Abstract
The effects of [Co2 (SCN) 4(hmt)2(H2O)6. H2O] (SC1) and [Co (CN)6 (Me3Sn)3(H2O). (qox)] (SCP2) MOF as corrosion inhibitors on C-steel in 0.5 M sulfuric acid solutions are illustrated utilizing mass reduction (MR), electrochemical [potentiodynamic polarization (PP), and AC electrochemical impedance (EIS)]. The experiments revealed that as the dose of these compounds rose, the inhibition efficacy (IE percent) of C-steel corrosion improved, reaching 80.7–93.1% at dose 25 × 10−6 M for SC1 and SCP2, respectively. IE percent, on the other hand, dropped as the temperature range grew. SC1was adsorbed physically and chemically (mixed adsorption) but SCP2 was adsorbed physically on the surface of C-steel and conformed to the Langmuir adsorption isotherm equation. The PP studies revealed that these compounds act as mixed kind inhibitors. To establish the morphology of the inhibited C-steel surface, scanning electron microscopy (SEM), energy transmitted X-ray (EDX), and atomic force microscopy (AFM) studies were used. All tested experiments were in good agreement.
Similar content being viewed by others
Introduction
Because the use of toxic chemicals as inhibitors has been prohibited due to environmental concerns, there is a strong interest in replacing dangerous inhibitors with non-hazardous alternatives1,2,3,4,5,6,7,8,9. Sulfuric acid is primarily used in the manufacture of fertilizers. It is commonly used in the production of chemicals, medicines and used as pickling agent for metals to remove scales. Corrosion inhibitors have been widely researched in various sectors to reduce the dissolving rate of metal in contact with corrosive environments10,11,12,13,14. The capacity of corrosion inhibitors to absorb on metal surfaces was shown to be associated with their high efficiency15. Adsorption will also be based on the predicted interaction of the inhibitor's-orbitals with the surface atoms' d-orbitals. This interaction increases adsorption on the C-steel surface, resulting in film-protective corrosion16. The authors concentrated their efforts on utilizing organic compounds as inhibitors that can be obtained, are environmentally friendly, inexpensive, and have renewable sources of obtaining them, and contain heteroatoms such as O, N, S, and multiple bonds in their molecular structure, giving them a strong affinity to inhibit metal corrosion in acid solutions17,18,19,20,21. Earlier studies shown that organic compounds containing heteroatoms such as N, O, S, and others, as well as aromatic rings, work as excellent corrosion-protecting chemicals. The MOF looks to be a potential inhibitor because it mixes of metal ions with an organic framework. The addition of more electropositive metals to the organic framework enhances sacrificial efficacy. The organic structure, on the other hand, forms a protective covering over the metal surface, slowing corrosion. A few studies in the literature22 showed that MOF can be used as an efficient corrosion inhibitor reported a 3D network of silver-based MOFs that were found to be suitable for preventing C-steel corrosion in 1 M HCl solution. In another investigation, metal organic frameworks based on both silver and nitrogen donors were shown to be efficient Cu corrosion inhibitors in HCl solution23. MOFs with organic ligands comprising substituted aryl, heteroaryl, or heterocyclic compounds with an exocyclic sulphur group have also been described for use as corrosion inhibitors in metals and alloys24. A new MOF from Cd has been reported in the literature25. The impact of Co, Ni, and Cu metal-based MOFs on mild steel corrosion prevention was reported in 201726. MOF research has resulted in the use of hydrophobic MOFs like ZIF-8 in the anticorrosion sector27. ZnAl-CO3 layered double hydroxide precursor buffer layers were transformed to well inter grown ZIF-8 coatings in that study28. A recent research created an anticorrosive coating for the petrochemical industry using Samarium (III) nitrate and [bis(phosphonomethyl) amino] methyl phosphonic acid (ATMP) to preserve mild steel in saline solutions29. According to a thermodynamic research30, the extremely excellent inhibitory property of MOF on metal surface was attributed to chemisorption of MOF on metal surface30.
MOFs derived from nanostructures have a higher specific surface area and good form, such as nano cages and hollow spheres, as compared to other nanostructures. This motivated us to investigate the use of SC1 and SCP2 as corrosion inhibitors for C-steel in 0.5 M H2SO4 solutions. In this study, we utilized the chemical approach (mass reduction method) and electrochemical methods (potentiodynamic polarization (PP) and electrochemical impedance (EIS)). Attenuated total reflection infrared (ATR-IR) and atomic force microscopy (AFM) were employed to assess the metal–organic and surface morphology of C-steel, respectively.
Experimental
Composition of C-steel samples
The experiments were performed with C-steel type C1018 with the following: composition (Table 1).
For mass reduction measurements, rectangular specimens with dimensions of 2 × 2x0.2 cm were utilized. The exposed surface area of carbon steel for electrochemical testing was 1 cm2.
Chemicals
By diluting the stock solution (1 × 10–3 M) of these compounds with double distilled water, various inhibitor concentrations “(5, 10, 15, 20, and 25 × 10–6 M) were produced. In 0.5 M H2SO4, the maximal soundness of a metal–organic compound was reported to be 25 × 10–6 M. The metal–organic complex employed in this work is highly soluble in water, has higher molecular weights, and includes a significant number of donating atoms (N and O)”.and easily available, non-toxic and their structures are listed in Table 2.
Preparation and characterization of inhibitors
Preparation of [Co2(SCN)4(hmt)2(H2O)6. H2O], (SC1)
In the presence of ultrasonic radiation, red crystals of [Co2(SCN)4(hmt)2(H2O)6. H2O], SC1, were produced. In an ultrasonic bath, a 0.118 g (0.5 mmol) solution of CoCl2.6H2O dissolved in 10-mL bidistilled H2O was gradually added to a stirred mixture of 0.099 g (0.5 mmol) trimethyl tin chloride and 0.097 g (1 mmol) KSCN in 10 mL of CH3CN/deionized H2O. Following a few minutes of magnetic stirring, a solution of 0.07 g (0.5 mmol) hexamethylenetetramine (hmt) in 10 mL H2O was added to the mixture drop by drop. The resulting mixture was ultrasonically treated for 6 h at 30 °C with a power of 60 W. Filtering, precipitation, washing with 10 mL H2O, and drying in the open air were then used to separate the precipitate SC1 had been obtained in the amount of 367 mg (94.2 percent). SC1, C16H40Co2N12O7S4, MW = 774.70 g/mol, calculated percent: C, 25.47, H, 5.28, N, 22.20, Co, 15.57, S, 16.91; found percent: C, 25.35, H, 5.36, N, 22.15, Co, 15.61, S, 16.97. The beam line (XRD1) of the Elettra Synchrotron in Trieste (Italy) was used to gather X-ray single-crystal diffraction data31. SC1, cm1 IR-data: 3900 (vOH) of H2O, 2952 (assym.CH), 2279 (sym.CH), 1457 (CH), and (δCH), and 697 (γCH) of hmt, 1012 and 1242 (νC-N), 2170 (υC≡N) thiocyanate,814 (υCS), 426 (υCo–N), 451 (υCo–Nhmt).
Synthesis of ∞ 3[Co (CN)6(Me3Sn)3(H2O). (qox)], (SCP2)
Self-assembly of the ternary adducts of K3[Co (CN)6] [104 mg (0.315 mmol)] in 10 mL H2O, Me3SnCl [189 mg (0.95 mmol)] in 10 mL H2O, and quinoxaline (qox) [41 mg (0.315 mmol)] in 10 mL acetonitrile yields white prismatic crystals. After filtration, washing with small quantity of cold H2O and acetonitrile and drying overnight, 169 mg (63.3% referred to K3[Co (CN)6]) of colorless crystals of SCP2 were obtained. Anal. Calc. for SCP2 (C23H35O1N8CoSn3) MW = 854.64 g mol−1, %: C, 32.32; H, 4.13; N, 13.11; Co, 6.90; Found: C, 32.10; H, 4.01; N, 13.06; Co, 7.30. FT-IR-data (cm−1): 3444 (νH2O), 2158 (νC≡N), 1631 (νC=C), 1401(νC=N), 792(γCH), 547(νSn-N), 427 (νCo-C). Data for X-ray single crystal diffraction are collected at the Elettra Synchrotron's beam line (XRD1) in Trieste, Italy31.
Methods
Mass reduction (MR) tests
The usual technique for measuring the dissolution rate and inhibition efficacy (percent IE) is MR approach in which a 2 × 2 × 0.2 cm2 piece of metal is used. The samples are cut and sanded as previously, then washed with double distilled water, dried, and weighed before being placed in solutions made from varying dosages of metal–organic compounds ranging from 5 × 10–6 to 5 × 10–6 M in a beaker containing 0.5 M H2SO4 and changing quantities of metal–organic compound inhibitors for 3 h. and metal–organic compound inhibitors in varying concentrations for 3 h. This happens in the presence of 0.5 M H2SO4 when compared to a sample put in a solution of 0.5 M sulfuric acid without the addition of metal–organic compounds. The samples are weighed before being re-immersed in respective solutions. The temperature varies between 298 and 318 K. After drying thoroughly, for 3 h, it was put in a beaker with 0.5 M H2SO4 and varying amounts of metal–organic inhibitors. All experiments were repeated three times for reproducibility.
Electrochemical tests
Measurements of PP
The capacity of PP was adjusted automatically from − 700 to + 700 mV against (Eocp). At a scan rate of 1 mVs-1, the power was measured.
Measurement of EIS
All open-circuit testing with EIS were carried out with AC signals ranging from 100 kHz to 0.1 Hz and peak amplitudes of 10 mV at open circuit potential (OCP). The equipment used in electrochemical experiments was a “Gamry Potentiostat/Galvanostat/ZRA” (PCI4-G750). Gamry comprises the DC105 DC Corrosion Program, the EIS300 EIS Program, and a data gathering computer. To plot and compute data, Echem Analyst version 5.5 was used”.
Morphology of the surface
Attenuated Total Reflection Infra-Red (ATR-IR) analysis
ATR-IR spectra were recorded in the spectral region “4000 to 500 cm−1” using the Attenuated Total Reflectance (ATR) technique on an FTIR-Spectrometer iS 10. (Thermo Fisher Scientific, USA). The FT-IR spectrum is a useful tool for comparing inhibitor and corrosion products following inhibitor adsorption. After immersion for a period, the FT-IR peak values for metal–organic and C-steel were obtained. After 24 h of immersion in the acid corrosive solution with 25 × 10−6 M of metal–organic, the peak values of the FT-IR were recorded for metal–organic and C-steel32.
Atomic force microscopy (AFM) analysis
AFM is a modified test that provides data on the surface of a C-steel sample with metric linear purity. Persecution is used to apply and appraise measured knowledge33. Adapted from the SPM management computer code34.
Results data and discussion
Crystal structure of ∞ 3[{Co(hmt)2(NCS)2(H2O)2} {Co (NCS)2(H2O)4 (H2O)], (SC1)
Sonochemical synthesis of the quaternary adducts hmt, CoCl2·6H2O, Me3SnCl, and KSCN in CH3CN/H2O resulted in red crystals of the tin-free empirical composition [Co2(SCN)4(hmt)2(H2O)6H2O], SC1. Table 3 contains the lattice constants and refinement parameters of SC1, whereas Table 4 has the bond lengths and angles. SC1's structure displays two unique complexes composed of two crystallographic and chemically distinct CoII atoms, one hmt molecule, two thiocyanate ligands, three coordinated water ligands, and one H2O molecule of crystallization (Fig. 1a). SC1's unit cell structure, on the other hand, consists of two neutral complexes with two different CoII atoms, four thiocyanate ligands, and one thiocyanate Two hmt ligands, six coordinated water molecules, and one uncoordinated water molecule (Fig. 1b). The Co1 atom exhibits octahedral shape based on bond lengths and angles (Table 4, Fig. 1b). The Co–N–C angle shows bent structure (163.93°). To create the OC-6 structure, the Co2 atom coordinates with two thiocyanate groups in apical positions and four water molecules in an equatorial plane geometry, which is maintained by bond lengths and angles (Table 4). The two CoII components form 1D chains connected by many strong H bonds (1.936–2.077). (See Fig. S1). As seen in Fig. S2, the structure of SC1 extends three dimensions via strong H-bonds. The lattice water molecule and the Co2 fragment, [Co (SCN)2(H2O)4,] are arranged in rows between the chains of [Co (SCN)2(hmt)2(H2O)2], and they are responsible for SC1's strong backing structure via strong H-bonds (1.911–2.994).
Crystal structure of 3 ∞[Co (CN)6(Me3Sn)3(H2O)(qox)], (SCP2)
SCP2's asymmetric unit is composed of one crystallographically independent CoIII center, six ordered cyanide ligands, three crystallographically distinct Me3Sn+ cations, and one coordinated water molecule, as well as qox as a guest molecule, as shown in Fig. 2, Table S3 (in the suplimintary file). The CoIII atom is six coordinated to the carbon end of the six ordered cyanide ligands with Co–C distances in the range of 1.872(5) – 1.884(6) Å, Table S4. Table S4 shows that the C–Co–C angles imply an octahedral shape of the CoIII core. The Co (CN)6 building blocks are the primary components that make up the host network that is bridged by the Me3Sn+ cations (in the suplimintary file). Tin atoms are coupled to the nitrogen ends of the cyanide groups, resulting in a trigonal bipyramidal structure. Surprisingly, the Sn3 atom has a distinct crystallographic structure than the Snl and Sn2 atoms. The Sn3 atom coordinates with three methyl ligands to create the Tp-3 configuration, whereas the N3 atom and one water molecule are located at axial locations, as shown in Fig. 2. As a result, the Me3Sn1 and Me3Sn2 cations act as connectors between the Co (CN)6 building blocks, resulting in 1D-coordinated chains (Fig. S3). while the Me3Sn3 cation structure ends with an H2O ligand, which helps in the formation of H-bonds (1.988–3.085) and—stacking (qox—O = 2.771). Surprisingly, five of the cyanide ligands behave as 2-ligands, while the C5N5 ligand has a free uncoordinated nitrogen end that may make H-bonds with the guest qox molecules (2.703–2.735) and water molecules (2.739). SCP2’s structure propagates three-dimensionally based on infinite, but nonlinearly coordinated –[–Co–CN–Sn–NC–Co]– chains that cross each other at quasi-octahedral Co sites, as seen in Fig. S4 (in the suplimintary file). Each qox guest molecule is linked by H-bonds (2.456) and π–π interaction (3.278), and the chains are connected by H-bonds through O1 and C5N5. The 3D-network structure comprises a deformed adamantoid [Co9(CN)18(Me3Sn)9] ring, as illustrated in Fig. S5. The network space comprises methyl groups and guest qox molecules in addition to the coordinated water group, resulting in a stunning structure.
Mass reduction (MR) tests
The mass loss which calculated from MR is given by Eq. (1):
W1, W2 are the weights of the C-steel specimens before and after reaction with solution.
Equation 2 was used to calculate the IE percentage:
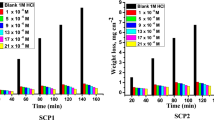
where ΔW and ΔWi represent the MR per unit area in the absence and presence of prepared samples, respectively. This measurement was performed in accordance with ASTM standard G 31–7235. The MR—time curves for C-steel in the presence and absence of changed dosages ranging from 5 × 10–6 to 25 × 10–6 M for SC1 and SCP2 are shown in Fig. 3. The kcorr grew as the temperature increased, therefore the kcorr increased while the IE percent decreased. The curves in the presence of inhibitors are lower than those in the absence of inhibitors. The higher IE percent with increased dosage of metal–organic compounds can be attributed to the formation of an inhibitor layer on the C-steel surface via adsorption. This layer is formed by the free electron pairs on the oxygen and nitrogen atoms of metal–organic compound molecules, as well as the π-electrons of aromatic rings. The reduction in IE percent with rising temperature is most likely due to a higher rate of desorption, which is physical adsorption; the IE percent order was: SC1 > SCP2 Table 5 for example, shows the IE percent and kcorr at various doses of metal–organic SC1 of C-steel at temperatures ranging from 298 to 318 K for 120 min immersion. As seen in the Table, raising the temperature lowers the % IE while raising the inhibitor doses raises it.
Temperature influence on corrosion procedure
The activation energy Ea*, which can be derived from Eq. (3), is an essential component that influences the speed of reaction and the kind of adsorption.
where kcorr is the corrosion rate. Figure 4 depicts Arrhenius diagrams for SC1 and SCP2 [log (kcorr) versus 1/T], where the E*a energy of the activation of the results was obtained in Table 6, It suggests that the surface reaction dominates the overall activity since the activation corrosion process (Ea*) is more than (20 kJ mol−1) and the activation energy increases as the dosage of metal–organic compound increases. Energy rises as the dose of metal–organic compound increases, it appears that the surface reaction dominates the overall activity. The adsorption nature of metal–organic compounds on C-steel causes this rise, which correlates to the physical adsorption of metal–organic compounds36,37,38,39. The transitional state equation was used to calculate the changes in entropy and enthalpy. The activation enthalpy (ΔH*) and entropy (ΔS*) increases for C-steel corrosion in 0.5 M H2SO4 are calculated using the equation below:
where symbol “h” is the Planck's constant and N is the Avogadro’s number. Graph of log (kcorr/T) versus (1/T) for unprotected C-steel at 0.5 M H2SO4 and in the existence of metal–organic compounds is shown in Fig. 5, which gave straight lines with slope equal (− ΔH*/2.303R) and an intercept equal (log R/Nh − ΔS*/2.303R) from which ΔH* and ΔS* data were calculated and depicted in Table 6. Negative results for (ΔH*) on the C-steel surface, indicating that the reaction that occurs during the dissolving process is exothermic, and it is known that they may be used to chemical and physical adsorption40,41,42. The mean values (ΔS*)” are both high and negative, indicating that the activated complex is associated rather than dissociated during the rate-determining stage.
Adsorption isotherm behavior
Studding of adsorption isotherms help us to explain the reaction occurred among the C-steel surface and metal–organic additives. It is deduced that θ increased with raising the inhibitor dose; this is because of the adsorption of metal–organic additive molecules on the C-steel surface. It is also supposed that the adsorption of the studied metal–organic additives is proceeding with the monolayer adsorption so that the adsorption process may obeys Langmuir isotherm. The Cinh/relationship dependence for SC1 and SCP2 is shown in Fig. 6, because of the dosage of metal–organic compounds (Cinh) obeying Langmuir isotherm adsorption.
where Kads is the equilibrium adsorption constant intricate in chemical reaction
In which the free adsorbent energy is stimulated by a 55.5 dosage of molar water in solution. The data pattern revealed a negative sign of \(\Delta G_{ads}^{ \circ }\) due to the spontaneous and stable adsorbed layer on the metal surface43. The adsorption characteristics for the metal–organic compounds found are shown in Table 7. The free energy findings show that the kind of adsorption for SC1 is physical and chemical adsorption but physical in case of SCP2, since it is known that negative values are greater than 20 kJ mol−1 and less than 40 kJ mol−1 for SC1. The \(\Delta G_{ads}^{ \circ }\) values ranged between − 22.7 and − 23.1 kJ mol−1, suggesting physical and chemical adsorption (mixed adsorption), but between 21 anf 21.5 kJ mol−1 for SCP2 which showed that it adsorbed on C-steel surface physically. The enthalpy of adsorption, \(\Delta H_{ads}^{ \circ }\), was determined using the Vant Hoff equation:
Figure 7 shows plotting of log Kads with 1/T for C-steel in 0.5 M H2SO4 with SC1. The negative sign of the \(\Delta H_{ads}^{ \circ }\) value indicates that the adsorption process is exothermic. Adsorption can be physical or chemical in an exothermic process, while it can only be chemical in an endothermic process. Finally, the following equation may be used to calculate \(\Delta S_{ads}^{ \circ }\).
Table 7 shows the values for \(\Delta S_{ads}^{ \circ }\). The negative sign of \(\Delta S_{ads}^{ \circ }\) values indicates that the order of the adsorbed molecules at the solid/liquid contact is decreasing.
Electrochemical measurements
PP measurements
PP diagrams of C-steel in 0.5 M sulfuric acid in the existence and absence of altered doses of metal–organic compounds at 298 K are shown in Fig. 8. From this Figure we see that Tafel extrapolation obtained the electrochemical parameters at Ecorr and were depicted in Table 8. The current density reduced as the accumulation of inhibitors increased. According to the results of the tests, βc is somewhat greater than βa, suggesting that the inhibitors favor cathodic rather than nodic action. As a result, these inhibitors function like a combination of inhibitors. Also, Ecorr change slightly (less than ± 85 mV) which confirm that these compounds exert on both cathodic (hydrogen reduction) and anodic (metal dissolution) processes. The efficacy of inhibition (IE%) was determined from the curves of polarization as in Eq. (9):
where icorr and\(i_{corr}^{ \circ }\), respectively, are the current densities of corrosion with and without of metal–organic compounds (SC1 & SCP2)44,45. The parallel Tafel lines with and without inhibitors indicate that there is no change in corrosion mechanism.
Electrochemical impedance spectroscopy (EIS) measurements
Figures 9 and 10 show the C-steel Nyquist and Bode diagrams at OCP in the presence and absence of different dosages of metal–organic SC1 and SCP2 at 298 K. The circuit that represents metal organic compounds and electrolyte is presented in Fig. 11, with Rs as the solution resistance. The impedance spectra show that the diameter decreases as the dose of studied inhibitors rises. The interfacial capacitance Cdl values can be estimated from CPE parameters (Y0 and n) and is defined in Eq. (10)46,47,48,49,50:
where Y0 is the CPE magnitude, and n is the variance CPE data of the: − 1 < n < 1. Using Eq. (10). Table 9 shows the impedance data that established the data of Rct increasing with increasing the dosage of the metal–organic compounds, pointing to an increase in IE percent. This might be due to an increase in the thickness of the adsorbed layer caused by increasing the metal–organic compound dosages. The Table also shows that (n) value varies directly with SC1 and SCP2 dosages. (n) value is a measure of surface roughness51, and its rise might indicate a reduction in the heterogeneity of the metal surface caused by SC1 and SCP2 adsorption. The inclusion of SC1 and SCP2 results in lower Cdl values, which the Helmholtz model ascribed to an increase in the thickness of the electric double layer or/and a drop in the local dielectric constant52:
where ε is the dielectric constant of the medium, ε° is vacuum permittivity, A is the electrode area and δ is the thickness of the protective layer. Bode graphs (Fig. 11) in the presence of inhibitors revealed that the Bode amplitude value increases over the whole frequency range with the addition of SC1 and SCP2. Equation 12 was used to get the percent IE and θ from the impedance testing:
where \(R_{p}^{ \circ }\) and Rp are the resistances unprotected and protected metal–organic compounds, individually. Table 10 shows the values of parameters such as Rs and Rct obtained from EIS fitting, as well as the derived parameters Cdl and IE percent. The usual criteria for evaluating the best fit of these compounds were followed: the chi-square errors were low (χ2 ≈ 10–4) and the allowable errors of elements in fitting mode were low (5%). As a result, the utilised circuit is acceptable in this situation.
Surface analysis
AFM analysis
AFM in Table 10 and Fig. 12 measured the surface roughness of C-steel in 0.5 M H2SO4 in the presence and absence of 25 × 10–6 M. Where, (a) shows blank, (b) C-steel free (c) C-steel with SC1 and SCP2 at 25 × 10–6 M.
The roughness calculated from AFM image are summarized in Table 10. The values displayed that the roughness rises with adding H2SO4 due to the corrosion occurs on the C-steel surface but decreased with adding the prepared53.
FT-IR analysis
Fourier transform infrared spectroscopy (FT-IR) identifies chemical bonds in a molecule by producing an infrared absorption spectrum. “FT-IR spectrum of the corrosion product at C-steel surface in 0.5 M H2SO4 does not show any useful adsorption peaks54. FT-IR fingerprint spectra of the stock metal–organic SC1and the C-steel surface after dipping in 0.5 M H2SO4 + 25 × 10–6 M of metal–organic SC1 for 24 h was obtained and compared to each other it was obviously clear that the same fingerprint of metal–organic SC1solution present on C-steel surface except the absence of some functional group and it suggested to be due to reaction with H2SO4. From Fig. 13 there are small shift in the peaks at C-steel surface from the original peak of the stock inhibitor solution”, these shifts indicate that there is interaction between C-steel and metal–organic (SC1&SCP2).
Corrosion inhibition mechanism analysis
Metal–organic compound inhibitors prevent C-steel corrosion primarily by adsorption on the C-steel surface, where it moves H2O molecules, forming a tight barrier layer55. Adsorption is related to inhibitor functional groups such as O, N and S, as well as the potential electronic density and steric effect of active centers, which can donate their lone electron to the d-orbital of Fe, forming a chemical bond that is characteristic of chemical adsorption as in case of SC1 and this confirmed from the values of ΔGoads which are more than 20 kJ mol-1. On the other hand, the surface of the C-steel sample is positively charge in aqueous acid solution56. The SO42− ions get adsorbed on C-steel sample and turn it as negatively charged surface, the protonated inhibitor metal–organic molecules (cationic) get adsorbed on the negatively charged metal surface by an electrostatic attraction. The protonated molecules may adsorb on C-steel samples, resulting in physical adsorption (Fig. 14) also this confirmed from the values of ΔGoads which are about 20 kJ mol-1. The order of percent IE is as follows: SC1 (93.1%) > SCP2 (88.7%). This due to the presence of more donating atoms (12 N, 7 O and 4 S) in SC1 than in SCP2 (8 N and 1 O).
Conclusions
The metal–organic compounds investigated have a high inhibition efficiency ranging from 93.1 to 91.2% at 25 × 10−6 based on measurements of mass reduction as it gives linear variation of mass reduction over time. Electrochemical measurements also provide high inhibition efficiency as Tafel lines moved to higher potential regions and the EIS analysis showed a rise in Rct and a lowered in Cdl as the dose of the inhibitors improved. The investigated compounds adsorption obeyed Langmuir isotherm. Thermodynamic and kinetic parameters indicated that the metal–organic compound act as mixed kind as the adsorption is spontaneous and involving physical adsorption.
Change history
28 September 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-022-21190-8
References
Liao, L. L., Mo, S., Lei, J. L., Luo, H. Q. & Li, N. B. Application of a cosmetic additive as an eco-friendly inhibitor for mild steel corrosion in HCl solution. J. Colloid Interface Sci.474, 68–77 (2016).
Raja, P. B. & Sethuraman, M. G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett.62, 113–116 (2008).
Fouda, A. S., El-Wakeel, A. M., Shalabi, K. & El-Hossiany, A. Corrosion inhibition for carbon steel by levofloxacin drug in acidic medium. Elixir Corros. Day83, 33086–33094 (2015).
Lukovits, I., Palfi, K. & Kalman, E. Corrosion, 53 (1997) 915. 74-P. Zhao, Q. Liang, Y. Li. Appl. Surf. Sci252, 1596 (2005).
Fouda, A. S., Shalabi, K. & El-Hossiany, A. Moxifloxacin antibiotic as green corrosion inhibitor for carbon steel in 1 M HCl. J. Bio- Tribo-Corros.2(3), 1–13 (2016).
Fouda, A. S., El-Gharkawy, E. S., Ramadan, H. & El-Hossiany, A. Corrosion resistance of mild steel in hydrochloric acid solutions by clinopodium acinos as a green inhibitor. Biointerface Res. Appl. Chem.11, 9786–9803 (2021).
Fouda, E.-A., El-Hossiany, A. & Ramadan, H. Calotropis Procera plant extract as green corrosion inhibitor for 304 stainless steel in hydrochloric acid solution. Zast. Mater.58, 541–555 (2017).
Keleş, H., Keleş, M., Dehri, I. & Serindağ, O. The inhibitive effect of 6-amino-m-cresol and its Schiff base on the corrosion of mild steel in 0.5 M HCI medium. Mater. Chem. Phys.112, 173–179 (2008).
Fouda, A. S., Abdel-Latif, E., Helal, H. M. & El-Hossiany, A. Synthesis and characterization of some novel thiazole derivatives and their applications as corrosion inhibitors for zinc in 1 M hydrochloric acid solution. Russ. J. Electrochem.57, 159–171 (2021).
Fouda, A. S., Ibrahim, H., Rashwaan, S., El-Hossiany, A. & Ahmed, R. M. Expired drug (pantoprazole sodium) as a corrosion inhibitor for high carbon steel in hydrochloric acid solution. Int. J. Electrochem. Sci13, 6327–6346 (2018).
Fouda, A. S., El-Azaly, A. H., Awad, R. S. & Ahmed, A. M. New benzonitrile azo dyes as corrosion inhibitors for carbon steel in hydrochloric acid solutions. Int. J. Electrochem. Sci9, 1117–1131 (2014).
Fouda, A. S., Eissa, M. & El-Hossiany, A. Ciprofloxacin as eco-friendly corrosion inhibitor for carbon steel in hydrochloric acid solution. Int. J. Electrochem. Sci.13, 11096–11112 (2018).
Deyab, M. A., Dief, H. A. A., Eissa, E. A. & Taman, A. R. Electrochemical investigations of naphthenic acid corrosion for carbon steel and the inhibitive effect by some ethoxylated fatty acids. Electrochim. Acta52, 8105–8110 (2007).
Benabbouha, T. et al. Thermodynamic and electrochemical investigation of 2-mercaptobenzimidazole as corrosion inhibitors for mild steel C35E in hydrochloric acid solutions. Int. J. Sci. Eng. Investig.6(60), 136–143 (2017).
Bentiss, F., Lagrenee, M., Traisnel, M. & Hornez, J. C. The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros. Sci.41, 789–803 (1999).
Fouda, A. S., Ahmed, R. E. & El-Hossiany, A. Chemical, electrochemical and quantum chemical studies for famotidine drug as a safe corrosion inhibitor for α-brass in HCl solution. Prot. Met. Phys. Chem. Surfaces57, 398–411 (2021).
Fouda, A. S., Etaiw, S. H. & Wahba, A. Effect of acetazolamide drug as corrosion inhibitor for carbon steel in hydrochloric acid solution. Nat Sci13, 1–8 (2015).
Kermannezhad, K., Chermahini, A. N., Momeni, M. M. & Rezaei, B. Application of amine-functionalized MCM-41 as pH-sensitive nano container for controlled release of 2-mercaptobenzoxazole corrosion inhibitor. Chem. Eng. J.306, 849–857 (2016).
Wang, L. Inhibition of mild steel corrosion in phosphoric acid solution by triazole derivatives. Corros. Sci.48, 608–616 (2006).
Ramesh, S. & Rajeswari, S. Corrosion inhibition of mild steel in neutral aqueous solution by new triazole derivatives. Electrochim. Acta49, 811–820 (2004).
Fouda, A. S., Al-Hazmi, N. E., El-Zehry, H. H. & El-Hossainy, A. Electrochemical and surface characterization of chondria macrocarpa extract (CME) as save corrosion inhibitor for aluminum in 1M HCl medium. J. Appl. Chem.9, 362–381 (2020).
Etaiw, S. H., Fouda, A. S., Amer, S. A. & El-bendary, M. M. Structure, characterization and anti-corrosion activity of the new metal–organic framework [Ag (qox)(4-ab)]. J. Inorg. Organomet. Polym. Mater.21, 327–335 (2011).
Fouda, A. S., Etaiw, S. H., El-bendary, M. M. & Maher, M. M. Metal-organic frameworks based on silver (I) and nitrogen donors as new corrosion inhibitors for copper in HCl solution. J. Mol. Liq.213, 228–234 (2016).
Mardel, J. I. et al. US20200339824-Polymeric Agents And Compositions For Inhibiting Corrosion, The Boeing Company Commonwealth Scientific And Industrial Research Organisation (2020).
Etaiw, S. H., El-bendary, M. M., Fouda, A. S. & Maher, M. M. A new metal-organic framework based on cadmium thiocyanate and 6-methylequinoline as corrosion inhibitor for copper in 1 M HCl solution. Prot. Met. Phys. Chem. Surfaces53, 937–949 (2017).
Čačić, M., Pavić, V., Molnar, M., Šarkanj, B. & Has-Schön, E. Design and synthesis of some new 1, 3, 4-thiadiazines with coumarin moieties and their antioxidative and antifungal activity. Molecules19, 1163–1177 (2014).
Zhang, M., Ma, L., Wang, L., Sun, Y. & Liu, Y. Insights into the use of metal–organic framework as high-performance anticorrosion coatings. ACS Appl. Mater. Interfaces10, 2259–2263 (2018).
Li, W., Ren, B., Chen, Y., Wang, X. & Cao, R. Excellent efficacy of MOF films for bronze artwork conservation: the key role of HKUST-1 film nanocontainers in selectively positioning and protecting inhibitors. ACS Appl. Mater. Interfaces10, 37529–37534 (2018).
Dehghani, A., Poshtiban, F., Bahlakeh, G. & Ramezanzadeh, B. Fabrication of metal-organic based complex film based on three-valent samarium ions-[bis (phosphonomethyl) amino] methylphosphonic acid (ATMP) for effective corrosion inhibition of mild steel in simulated seawater. Constr. Build. Mater.239, 117812 (2020).
Zafari, S., Shahrak, M. N. & Ghahramaninezhad, M. New MOF-based corrosion inhibitor for carbon steel in acidic media. Met. Mater. Int.26, 25–38 (2020).
Lausi, A. et al. Status of the crystallography beamlines at Elettra. Eur. Phys. J. Plus130, 1–8 (2015).
Jafari, H., Akbarzade, K. & Danaee, I. Corrosion inhibition of carbon steel immersed in a 1 M HCl solution using benzothiazole derivatives. Arab. J. Chem.12, 1387–1394 (2019).
Khaled, M. A., Ismail, M. A., El-Hossiany, A. A. & Fouda, A.E.-A.S. Novel pyrimidine-bichalcophene derivatives as corrosion inhibitors for copper in 1 M nitric acid solution. RSC Adv.11, 25314–25333 (2021).
Binnig, G., Quate, C. F. & Gerber, C. Atomic force microscope. Phys. Rev. Lett.56, 930 (1986).
Fouda, A. S., El-Ghaffar, M. A., Sherif, M. H., El-Habab, A. T. & El-Hossiany, A. Novel anionic 4-tert-octyl phenol ethoxylate phosphate surfactant as corrosion inhibitor for C-steel in acidic media. Prot. Met. Phys. Chem. Surf.56, 189–201 (2020).
Ansari, K. R. & Quraishi, M. A. Experimental and quantum chemical evaluation of Schiff bases of isatin as a new and green corrosion inhibitors for mild steel in 20% H2SO4. J. Taiwan Inst. Chem. Eng.54, 145–154 (2015).
Martinez, S. & Stern, I. Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl. Surf. Sci.199, 83–89 (2002).
Ahamad, I., Prasad, R. & Quraishi, M. A. Inhibition of mild steel corrosion in acid solution by Pheniramine drug: Experimental and theoretical study. Corros. Sci.52, 3033–3041 (2010).
Bockris, J. & Swinkels, D. A. J. Adsorption of n-decylamine on solid metal electrodes. J. Electrochem. Soc.111, 736 (1964).
Fouda, A. S., El-Maksoud, S. A., El-Hossiany, A. & Ibrahim, A. Evolution of the corrosion-inhibiting efficiency of novel hydrazine derivatives against corrosion of stainless steel 201 in acidic medium. Int. J. Electrochem. Sci.14, 6045–6064 (2019).
Saleh, M. M. & Atia, A. A. Effects of structure of the ionic head of cationic surfactant on its inhibition of acid corrosion of mild steel. J. Appl. Electrochem.36, 899–905 (2006).
Ameh, P. O. & Eddy, N. O. Commiphora pedunculata gum as a green inhibitor for the corrosion of aluminium alloy in 0.1 M HCl. Res. Chem. Intermed.40, 2641–2649 (2014).
Fouda, A. S., Abdel Azeem, M., Mohamed, S. A., El-Hossiany, A. & El-Desouky, E. Corrosion inhibition and adsorption behavior of Nerium Oleander extract on carbon steel in hydrochloric acid solution. Int. J. Electrochem. Sci.14, 3932–3948 (2019).
Satapathy, A. K., Gunasekaran, G., Sahoo, S. C., Amit, K. & Rodrigues, P. V. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci.51, 2848–2856 (2009).
Fouda, A. S., Abd El-Maksoud, S. A., El-Hossiany, A. & Ibrahim, A. Corrosion protection of stainless steel 201 in acidic media using novel hydrazine derivatives as corrosion inhibitors. Int. J. Electrochem. Sci.14, 2187–2207 (2019).
Elayyachy, M. et al. New bipyrazole derivatives as corrosion inhibitors for steel in hydrochloric acid solutions. Mater. Chem. Phys.93, 281–285 (2005).
Motawea, M. M., El-Hossiany, A. & Fouda, A. S. Corrosion control of copper in nitric acid solution using chenopodium extract. Int. J. Electrochem. Sci.14, 1372–1387 (2019).
El-Haddad, M. N. & Fouda, A. S. Corrosion inhibition and adsorption behavior of some azo dye derivatives on carbon steel in acidic medium: synergistic effect of halide ions. Chem. Eng. Commun.200, 1366–1393 (2013).
Fouda, A. S., Rashwan, S., El-Hossiany, A. & El-Morsy, F. E. Corrosion inhibition of zinc in hydrochloric acid solution using some organic compounds as eco-friendly inhibitors. J. Chem. Biol. Phys. Sci.9, 1–24 (2019).
Fouda, A. S., El-Dossoki, F. I., El-Hossiany, A. & Sello, E. A. Adsorption and anticorrosion behavior of expired meloxicam on mild steel in hydrochloric acid solution. Surf. Eng. Appl. Electrochem.56, 491–500 (2020).
Kuş, E. & Mansfeld, F. An evaluation of the electrochemical frequency modulation (EFM) technique. Corros. Sci.48, 965–979 (2006).
Fouda, A. S., Abd El-Maksoud, S. A., Belal, A. M., El-Hossiany, A. & Ibrahium, A. Effectiveness of some organic compounds as corrosion inhibitors for stainless steel 201 in 1M HCl: Experimental and theoretical studies. Int. J. Electrochem. Sci.13, 9826–9846 (2018).
Cook, T. R., Zheng, Y.-R. & Stang, P. J. Metal–organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal–organic materials. Chem. Rev.113, 734–777 (2013).
Noor, E. A. & Al-Moubaraki, A. H. Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4 [4′(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys.110, 145–154 (2008).
Elgyar, O. A., Ouf, A. M., El-Hossiany, A. & Fouda, A. S. The inhibition action of viscum album extract on the corrosion of carbon steel in hydrochloric acid solution. Biointerface Res. Appl. Chem.11, 14344–14358 (2021).
Hass, F., Abrantes, A. C. T. G., Diógenes, A. N. & Ponte, H. A. Evaluation of naphthenic acidity number and temperature on the corrosion behavior of stainless steels by using Electrochemical Noise technique. Electrochim. Acta124, 206–210 (2014).
Author information
Authors and Affiliations
Contributions
A.B. Wrote the main manuscript text, C,. prepared Figures and does the experimental work, All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-022-21190-8"
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fouda, A.EA.S., Etaiw, S.E.H. & Hassan, G.S. RETRACTED ARTICLE: Chemical, electrochemical and surface studies of new metal–organic frameworks (MOF) as corrosion inhibitors for carbon steel in sulfuric acid environment. Sci Rep 11, 20179 (2021). https://doi.org/10.1038/s41598-021-99700-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99700-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.