50 Hz, 1H, H-6′); 7.23–7.19 (m, 2H, H-4′, H-5′); 7.11–6.88 (m, 3H, H-4, H-6, H-7); 6.00–5.94 (m, 1H, CH=CH2); 5.09–5.01 (m, 2H, CH=CH2); 4.87, 4.52 (s, 0.60H, 1.40H, CH2–CONH); 3.68 (s, 2H, H-3a, H-3b); 3.40, 3.36 (d, J = 6.50 Hz, 2H, CH2–CH). 13C-NMR (125 MHz, DMSO-d6): δ 159.59, 157.80, 155.91, 154.85, 150.24, 146.63, 141.37, 141.10, 136.97, 132.24, 129.76, 128.67, 127.80, 127.61, 126.98, 126.91, 126.78, 126.70, 120.22, 119.60, 119.07, 117.73, 116.30, 116.19, 114.14, 114.04, 113.96, 113.85, 112.83, 112.63, 112.45, 110.14, 110.78, 109.87, 109.81, 42.02, 41.27, 40.60, 35.89, 33.75. HR-MS (ESI) m/z: 368.1403 [M + H]+. HR-MS (ESI) m/z calculated for C20H19FN3O3 [M + H]+ 368.1410. Found 368.1403.
(Z)-2-(2-Oxoindolin-1-yl)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (5a)
Yellow solid; Yield: 58%. mp: 191–192 °C. Rf = 0.61 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3123 (NH); 3086 (CH aren); 2984, 2920, 2851, 2806 (CH, CH2); 1695, 1663 (C=O); 1612 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 12.67 (s, 1H, NH-1′); 11.29 (s, 1H, CONH); 7.60 (s, 1H, H-4′); 7.41 (t, J = 7.50 Hz, 1H, H-6′); 7.31 (d, J = 7.50 Hz, 1H, H-7); 7.24 (t, J = 7.75 Hz, 1H, H-5); 7.12 (t, J = 7.50 Hz, 1H, H-5′); 7.03 (t, J = 8.50 Hz, 1H, H-6); 7.02 (d, J = 8.50 Hz, 1H, H-7′); 6.79 (d, J = 7.50 Hz, 1H, H-4); 5.05, 4.73 (s, 1.60H, 0.40H, CH2–CONH); 3.67 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.19, 163.00, 143.11, 132.33, 127.97, 124.96, 124.76, 123.12, 122.50, 121.40, 120.10, 111.70, 109.29, 35.45. HR-MS (ESI) m/z: 335.1141 [M + H]+. HR-MS (ESI) m/z calculated for C18H15N4O3 [M + H]+ 335.1144. Found 335.1141.
(Z)-N'-(5-Chloro-2-oxoindolin-3-ylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (5b)
Yellow solid; Yield: 64%. mp: 201–202 °C. Rf = 0.65 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3063 (CH aren); 2976 (CH, CH2); 1701, 1668 (C=O); 1614 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 7.61 (s, 1H, H-4′); 7.44, 7.42 (dd, J = 8.25 Hz, J′ = 2.25 Hz, 1H, H-6′); 7.31 (d, J = 7.50 Hz, 1H, H-7), 7.25 (t, J = 7.75 Hz, 1H, H-5), 7.04 (t, J = 7.50 Hz, 1H, H-6); 7.02 (d, J = 8.00 Hz, 1H, H-7′); 6.97 (d, J = 8.00 Hz, 1H, H-4); 5.00 (s, 2H, CH2–CONH); 3.67 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 175.20, 162.99, 142.23, 131.60, 127.97, 127.13, 124.98, 124.78, 122.52, 122.02, 120.99, 113.27, 109.24, 35.45. HR-MS (ESI) m/z: 369.0751 {[M + H]+, 35Cl}; 371.0715 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C18H14ClN4O3 [M + H]+ 369.0754. Found 369.0751.
(Z)-N'-(5-Methyl-2-oxoindolin-3-ylidene)-2-(2-oxoindolin-1-yl)acetohydrazide (5c)
Yellow solid; Yield: 67%. mp: 198–199 °C. Rf = 0.63 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3146 (NH); 2984, 2938 (CH, CH2); 1690, 1665 (C=O); 1612 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 13.21, 12.68 (s, 0.30H, 0.70H, NH-1′); 11.81 (s, 1H, CONH); 7.41 (s, 1H, H-4′); 7.31 (d, J = 7.00 Hz, 1H, H-6′); 7.24 (t, J = 7.75 Hz, 1H, H-5); 7.21 (d, J = 8.00 Hz, 1H, H-7); 7.04 (t, J = 7.50 Hz, 1H, H-6); 7.02 (d, J = 8.00 Hz, 1H, H-7′); 6.85 (d, J = 7.00 Hz, 1H, H-4); 5.04, 4.71 (s, 1.40H, 0.60H, CH2–CONH); 3.67 (s, 2H, H-3a, H-3b); 2.31 (s, 3H, CH3). 13C-NMR (125 MHz, DMSO-d6): δ 175.19, 169.37, 163.01, 144.84, 140.82, 135.71, 132.73, 132.24, 127.96, 124.97, 124.76, 122.47, 121.73, 120.09, 111.46, 109.28, 35.44, 20.98. HR-MS (ESI) m/z: 349.1304 [M + H]+. HR-MS (ESI) m/z calculated for C19H17N4O3 [M + H]+ 349.1301. Found 349.1304.
(Z)-2-(5-Fluoro-2-oxoindolin-1-yl)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (5d)
Yellow solid; Yield: 55%. mp: 215–216 °C. Rf = 0.63 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3246 (NH); 2982, 2886 (CH, CH2); 1732, 1694 (C=O); 1620 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 13.18, 12.68 (s, 0.30H, 0.70H, NH-1′); 11.29 (s, 1H, CONH); 7.61, 7.51 (d, J = 8.00 Hz, 0.70H, 0.30H, H-4′); 7.41 (t, J = 7.50 Hz, 1H, H-6′); 7.25, 7.24 (dd, J = 8.00 Hz, J′ = 1.50 Hz, 1H, H-7); 7.19, 7.17 (d, J = 8.50 Hz, 1H, H-6); 7.13, 7.12 (d, J = 8.25 Hz, 1H, H-4), 7.05 (t, J = 10.25 Hz, 1H, H-5′); 6.97 (d, J = 7.50 Hz, 1H, H-7′); 5.18, 5.15 (s, 1.40H, 0.60H, CH2–CONH); 4.86 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 162.97, 161.49, 143.11, 132.35, 123.13, 121.42, 120.07, 113.56, 113.36, 111.70, 110.70. HR-MS (ESI) m/z: 353.1043 [M + H]+. HR-MS (ESI) m/z calculated for C18H14FN4O3 [M + H]+ 353.1050. Found 353.1043.
(Z)-N'-(5-Chloro-2-oxoindolin-3-ylidene)-2-(5-fluoro-2-oxoindolin-1-yl)acetohydrazide (5e)
Yellow solid; Yield: 64%. mp: 221–222 °C. Rf = 0.65 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3065 (CH aren); 2978 (CH, CH2); 1701, 1668 (C=O); 1622 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 12.57 (s, 1H, NH-1′); 11.40 (s, 1H, CONH); 7.62 (s, 1H, H-4′); 7.45, 7.43 (dd, J = 8.50 Hz, J′ = 2.00 Hz, 1H, H-6′); 7.22 (d, J = 7.50 Hz, 1H, H-7); 7.09 (td, J = 9.00 Hz, J′ = 2.00 Hz, 1H, H-6); 7.04, 7.03 (d, J = 8.50 Hz, 1H, H-4); 6.98 (d, J = 8.00 Hz, 1H, H-7′); 5.05 (s, 2H, CH2–CONH); 3.71 (s, 2H, H-3a, H-3b). 13C-NMR (125 MHz, DMSO-d6): δ 174.98, 169.50, 162.74, 159.71, 157.83, 141.75, 131.64, 127.28, 126.91, 126.84, 121.87, 121.03, 114.06, 113.98, 113.21, 112.84, 112.65, 110.00, 109.93, 35.83. HR-MS (ESI) m/z: 387.0657 {[M + H]+, 35Cl}; 389.0627 {[M + H]+, 37Cl}. HR-MS (ESI) m/z calculated for C18H13ClFN4O3 [M + H]+ 387.0660. Found 387.0657.
(Z)-2-(5-Fluoro-2-oxoindolin-1-yl)-N'-(5-methyl-2-oxoindolin-3-ylidene)acetohydrazide (5f)
Yellow solid; Yield: 68%. mp: 217–218 °C. Rf = 0.63 (DCM:MeOH = 14:1). IR (KBr, cm−1): 3177 (NH); 3065 (CH aren); 2922 (CH, CH2); 1678, 1667 (C=O); 1628, 1609 (C=C). 1H-NMR (500 MHz, DMSO-d6): δ 13.20, 12.67 (s, 1H, NH-1′); 11.18 (s, 1H, CONH); 7.40 (s, 1H, H-4′); 7.21 (d, J = 7.50 Hz, 2H, H-6′, H-7); 7.09 (td, J = 9.13 Hz, J′ = 2.25 Hz, 1H, H-6); 7.05, 7.03 (d, J = 8.00 Hz, 0.70H, 0.30H, H-4); 6.85 (d, J = 7.50 Hz, 1H, H-7′); 5.04, 4.70 (s, 1.40H, 0.60H, CH2–CONH), 3.71(s, 2H, H-3a, H-3b), 2.31 (s, 3H, CH3). 13C-NMR (125 MHz, DMSO-d6): δ 174.97, 163.07, 157.83, 140.83, 132.74, 132.24, 126.89, 121.72, 120.08, 114.16, 113.98, 112.83, 111.47, 110.05, 109.99, 35.83, 20.97. HR-MS (ESI) m/z: 367.1203 [M + H]+. HR-MS (ESI) m/z calculated for C19H16FN4O3 [M + H]+ 367.1206. Found 367.1203.
Bioactivity
Two series of compounds synthesized (4a–p and 5a–f) were evaluated for their cytotoxicity against three human cancer cell lines, including SW620 (colon cancer), PC3 (prostate cancer), NCI-H23 (lung cancer), using SRB method as described previously22 with slight modifications23,24,25,26. 5-Fluorouracil (5-FU) and PAC-1 were included in the assay as positive controls. The results expressed as IC50 values are summarized in Table 1.
From the results in Table 1, all compounds exhibited strong cytotoxicity against three cancer cell lines. Overally, 5-fluorinated compounds (4i–p and 5d–f) were slightly more cytotoxic than non-fluorinated ones (4a–h, 5a–c). For compounds in series 4a–p, it was observed that, electron-releasing substituents (–OCH3, OH) were generally better than electron-withdrawing groups (–Cl) for cytotoxicity. Especially, compounds with 2-OH substituents produced the best cytotoxicity. The addition of either 4-OCH3 or 3-allyl groups further enhanced cytotoxicity of the related compounds (4g, 4h and 4o, 4p). Very interestingly, a 2-hydroxy-4-methoxy substituted pattern was shown to be more favorable for cytotoxicity than 2-hydroxy-3-allyl substituent, which was present in PAC-1. Compound 4o, which was 5-fluorinated on the 2-oxoindoline part and bearing 2-hydroxy-4-methoxy substituent on the phenyl ring, was the most potent one in term of cytotoxicity. Its IC50 values were 1.88 ± 0.02, 1.83 ± 0.07, and 1.00 ± 0.01 μM in SW620, PC-3, and NCI-H23 cell lines, respectively. These values were approximately three- to five-fold lower than that of PAC-1. However, our compounds showed much higher IC50 values on normal mesenchymal stem cells than on cancer cells (Table 1).
Next, we selected 5 representative compounds, including 4f, 4h, 4n, 4o and 4p, to investigate their effects on the caspase activity, cell cycle, and apoptosis. At first, we tried to use the extract of SW620, PC-3, and NCI-H23 in caspase activation assay, but these extracts did not show caspase activity. Thus, we used the extract of U937 cells in caspase-3 activation assay, referring to our previous study31. As reported, PAC-1 activated caspase-3 in 24-h-caspase-3 assay in U937 cells at 50 µM higher concentration than an IC50 value of 8.47 µM in 48-h-cytotoxicity assay. However, our compounds unexpectedly were not recorded to activate caspase-3 activity. It was likely that the compounds might activate other caspases and eventually caused effects on the cell cycle and apoptosis. Next, we investigated the effect of PAC-1 and our chemicals on cell cycle and apoptosis by using U937 cells31. In the cell cycle analysis, U937 cells were treated with 50 µM of compounds for 24 h, stained with propidium iodide (PI) in the presence of RNase, and then analyzed for DNA content by using flow cytometry. PAC-1 was used in parallel as a positive control. The results illustrated in Fig. 3 indicate that the compounds tended to cause accumulation of cells in S phase, although PAC-1 caused the accumulation of cells in G0/G1 phase. Compound 4o inhibited cell cycles at 5 µM, which was close to IC50 value (Fig. 4). In the Annexin V-FITC/PI apoptotic analysis, compounds 4f, 4h, 4n, 4o and 4p also induced early, and more substantially, late apoptosis (Fig. 5). The effects were more prominent with compounds 4h, 4o and 4p (Fig. 5). Compound 4o increased late apoptotic cell population at 5 µM, which was close to IC50 value (Fig. 6). Regarding the effects of the compounds on cellular morphology, SW620 cells treated with PAC-1 and our compounds showed morphology of apoptotic cells (Figs. 7 and 8).
Cell cycle analysis after treatment with some compounds. U937 human lymphoma cells were treated with compounds (50 µM) for 24 h. The harvested cells were stained with propidium iodide (PI) in the presence of RNase and then were analyzed for DNA content by using flow cytometry. Ten thousand cells were acquired (n = 3). UN untreated, VH vehicle (DMSO. 0.1%). Representative histograms (A) and bar graphs (B) are shown. p values were calculated using one-way ANOVA in GraphPad Software (San Diego, CA, USA). *p < 0.01 vs VH control.
Cell cycle analysis under treatment with different concentrations of compound 4o. U937 human lymphoma cells were treated with compound 4o at 5, 10 and 30 µM or PAC-1 (30 µM) for 24 h. The harvested cells were stained with propidium iodide (PI) in the presence of RNase and then were analyzed for DNA content by using flow cytometry. Ten thousand cells were acquired (n = 3). UN untreated, VH vehicle (DMSO. 0.1%). Representative histograms (A) and bar graphs (B) are shown. p values were calculated using one-way ANOVA in GraphPad Software (San Diego, CA, USA). *p < 0.01 vs VH control.
Apoptosis (Annexin V/PI) analysis of cells after treatment with some compounds. U937 cells were treated with compounds (50 µM) for 24 h. The harvested cells were incubated with Annexin V-FITC and PI and analyzed by using flow cytometry. Ten thousand cells were acquired (n = 3). UN untreated, VH vehicle (DMSO. 0.1%). Representative dot plots (A). Live cells, left down in dot plot. Early apoptotic cells, right down. Necrotic cells, left up, Late apoptotic cells, right up. Bar graph of late apoptotic cells (B). p values were calculated using one-way ANOVA in GraphPad Software (San Diego, CA, USA). *p < 0.01 vs VH control.
Apoptosis (Annexin V/PI) analysis of cells after treatment with different concentrations of compound 4o. U937 cells were treated with compound 4o at 5, 10 and 30 µM or PAC-1 (30 µM) for 24 h. The harvested cells were incubated with Annexin V-FITC and PI and analyzed by using flow cytometry. Ten thousand cells were acquired (n = 3). UN untreated, VH vehicle (DMSO. 0.1%). Representative dot plots (A). Live cells, left down in dot plot. Early apoptotic cells, right down. Necrotic cells, left up, Late apoptotic cells, right up. Bar graph of late apoptotic cells (B). p values were calculated using one-way ANOVA in GraphPad Software (San Diego, CA, USA). *p < 0.01 vs VH control.
Morphology changes of cells treated with compound 4f, 4h, 4n, 4o, 4p or PAC-1. SW620 at 2.5 × 105 cells/mL (500 µL in 24 well) were incubated for 24 h and then further treated with compounds or PAC-1 (50 μM) for 24 h. Cells were photographed using an Imaging Device (Celldiscoverer 7) with ×20 (A) and ×40 (B) lens. Scale bar: 50 mm. UN untreated, VH vehicle (DMSO. 0.1%). Representative images are shown.
Morphology changes of cells treated with compound 4o or PAC-1. SW620 at 2.5 × 105 cells/mL (500 µL in 24 well) were incubated for 24 h and then further treated with 4o (5, 10, and 30 μM) or PAC-1 (30 μM) for 24 h. Cells were photographed using an Imaging Device (Celldiscoverer 7) with ×20 (A) and ×40 (B) lens. Scale bar: 50 mm. UN untreated, VH vehicle (DMSO. 0.1%). Representative images are shown.
Conclusions
In conclusion, two series of novel (E)-N'-arylidene-2-(2-oxoindolin-1-yl)acetohydrazides (4a–p) and (Z)-2-(5-substituted-2-oxoindolin-1-yl)-N'-(2-oxoindolin-3-ylidene)acetohydrazides (5a–f) were designed and synthesized. Biological results revealed that the significant cytotoxicity against three human cancer cell lines SW620, PC-3, and NCI-H23 of these compounds were obtained. Under our conditions, compounds 4f–h and 4n–p, exhibited cytotoxicity equal to superior to positive control PAC-1. In particular, compound 4o was the most potent with cytotoxicity up to three- to five-fold stronger than PAC-1 in three cancer cell lines tested. Cell cycle and apoptosis analysis showed that representative compounds 4f, 4h, 4n, 4o and 4p (especially 4o) accumulated U937 cells in the S phase and substantially induced late cell apoptosis. Collectively, the results show that compound 4o would serve as a template for further design and development of novel anticancer agents.
References
Fouad, Y. A. & Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 7(5), 1016–1036 (2017).
Nam, N. H. & Parang, K. Current targets for anticancer drug discovery. Curr. Drug Targets 4(2), 159–179 (2003).
Storey, S. Targeting apoptosis: Selected anticancer strategies. Nat. Rev. Drug Discov. 7, 971–972 (2008).
Lain, S. et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell 13(5), 454–463 (2008).
Flygare, J. A. et al. Discovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152). J. Med. Chem. 55(9), 4101–4113 (2012).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202 (2013).
Shalini, S., Dorstyn, L., Dawar, S. & Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 22, 626–539 (2015).
Li, J. & Yuan, J. Caspases in apoptosis and beyond. Oncogene 27, 6194–6206 (2008).
Boudreau, P. M. W., Peh, J. & Hergenrother, P. J. Procaspase-3 overexpression in cancer: A paradoxical observation with therapeutic potential. ACS Chem. Biol. https://doi.org/10.1021/acschembio.9b00338 (2019) (in press).
Nakagawara, A. et al. High levels of expression and nuclear localization of interleukin-1 β converting enzyme (ICE) and CPP32 in favorable human neuroblastomas. Cancer Res. 57(20), 4578–4584 (1997).
O’Donovan, N. et al. Caspase 3 in breast cancer. Clin. Cancer Res. 9(2), 738–742 (2003).
Krepela, E., Procházka, J., Liu, X., Fiala, P. & Kinkor, Z. Increased expression of Apaf-1 and procaspase-3 and the functionality of intrinsic apoptosis apparatus in non-small cell lung carcinoma. bchm. 385(2), 153–168 (2004).
Persad, R. et al. Overexpression of caspase-3 in hepatocellular carcinomas. Mod. Pathol. 17, 861–867 (2004).
Izban, K. F. et al. Characterization of the Interleukin-1β-converting enzyme/Ced-3-family protease, caspase-3/CPP32, in Hodgkin’s disease: Lack of caspase-3 expression in nodular lymphocyte predominance Hodgkin’s disease. Am. J. Pathol. 154(5), 1439–1447 (1999).
Putt, K. S. et al. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat. Chem. Biol. 2(10), 543–550 (2006).
Howard, S. R. & Paul, J. H. Derivatives of procaspase-activating compound 1 (PAC-1) and their anticancer activities. Curr. Med. Chem. 23(3), 201–241 (2016).
Peng, X. et al. Aroylhydrazone derivative as fluorescent sensor for highly selective recognition of Zn2+ ions: Syntheses, characterization, crystal structures and spectroscopic properties. Dalton Trans. 40(19), 5271–5277 (2011).
Peterson, Q. P. et al. PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition. J. Mol. Biol. 388(1), 144–158 (2009).
Huan, L. C. et al. (E)-N’-Arylidene-2-(4-oxoquinazolin-4(3H)-yl) acetohydrazides: Synthesis and evaluation of antitumor cytotoxicity and caspase activation activity. J. Enzyme Inhib. Med. Chem. 34(1), 465–478 (2019).
Huan, L. C. et al. (E)-N′-Arylidene-2-(3-oxo-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)acetohydrazides: Synthesis and evaluation of caspase activation activity and cytotoxicity. Chem. Biodivers. 15(10), e1800322 (2018).
Pakravan, P., Kashanian, S., Khodaei, M. M. & Harding, F. J. Biochemical and pharmacological characterization of isatin and its derivatives: From structure to activity”. Pharmacol. Rep. 65, 313–335 (2013).
Skehan, P. et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer. Inst. 82(13), 1107–1112 (1990).
Dung, D. T. M. et al. Novel 3-substituted-2-oxoindoline-based N-hydroxypropenamides as histone deacetylase inhibitors and antitumor agents. Med. Chem. 11, 725–735 (2015).
Nam, N. H. et al. Design of tetrapeptide ligands as inhibitors of the Src SH2 domain. Bioorg. Med. Chem. 12, 779–787 (2004).
Nam, N. H. et al. Synthesis and cytotoxicity of 2, 5-dihydroxychalcones and related compounds. Arch. Pharm. Res. 27, 581–588 (2004).
Min, B. S. et al. Furo-1, 2-naphthoquinones from Crataegus pinnatifida with ICAM-1 expression inhibition activity. Planta Med. 70, 1166–1169 (2004).
Wu, L. et al. Multidrug-resistant phenotype of disease-oriented panels of human tumor cell lines used for anticancer drug screening. Cancer Res. 52(11), 3029–3034 (1992).
Anh, D. T. et al. Design, synthesis and evaluation of novel indirubin-based N-hydroxybenzamides, N-hydroxypropenamides and N-hydroxyheptanamides as histone deacetylase inhibitors and antitumor agents. Bioorg. Med. Chem. Lett. 30(22), 127537 (2020).
Anh, D. T. et al. Novel 4-oxoquinazoline-based N-hydroxypropenamides as histone deacetylase inhibitors: Design, synthesis, and biological evaluation. ACS Omega 6(7), 4907–4920 (2021).
Lin, W.-J., Shia, K.-S., Song, J.-S., Wub, M.-H. & Li, W.-T. Synthesis of (E)-oxindolylidene acetate using tandem palladium-catalyzed Heck and alkoxycarbonylation reactions. Org. Biomol. Chem. 14, 220–228 (2016).
Dung, D. T. M. et al. Design, synthesis and evaluation of novel (E)-N′-(3-Allyl-2-hydroxy)benzylidene-2-(4-oxoquinazolin-3(4H)-yl)acetohydrazides as antitumor agents. Arch. Pharm. 355, e2100216 (2021).
Acknowledgements
We acknowledge the principal financial supports from the National Foundation for Science and Technology of Vietnam (NAFOSTED, Grant number 104.01-2019.300). The work was also partly supported by a grant funded by the Korean Government (NRF, Grant number 2017R1A5A2015541 & 2020R1A2C2004200) and small grant from Hanoi University of Pharmacy.
Author information
Authors and Affiliations
Contributions
N.-H.N and S.-B.H. proposed the work. N.-H.N., T.T.T., D.T.M.D., D.T.P.P. and D.T.A. mainly developed the synthesis studies, S.-B.H., E.J.P., I.H.N., J.H.K. and J.S.K. performed the biological testing assays. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dung, D.T.M., Park, E.J., Anh, D.T. et al. Design, synthesis and evaluation of novel 2-oxoindoline-based acetohydrazides as antitumor agents. Sci Rep 12, 2886 (2022). https://doi.org/10.1038/s41598-022-06887-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06887-0
This article is cited by
-
Evaluation of 1,10-phenanthroline-based hydroxamate derivative as dual histone deacetylases/ribonucleotide reductase inhibitor with antitumor activities
DARU Journal of Pharmaceutical Sciences (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.