Abstract
Soil transmitted helminth (STH) infections are among the most common human infections worldwide with over 1 billion people affected. Many estimates of STH infection are often based on school-aged children (SAC). This study produced predictive risk-maps of STH on a more finite scale, estimated the number of people infected, and the amount of drug required for preventive chemotherapy (PC) in Ogun state, Nigeria. Georeferenced STH infection data obtained from a cross-sectional survey at 33 locations between July 2016 and November 2018, together with remotely-sensed environmental and socio-economic data were analyzed using Bayesian geostatistical modelling. Stepwise variable selection procedure was employed to select a parsimonious set of predictors to predict risk and spatial distribution of STH infections. The number of persons (pre-school ages children, SAC and adults) infected with STH were estimated, with the amount of tablets needed for preventive chemotherapy. An overall prevalence of 17.2% (95% CI 14.9, 19.5) was recorded for any STH infection. Ascaris lumbricoides infections was the most predominant, with an overall prevalence of 13.6% (95% CI 11.5, 15.7), while Hookworm and Trichuris trichiura had overall prevalence of 4.6% (95% CI 3.3, 5.9) and 1.7% (95% CI 0.9, 2.4), respectively. The model-based prevalence predictions ranged from 5.0 to 23.8% for Ascaris lumbricoides, from 2.0 to 14.5% for hookworms, and from 0.1 to 5.7% for Trichuris trichiura across the implementation units. The predictive maps revealed a spatial pattern of high risk in the central, western and on the border of Republic of Benin. The model identified soil pH, soil moisture and elevation as the main predictors of infection for A. lumbricoides, Hookworms and T. trichiura respectively. About 50% (10/20) of the implementation units require biannual rounds of mass drug administration. Approximately, a total of 1.1 million persons were infected and require 7.8 million doses. However, a sub-total of 375,374 SAC were estimated to be infected, requiring 2.7 million doses. Our predictive risk maps and estimated PC needs provide useful information for the elimination of STH, either for resource acquisition or identifying priority areas for delivery of interventions in Ogun State, Nigeria.
Similar content being viewed by others
Introduction
Soil transmitted helminth (STH) infections are among the most common human infections worldwide and have been classified as neglected tropical diseases by the WHO1,2. Most of the world’s STH infections are caused by four common parasitic helminths; Ascaris lumbricoides (roundworm), Trichuris trichiura (whipworm), Necator americanus and Ancylostoma duodenale (hookworms), whose life cycle are linked with the soil environment and a definitive human host2,3. STH infections are widely distributed in the tropical and subtropical regions, with the greatest numbers occurring in sub-Saharan Africa, the Americas, China and East Asia1,3. About 5 billion people are at risk, and 1.5 billion of the worlds’ population are currently infected1,3. Infections are predominantly abundant in areas characterized with poverty, favorable climate and lack of access to basic infrastructural amenities such as potable water supply, sanitation and hygiene facilities4,5,6,7,8,9.
Efforts targeted at controlling STH have been through preventive chemotherapy (PC), and involves large-scale administration of albendazole or mebendazole medicines with a major focus on school-aged children (SAC) in endemic communities1,10. The World Health Organization (WHO) recommends PC, either once a year (annually), when the baseline prevalence of infections is between 20 and 50%, or twice a year (biannually) when the prevalence is above 50%10. PC is expensive, costing donor agencies and developing economies billions of dollars11. For instance, since 2010, the World Health Organization (WHO) has coordinated the annual distribution of 600 million medicines for PC in endemic countries, with albendazole, donated by GlaxoSmithKline, and mebendazole, donated by Johnson & Johnson12.
In line with elimination goals, the WHO stipulates that endemic countries must consistently treat and protect at least 75% of its population that requires treatment. Globally, a total of 576 million (59.9%) of the estimated 1.1 billion children requiring albendazole or mebendazole medicines were treated in 2018, and about 71.3% of the implementation units (IUs) achieved the 75% effective coverage target for SAC12,13. However, the recently published 2020–2030 NTD road map, emphasized more specific targets by 2030 which includes (1) eliminating STH as a public health problem (< 2% proportion of moderate and heavy intensity infections) in 96 countries; (2) reducing by 50% the number of tablets required during PC for STH and (3) increasing domestic financial support for PC for STH, with 25 countries deworming children using domestic funds12,14,15.
These targets call for more refined commitments in the planning and delivery approaches for large-scale administration of PC tablets to SAC, most especially in areas where prevalence estimates are high16,17,18. It is therefore important to constantly delineate highly endemic areas using parasitological surveys, and produce risk maps for unsampled areas using geostatistical methods to support targeting and delivery of tablets for PC19,20,21. Approaches combining parasitological surveys, geographical information systems (GIS), remote sensing (RS), spatial and geostatistical analysis have been explored extensively to model and predict the risk of helminth infections on a national scale in China20, Cote’d voire21, Cambodia22, South America23 and Nigeria24. Yet, there is paucity of studies investigating risk factors explaining spatial distribution of STH at a more finite scale such as the implementation units (IUs). Model based geostatistical approaches are useful in understanding the variations that exist within IUs, which are necessary for targeting intervention in hotspot area with high prevalence.
Nigeria is endemic for all the four common STH infections, and leading in terms of burden, and the number of people infected in sub-Saharan Africa25,26. Information on the spatial distribution and risk of STH infections is needed to facilitate targeting of control efforts in the context of resource scarcity. The few published studies in the country, have used secondary survey data to produce county-level risk maps24,27,28 and annual drug requirements24. Since implementation of PC occur in a more finite scale at IUs (referred to as local government areas (LGAs) in Nigeria), we hypothesize that risk maps made at these levels using more recent parasitological data will offer more robust insight into disease distribution and risk factors associated with such distributions. In addition, data generated at such IUs would be useful to estimate number of people at risk or infected with STH, rounds of MDA and the drug requirements for PC.
We therefore present findings from a geostatistical analysis of soil-transmitted helminth infection data that were obtained from a state-wide community-based survey in Ogun State, Nigeria. The aims of this study were (1) to map and predict the spatial distribution of soil-transmitted helminth infections at 2 km spatial scale using a Bayesian geostatistical approach; (2) identify the most important climatic, environmental and socioeconomic determinants of soil-transmitted helminth infections (3) calculate the number of persons infected and; (4) estimate the annual drug requirements for preventive chemotherapy according to guidelines put forward by the World Health Organization (WHO).
Methods
Study area, design and population
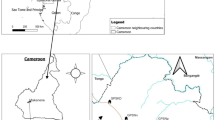
This study was conducted in Ogun State, Nigeria (Fig. 1). Details of the study area, design and population surveyed have been described elsewhere19. In brief, the study was carried out between July 2016 and November 2018 spanning across both wet and dry season. We designed a cross-sectional survey, and employed a systematic grid sampling method in the selection of communities to ensure an unbiased representation across the state19. A total of 1499 children and adults, from 33 spatially selected communities participated in the study19. In each community, all households and their occupants were considered eligible for participation and invited to participate in the study.

Available at https://www.R-project.org/.
Map of the Ogun State showing the 20 implementation units (IUs) with Nigeria as inset. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
STH Infection data
The field and laboratory procedures have been previously described in19. In brief, stool container was distributed to consenting household members in advance. Participants’ unique identifiers were marked on the containers and detailed instructions of how to collect a fresh morning stool sample were given. Stool samples were processed in a designated area provided by the community leader. Duplicate sediment slides were prepared from 1 g of each stool using SAF-Ether concentration method19,29. The slides were examined under a light microscope by experienced laboratory technicians 2 h post sample collection. Infection was defined as the presence of at least one helminth egg on one of the two slides. The parasites’ eggs were counted for each species, and number of eggs per species and per stool examined was recorded for each participant19.
Environmental and socio-economic predictors
Nine environmental variables (elevation, enhanced vegetation index (EVI), normalized difference vegetation index (NDVI), land surface temperature for day (LSTD), land surface temperature for night (LSTN), rainfall, population, soil pH, and soil moisture; three socio-economic variables (night light emission (NLE), improved access to sanitation facilities and improved access to drinking water facilities) were used in the analysis. NLE was used as a proxy for urbanization and economic growth. These variables were chosen because they are either directly associated with prevalence of STH infections or they serve as proxy for other factors that are known to influence STH transmission30. All environmental and socio-economic data were obtained from open-access remote sensing data sources between 2016 and 2018 (Table 1). We resampled all the covariates to a spatial resolution of 2 km by 2 km using bilinear interpolation for continuous surface which is required to produce the spatial prediction of prevalence at every location in Ogun state.
Population data
Population data of Nigeria at 100 m spatial resolution was obtained from World Pop31 population database. The total population count of people in Ogun state in 2017 was 5,103,988 out of 197,259,740 people in Nigeria. The number of school-aged children in Ogun state in 2017 was computed to be 1,913,868, which represents 37.5% of the total population of all persons.
Variable selection procedures for the geostatistical model
To select the best set of covariates for the geostatistical model, we first examined the covariates for correlation using Pearson’s rank correlation index. Pairs of covariates with high correlation values (Pearson correlation > 0.7) were identified and only one of the correlated variables was included in the modelling process. The one included was chosen by visualizing the association via a scatterplot. Then we used both forward and backward stepwise selection to select a parsimonious set of covariates required for the prediction of STH among all the candidate set of covariates. This is achieved by fitting a non-spatial generalized linear model relating the prevalence of each STH species with the covariates. The final set of covariates result in a model with the lowest Akaike information criterion (AIC) and a further inclusion of any of the covariates does not improve the performance of the model.
Geostatistical modelling
The prevalence survey data available for this analysis are, at any geographical location x, the number of individuals tested, m and the number of people tested positive for each of the STH species, \(y_{k}\), where \(y_{1}\) is the number of people that tested positive for Ascaris, \(y_{2}\) for Hookworm, and \(y_{3}\) for Trichuris. The sampling distribution of \(y_{k}\) is binomial with number of trials m and probability of positive outcome \(P_{k} \left( x \right)\), the prevalence at x. The variation of \(P\left( x \right)\) was modelled using a combination of socio-economic and environmental covariate effects d(x); unexplained residual spatial variation, S(x). Therefore, we developed a binomial logistic geospatial model given as
\(S\left( x \right)\) is modelled as a zero-mean discretely indexed Gaussian Markov Random Field (GMRF) defined on the triangulation of the domain of interest, such that the correlation between any two locations \(x_{i}\) and \(x_{j}\) is modelled using Matérn correlation function. S(x) serves two purposes in the model; (1) it helps to capture the geographical variation; and (2) it helps to predict prevalence at unobserved locations. The Matérn covariance function is given as
where \(d = x_{i} - x_{j}\), \(\kappa\) is a scaling parameter, \(K_{\nu }\) is the modified Bessel function of second kind and order \(\nu > 0\) and \(\Gamma \left( . \right)\) is the Gamma-function, \(\sigma^{2}\) is the variance, and the spatial range \(\rho = \sqrt 8 /\kappa\), the distance at which the spatial correlation is becomes negligible (< 0.1).
The model was fitted using the Integrated Nested Laplace Approximation (INLA) 36,37 and the Stochastic Partial Differential Equation (SPDE)38 approaches. INLAallows us to perform a fast Bayesian inference. Because no prior information was available, an independent vague zero-mean Gaussian prior distribution was assigned to the fixed and random effects parameters. Posterior distributions were obtained for all the parameters and were summarised to obtain the mean and 95% credible interval (CI). Prediction of the prevalence of each of the STH species were provided at 2 km spatial resolution throughout the study area. We then estimate the prevalence of any STH prevalence by assuming that each species is independent38. The predicted prevalence was represented as the posterior mean.
Model validation
We validated our geostatistical model by assessing the predictive performance of the model using the 5-folds cross-validation. All survey data were randomly splitted into 5 groups. We hold-out each unique group then fit the model on the remaining groups and evaluate the predictive performance of the model on the hold-out group. The withheld data was matched with the predictions to summarize the performance of the model using the correlation, bias, root mean square error (RMSE) and the coverage probability.
Estimating the amount of the anthelmintic treatment required
We estimated the amount of anthelmintic treatment (albendazole or mebendazole) needed to treat the population annually at the local government areas in Ogun state. According to the WHO STH treatment decision tree39, prevalence of STH should be examined after 5–6 rounds of annual or biannual PC. Subsequent chemotherapy campaigns after this evaluation should continue according to a set of endemicity classes defined by the following prevalence thresholds; suspend PC if prevalence is < 2%; biennial PC if prevalence is between 2 and 10%; annual PC if prevalence is between 10 and 20%; biannual PC if prevalence is between 20 and 50%; triannual PC is prevalence is greater than 50%39. Hence, we computed the total number of anthelmintic drugs by classifying each pixel according to the treatment decision, thereby estimating the number of MDA rounds. Then multiply the number of MDA rounds by the total population of that pixel. Hence, we aggregate across the pixels the number of anthelmintic drugs over the local government areas. Also, we estimated the number of anthelmintic drugs required to treat school-aged children (SAC) by multiplying the number of MDA rounds per pixel by the population of SAC per pixel. Then we constructed the local government level estimate by aggregating across the pixels.
Estimating the number of people infected with STH
To estimate the number of people infected with STH parasites, we multiplied the prevalence at each pixel by the total population at that pixel. Also, to estimate the number of SAC infected with STH parasite, we multiplied the prevalence at each pixel by the population of SAC at that pixel. Hence, to construct the local government level estimate, we aggregate the values across the pixels. The 95% confidence interval of the estimate was constructed by using the prevalence values at the 2.5% and 97.5% quantiles.
Ethical approval
Ethical clearance for this study (HPRS/381/183) was obtained from the Ethics review committee of Department of Planning, Research and Statistics, Ogun State Ministry of Health, Oke Imosan Abeokuta, Nigeria. Prior to data collection, visitations were made to the LGAs and the selected communities were the objectives and study procedures were explained and permissions for field survey were sought. Written informed consents were obtained from household heads and corresponding occupants of their households. Children below age sixteen, completed assent forms through their parents or guardians. All methods including recruitment of participants, collection of participant’s data and samples, laboratory analysis and data management were performed in accordance with the 1964 Declarations of Helsinki.
Results
Data summaries
A total of 1027 infection data was included in this survey. The demographic characteristics of the study population have been described elsewhere19. However, Table 2 summarizes the soil-transmitted helminths species-specific prevalence among the examined participants. In short, an overall prevalence of 17.2% (95% confidence interval (CI) 14.9, 19.5) was recorded for any STH infection. Ascaris lumbricoides infections was the most predominant, with an overall prevalence of 13.6% (95% CI 11.5, 15.7), while Hookworm and Trichuris trichiura had overall prevalence of 4.6% (95% CI 3.3, 5.9) and 1.7% (95% CI 0.9, 2.4), respectively. The geographical distribution of the empirical prevalence for each soil-transmitted helminth species has been described elsewhere19.
Geostatistical variable selection, model parameter estimates and model validation
Following the variable selection, Soil pH, soil moisture and elevation were selected for Ascaris, Hookworms, and Trichuris infections respectively (Table 3). The selected covariates were used to build predictive risk models specific to each of the three soil transmitted helminth species. A negative association exist between Ascaris lumbricoides infection and soil pH (odds ratio (OR) = − 0.05; 95% credible interval (CrI) − 0.071, − 0.029). Residual spatial correlation was estimated to be 12.813 km (95% CrI 5.199, 26.760 km). Similarly, a negative association was observed between Hookworms and soil moisture (odds ratio (OR) = − 0.027; 95% credible interval (CrI) − 0.042, − 0.015). Residual spatial correlation was estimated to be 17.823 km (95% CrI 4.834, 50.090 km). For Trichuris trichiura, infection risk was also negatively associated with elevation (odds ratio (OR) = − 0.027; 95% credible interval (CrI) − 0.042, − 0.015). Residual spatial correlation was estimated to be 5.427 km (95% CrI 2.789, 17.190 km). The predictive performance of the model based on a 5-folds cross-validation showed that; for Ascaris lumbricoides, the in-sample observed data and the predictions has a coefficient correlation of 0.82; a RMSE of 0.04; and a bias of − 0.005; and coverage probability of 0.86, respectively; for Hookworms, the in-sample observed data and the predictions has a coefficient correlation of 0.83; a RMSE of 0.06; and a bias of − 0.001; and coverage probability of 0.88, respectively; and for Trichuris, the in-sample observed data and the predictions has a coefficient correlation of 0.83; a RMSE of 0.07; and a bias of -0.01; and coverage probability of 0.89, respectively.
Predictive risk maps of soil-transmitted helminth infections
Overall predicted mean prevalence of STH infections in Ogun State is 19.2% (95% CrI 1.82, 58.9) and ranges from 7.0 to 38% across the IUs. However, by species, predicted mean prevalence for Ascaris lumbricoides was 12.4% (95% CrI 0.98, 44.9), ranging from 5.0 to 23.8% across the IUs. For hookworms, the predicted mean prevalence was 6.2% (95% CrI 0.73, 22.5), and ranges between 2.0 and 14.5% across the IUs. The predicted mean prevalence of 1.9% (95% CrI 0.13, 8.84) was estimated for Trichuris trichiura, with a range between 0.1 and 5.7% across the IUs (Table 4). Figure 2 present the overall and species-specific predictive risk maps of soil-transmitted helminth infections. Predictive risk map for overall STH infection shows a high prevalence (> 20%) for LGAs in the central and western part of the state. Pockets of very high prevalence (> 40%) were also predicted for the LGAs around the boundary regions in the south-western part of the state. However, predicted prevalence were predominantly between 12 and 15% in the eastern part of the state. For Ascaris lumbricoides, pockets of high prevalence (> 20%) was predicted in the central and western part of Ogun State, with hotspots in the LGAs located close the border regions in the south-western part of the country. Moderate to high prevalence (5–20%) were also predicted in these regions. However, low predicted prevalence (5–10%) were observed in the eastern part of the country, with a sparse predicted prevalence within 10–12% around the border regions (Fig. 2). For hookworms, the predicted risk map shows that most regions have prevalence below 20%, except some pocket areas in Ipokia LGA, around the boundary lines. Predicted prevalence were predominantly between 5 and 10% in the central and western part of the state. However, predicted prevalence were lower (2–5%) in the eastern regions (Fig. 2). For Trichuris trichiura infections, most of the LGAs in the northern part of the state had predicted prevalence value between 0 and 1%. Similarly, in the southern region, predicted prevalence were predominantly between 5 and 10% (Fig. 2). The uncertainty of these estimates is presented in the standard error maps which can be found in Supplementary Fig. S1.

Available at https://www.R-project.org/.
Map showing the predicted risk of soil transmitted helminth infections in Ogun State. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Estimates of number of people infected and annual drug requirements
Table 5 shows the number of infected individuals, estimated rounds of MDA and drug requirements for combined treatment of entire population and school-aged population across the IUs in the State. About 55% (11/20) of the IUs in the State requires biannual rounds of MDA, while 35% (7/20) and 10% (2/20) requires annual and biennial MDA rounds respectively. For the entire population, comprising of preschool-aged children, school-aged children and adults, a total of 1,099,461 persons are estimated to be infected, requiring 7,866,374 drugs. By IUs, about 10 LGAs has over 50,000 infected persons each, requiring more than 300,000 albendazole or mebendazole tablets. Ado Odo-Ota LGA has the highest number of infected population (168,591 persons) and requires 1,204,423 albendazole or mebendazole tablets. The least number of infected population (9103 persons) were estimated for Ijebu North-east, requiring 64,181 tablets.
However, for school-aged population, a total of 375,374 were infected, requiring 2,685,618 drugs for preventive chemotherapy. Ten LGAs in the central and western part of the state had over 10,000 infected school-aged children, and requires over 100,000 albendazole or mebendazole tablets each, for mass administration campaigns. The highest number of infected school-aged children (56,556) were estimated for Ado-Odo Ota LGA, requiring a total of 404,123 albendazole or mebendazole tablets. The least number of infected school-aged population (3118) were estimated for Ijebu North-east, requiring 21,980 tablets.
Discussion
In this study, we utilized soil-transmitted helminth infection data from a state-wide cross-sectional survey to produce model-based estimates of infection risk, number of people infected, rounds of MDA and annual drug requirements for preventive chemotherapy. Empirical prevalence estimates for each of the three STH were below 20%, with Ascaris lumbricoides been the most predominant species (13.6%), followed by hookworm (4.6%) and Trichuris trichiura (1.7%)19. However, based on our model predictions, prevalence ranged from 5.0 to 23.8% for Ascaris lumbricoides, from 2.0 to 14.5% for hookworms, and from 0.1 to 5.7% for Trichuris trichiura across the IUs. However, location-specific predictions shows that overall STH and Ascaris infections were as high as 53% and 34% respectively, and greatest around the border of Republic of Benin in the west. Also, heavy risk approaching thresholds level necessitating preventive chemotherapy were observed in the central and western region. The risk of hookworms, also exhibit a similar pattern, however the predicted prevalence was further reduced below PC threshold levels. Majority of the LGAs were at very low risk of Trichuris infection. The spatial patterns observed in this study is in-line with the findings of24 for the three STH species, except for Ascaris lumbricoides where additional risk was reported in the eastern part of the state. This observation might be explained by the differences in composition of the study population (total population versus SAC only) and sampling point (communities versus school) in this present study19,24.
Furthermore, our results indicate the influence of some environmental covariates on transmission of soil transmitted helminth infections. For example, soil pH was negatively associated with Ascaris, suggesting that as pH of the soil increases, the survivability of Ascaris egg reduces. The effect of elevated pH on inactivation of Ascaris eggs have been previously reported40. Similarly, soil moisture was negatively associated with the risk of hookworm infection. This finding corroborates the observation of41. Temperature and moisture are determining factors in the development of helminth eggs42, with rainfall playing a major role in the restoration of the latter43. However, there are presumptions that heavy rainfalls might wash out soil transmitted helminth eggs from the soil23,42,44. This might explain the negative relationship between soil moisture and hookworm infections. Also, elevation was negatively associated with Trichuris. This supports already established evidence that the risk of Trichuris trichiura is rare or absent as altitude increases45,46. Our findings are also in-line with previous reports from Bolivia47 and Nigeria24.
STH infections thrives in areas lacking sanitation, potable water source, personal and domestic hygiene4,7,9,19, hence we expected infections to be associated with socio-economic predictors such as access to improved sanitation and drinking water facilities. However, none of the socioeconomic variables were picked during the geostatistical variable selection process. This finding is similar with those reported in Cambodia22, India48, Ethiopia49 and Australia50. Reasons for the no-effect association may not be limited to; (1) probable loss of variability as a result of household data aggregation for community analysis22, (2) insufficient coverage of water and sanitation resource facilities48, (3) Lack of standard and better water, sanitation and hygiene assessment tool leading to information bias49 and latrine efficiency in containing excreta49.
Prior efforts to model the treatment needs and number of SAC infected in Ogun State, were based on national survey data collected across 555 locations in Nigeria, with less than 20 location-specific data in Ogun State24. Indeed, these data may not reflect the actual situation of infected SAC and drug requirements for PC in the state24. Our study therefore presents, a robust estimate for the state, using more recent survey data collected across 1027 locations in the State. Based on our estimate, about 55% (11/20) of the IUs in the State requires biannual rounds of MDA, while 35% (7/20) and 10% (2/20) requires annual and biennial MDA rounds respectively. We therefore estimated a total of 1.1 million infected persons (comprising pre-school aged children, school-aged children and adults) and a total of 7.8 million albendazole or mebendazole tablets in Ogun State. More specifically, we estimate that 375,374 SAC were infected and a total of 2.7 million albendazole or mebendazole tablets will be required for PC. These estimates are twice as high as the number of tablets reported in24 for the state.
This study has shown the predicted prevalence using a robust geostatistical approach, and as well the spatial pattern of disease spread. The empirical and predicted prevalence for Ascaris infections were above 20%, hence necessitating annual PC in most regions. However, there were significant reduction in the prevalence and spread of Hookworm and Trichuris infections. This observation reflects the yields of investment made by the WHO, donor agencies, and various governmental and non-governmental health development agencies supporting PC in the country.
Our predictive maps and estimated drug requirements are therefore important in planning, targeting and delivery of prioritized interventions. The maps can also be utilized for designing more robust spatial surveys to meet more specialized needs including evaluation of STH control programs or long-term surveillance. Furthermore, we believe our estimations on the number of pre-school aged children and adults infected are useful, in the phase of expanding PC to adult population, to sustain accrued gains in morbidity control and interruption of transmission51.
Conclusion
The work presented here contributes to the existing body of knowledge on model-based estimates of the geographical distribution of soil-transmitted helminth infection risk at more finite scale (i.e., scale smaller than the implementation units) in Ogun State in Nigeria. We used data generated across a community based cross-sectional study focusing on all sub-sets of a population (pre-school aged children, school-aged children and adults) to; (1) predict disease distributions, (2) identify associated environmental and socioeconomic risk factors, (3) estimate number of persons infected, and (4) estimate annual drug requirements. Our prediction maps provide useful information for identifying priority areas where interventions targeting soil transmitted helminthiasis are most urgently required. In addition, our estimations of drug needs are useful in the process of resource acquisition, planning and delivery of interventions.
Data availability
The datasets for environmental and socio-economic variables are publicly available in the remote sensing data repositories cited within the text. The primary STH infection datasets analyzed for this study have also been previously published and are available at https://doi.org/10.1371/journal.pone.0233423.s001.
Abbreviations
- PC:
-
Preventive chemotherapy
- STH:
-
Soil transmitted helminths
- WHO:
-
World Health Organization
- SAC:
-
School-aged children
- GIS:
-
Geographic information system
- RS:
-
Remote sensing
- IU:
-
Implementation units
- LGAs:
-
Local government areas
- SAF:
-
Sodium acetate acetic acid formaldehyde
- NDVI:
-
Normalized difference vegetation index
- EVI:
-
Enhanced vegetation index
- LSTD:
-
Land surface temperature for day
- LSTN:
-
Land surface temperature for night
- MDA:
-
Mass drug administration
- NLE:
-
Night light emission
- MODIS/TERRA:
-
Moderate resolution imaging spectroradiometer
- C3S:
-
Corpernicus climate change service
- WORLDPOP:
-
World population database
- LBD:
-
Local Burden of Disease Project
- AIC:
-
Akaike information criterion
- GMRF:
-
Gaussian Markov random field
- INLA:
-
Integrated nested Laplace approximation
- SPDE:
-
Stochastic partial differential equation
- CI:
-
Confidence interval
- OR:
-
Odd ratio
- BCI:
-
Bayesian credible interval
References
WHO. Soil-transmitted helminth infections. World Health Organization. 2020. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections. Accessed 5 April 2021 (2020).
Bethony, J. et al. Soil transmitted helminth infections: Ascariasis, trichuriasis and hookworm. Lancet 367(9521), 1521–1532 (2006).
De Silva, N. R. et al. Soil transmitted helminth infections: Updating the global picture. Trends Parasitol. 19(12), 547–551 (2003).
Mogaji, H. O. et al. A preliminary survey of School-based Water, Sanitation, Hygiene (WASH) Resources and soil-transmitted helminthiasis in eight public schools in Odeda LGA, Ogun State. Nigeria. Parasitol. Open. 3(16), 1–10 (2017).
Acka, C. A. et al. Parasitic worms: knowledge, attitudes, and practices in western Côte d’Ivoire with implications for integrated control. PLoS Negl Trop Dis. 4(12), e910 (2010).
Belizario, V. Y. Jr., Totañes, F. I. G., de Leon, W. U., Lumampao, Y. F. & Ciro, R. N. T. Soil transmitted helminth and other intestinal parasitic infections among school children in indigenous people communities in Davao del Norte. Philippines. Acta Trop. 120(Suppl 1), 12–18 (2011).
Ziegelbauer, K. et al. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 9, e1001162 (2012).
Schmidlin, T. et al. Effects of hygiene and defecation behavior on helminths and intestinal protozoa infections in Taabo, Côte d’Ivoire. PLoS one 8, e65722 (2013).
Strunz, E. C. et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 11, e1001620 (2014).
WHO. Eliminating Soil-Transmitted Helminthiases as a Public Health Problem in Children. Progress Report 2001–2010 and Strategic Plan 2011–2020. World Health Organization, Geneva. https://apps.who.int/iris/bitstream/handle/10665/44804/9789241503129_eng.pdf. Accessed 5 April 2021 (2012).
NTSDC. $812 Million NTD Funding Pledged, World Record Set for Drug Donations & Other NTD News. Neglected Tropical diseases Support Center. https://www.ntdsupport.org/news/812-million-ntd-funding-pledged-world-record-set-drug-donations-other-ntd-news. Accessed 5 April 2021 (2017).
Montresor, A. et al. The global progress of soil-transmitted helminthiases control in 2020 and World Health Organization targets for 2030. PLoS Negl Trop Dis. 14(8), e0008505. https://doi.org/10.1371/journal.pntd.0008505 (2020).
WHO. Schistosomiasis and soil-transmitted helminthiases: Number of people treated in 2018. Wkly. Epidemiol. Rec. 50, 601–12 (2019).
WHO. 2030 targets for soil-transmitted helminthiases control programmes. Geneva: World Health Organization; Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/bitstream/handle/10665/330611/9789240000315-eng.pdf?ua=1. Accessed 5 April 2021 (2019).
WHO. Ending the neglect to attain the Sustainable Development Goals: A road map for neglected tropical diseases 2021–2030. World Health Organization. https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs--NTDRoadmap.pdf?ua=1#:~:text=The%20road%20map%20for%202021,targets%20during%20the%20next%20decade. Accessed 5 April 2021 (2020).
Li, T., He, S. Y., Zhao, H., Zhao, G. H. & Zhu, X. Q. Major trends in human parasitic diseases in China. Trends Parasitol. 26, 264–270 (2010).
Wang, X. B. et al. Soil-transmitted helminth infections and correlated risk factors in preschool and school-aged children in rural southwest China. PLoS One. 7, e45939 (2017).
Zhou, X. N., Bergquist, R. & Tanner, M. Elimination of tropical disease through surveillance and response. Infect Dis Poverty. 2, 1 (2013).
Mogaji, H. O. et al. Distribution of ascariasis, trichuriasis and hookworm infections in Ogun State, Southwestern Nigeria. PLoS ONE 15(6), e0233423 (2020).
Lai, Y. S., Zhou, X. N., Utzinger, J. & Vounatsou, P. Bayesian geostatistical modelling of soil-transmitted helminth survey data in the People’s Republic of China. Parasit Vectors. 6, 359 (2013).
Yapi, R. B. et al. Bayesian risk profiling of soil-transmitted helminth infections and estimates of preventive chemotherapy for school-aged children in Côte d’Ivoire. Parasit Vectors. 9, 162 (2016).
Karagiannis-Voules, D. A. et al. Geostatistical modelling of soil-transmitted helminth infection in Cambodia: Do socioeconomic factors improve predictions. Acta Trop. 141, 2014–2212 (2014).
Chammartin, F. et al. Soil-transmitted helminth infection in South America: a systematic review and geostatistical meta-analysis. Lancet Infect. Dis. 13, 507–518 (2013).
Oluwole, A. S. et al. Bayesian geostatistical model-based estimates of soil-transmitted helminth infection in Nigeria, including annual deworming requirements. PLoS Negl. Trop. Dis. 9(4), e0003740 (2015).
Hotez, P. J. & Kamath, A. Neglected tropical diseases in sub-Saharan Africa: Review of their prevalence, distribution and disease burden. PLoS Negl. Trop. Dis. 3, e412 (2019).
Hotez, P. J., Asojo, O. A. & Adesina, A. M. Nigeria: “Ground Zero” for the high prevalence neglected tropical diseases. PLoS Negl. Trop. Dis. 6, e1600 (2012).
Yaro, C. A., Kogi, E. & Luka, S. A. Spatial distribution and modeling of soil transmitted helminthes infection in Nigeria. AID. 8, 82–107 (2018).
Ekpo, U. F. et al. Mapping and prediction of schistosomiasis in Nigeria using compiled survey data and Bayesian geospatial modelling. Geospat Health. 7, 355–366 (2013).
Endriss, Y., Elizabeth, E., Rohr, B., Rohr, H. & Weiss, N. Methods in Parasitology: SAF Method for Stool Specimen. Basel: Swiss Tropical Institute; (2005).
Deshpande, A. et al. Mapping geographical inequalities in access to drinking water and sanitation facilities in low-income and middle-income countries, 2000–17. Lancet Glob. 8(9), e1162–e1185 (2020).
WorldPop. Population Data. https://www.worldpop.org/project/categories?id=14. World Population Database: 2017–2018. Accessed 5 January, (2021).
MODIS. Land Surface Temperature, Elevation, Normalized Difference Vegetation Index and Rainfall Data. https://modis.gsfc.nasa.gov/data/dataprod/. Moderate Resolution Imaging Spectroradiometer (MODIS): 2017-2018. Accessed 5 January, (2021).
SoilGrids250m. Soil pH. https://soilgrids.org/. Soil Grids: 2017–2018. Accessed 5 January, (2021).
C3S. Soil Moisture. https://cds.climate.copernicus.eu/cdsapp#!/dataset/satellite-soil-moisture?tab=overview. Corpernicus Climate Change Service: 2017–2018. Accessed 5 January, (2021).
LBD. LMIC drinking water and sanitation geospatial estimates 2000–2017. https://cloud.ihme.washington.edu/s/bkH2X2tFQMejMxy. Local Burden of Disease project: 2000–2017. Accessed 5 January, (2021).
Rue, H., Martino, S. & Chopin, N. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. Ser. A Stat Soc. Ser. B 71(2), 319–392 (2009).
Moraga, P. Geospatial Health Data: Modeling and Visualization with R-INLA and Shiny (CRC Press, 2019).
Lindgren, F., Rue, H. & Lindström, J. An explicit link between Gaussian fields and Gaussian Markov random fields: The stochastic partial differential equation approach. J. R. Stat. Soc. Ser. A Stat. Soc. Ser. 73(4), 423–498 (2011).
WHO. Helminth control in school-age children: a guide for managers of control programmes. World Health Organization. https://www.who.int/neglected_diseases/resources/9789241548267/en/. Accessed 15 April 2021 (2011).
Senecal, J., Nordin, A. & Vinnerås, B. Fate of Ascaris at various pH, temperature and moisture levels. J. Water Health. 18(3), 375–382 (2020).
Chammartin, F. et al. Spatio-temporal distribution of soil-transmitted helminth infections in Brazil. Parasit. Vectors. 7, 440 (2014).
Brown, H. W. Studies on the rate of development and viability of the eggs of Ascaris lumbricoides and Trichuris trichiura under field conditions. J. Parasitol. 14, 1–15 (1927).
Shreve, F. Rainfall as a determinant of soil moisture. Plant World. 17(1), 9–26 (1914).
Gunawardena, G. S., Karunaweera, N. D. & Ismail, M. M. Wet-days: Are they better indicators of Ascaris infection levels?. J Helminthol. 78, 305–310 (2004).
Bundy, D. A. P. & Cooper, E. S. Trichuris and trichuriasis in humans. Adv. Parasitol. 28, 107–173 (1989).
Nolf, L. O. Experimental studies on certain factors influencing the development and viability of the ova of human Trichuris as compared with those of the human Ascaris. Am. J. Hyg. 16, 288–322 (1932).
Chammartin, F. et al. Modelling the geographical distribution of soil-transmitted helminth infections in Bolivia. Parasit. Vectors 6, 152 (2013).
Clasen, T. et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: A cluster-randomised trial. Lancet Glob. 2, e645–e653 (2014).
Oswald, W. E. et al. Association of community sanitation usage with soil-transmitted helminth infections among school-aged children in Amhara Region, Ethiopia. Parasit. Vectors 10, 91 (2017).
Campbell, S. J. et al. Water, Sanitation and Hygiene (WASH) and environmental risk factors for soil-transmitted helminth intensity of infection in Timor-Leste, using real time PCR. PLoS Negl Trop. 11(3), e0005393 (2017).
Karagiannis-Voules, D. A. et al. Spatial and temporal distribution of soil-transmitted helminth infection in sub-Saharan Africa: A systematic review and geostatistical meta-analysis. Lancet Infect. Dis. 15, 74–84 (2015).
de Silva, N. & Hall, A. Using the prevalence of individual species of intestinal nematode worms to estimate the combined prevalence of any species. PLoS Negl Trop Dis. 4(4), e655 (2010).
Acknowledgements
We are grateful to the community leaders and spokespersons that assisted with entry into the study locations. We also appreciate the study participants for their co-operation and participation. Our appreciation also goes to the Ogun State Ministry of Health for their assistance with field approvals and permits.
Author information
Authors and Affiliations
Contributions
H.O.M. and U.F.E. conceptualized the study. H.O.M., U.F.E., G.A.D., B.S.B. and S.B. designed the study. H.O.M., O.N.A., A.B.A. and O.O.J. downloaded remotely-sensed environmental and socioeconomic variables. H.O.M., O.N.A. and O.O.J. managed the data, O.O.J. performed all geostatistical analysis. H.O.M., A.B.A., O.O.J., U.F.E. interpreted the data. H.O.M. prepared the first draft of the manuscript, which was first reviewed by U.F.E., A.B.A., O.O.J. All authors contributed to the development of the final manuscript and approved its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mogaji, H.O., Johnson, O.O., Adigun, A.B. et al. Estimating the population at risk with soil transmitted helminthiasis and annual drug requirements for preventive chemotherapy in Ogun State, Nigeria. Sci Rep 12, 2027 (2022). https://doi.org/10.1038/s41598-022-06012-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06012-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.