Abstract
H2 is an important fermentation intermediate in anaerobic environments. Although H2 occurs at very low partial pressures in the environments, the culture and isolation of H2-utilizing microorganisms is usually carried out under very high H2 pressures, which might have hampered the discovery and understanding of microorganisms adapting to low H2 environments. Here we constructed a culture system designated the “iron corrosion-assisted H2-supplying (iCH) system” by connecting the gas phases of two vials (one for the iron corrosion reaction and the other for culturing microorganisms) to achieve cultures of microorganisms under low H2 pressures. We conducted enrichment cultures for methanogens and acetogens using rice paddy field soil as the microbial source. In the enrichment culture of methanogens under canonical high H2 pressures, only Methanobacterium spp. were enriched. By contrast, Methanocella spp. and Methanoculleus spp., methanogens adapting to low H2 pressures, were specifically enriched in the iCH cultures. We also observed selective enrichment of acetogen species by the iCH system (Acetobacterium spp. and Sporomusa spp.), whereas Clostridium spp. predominated in the high H2 cultures. These results demonstrate that the iCH system facilitates culture of anaerobic microorganisms under low H2 pressures, which will enable the selective culture of microorganisms adapting to low H2 environments.
Similar content being viewed by others
Introduction
Molecular hydrogen (H2) is an important intermediary metabolite and an energy carrier in anaerobic environments1,2,3. Because H2 is rapidly turned over in natural anaerobic environments, it occurs at very low partial pressures of only a few to several tens of pascals (Pa)4. In conventional studies, however, culture and isolation of H2-utilizing microorganisms have commonly been performed under high H2 partial pressures (100 kPa or more). Under such laboratory conditions, it is difficult to draw conclusions about the ecophysiology of H2-utilizing microorganisms in their natural environment, nor can microorganisms that have adapted to conditions with low H2 be isolated. In fact, the presence of uncultured H2-utilizing methanogens and acetogens in anaerobic environments where H2 concentrations are estimated to be quite low (i.e., environments with low available organic matter, including subsurface environments, peat soils, and deep-sea sediments) has been inferred by molecular environmental analyses such as metagenomics5,6,7.
Because H2 supplied at low partial pressure is rapidly consumed, sufficient microbial growth cannot be obtained in conventional batch culture systems. To date, several research groups have developed methods that can continuously supply H2 at low partial pressure to elucidate ecophysiology of hydrogenotrophic methanogens in low H2 environments. Morgan et al.8 reported a low-H2 culture of a hydrogenotrophic methanogen by using a continuous culture system with a continuous influx of a mixed gas containing H2. By using this system, the authors found that the expression of some metabolic enzymes in the methanogenic pathway is regulated by H2 concentration. Similar methods have frequently been utilized in subsequent studies on low H2 responses of hydrogenotrophic methanogens9,10. Sakai et al.11 developed the “coculture method”, in which methanogens are cocultured with heterotrophic H2-producing bacteria to achieve a continuous supply of H2 at low concentration. The coculture method enabled selective enrichment of uncultured hydrogenotrophic methanogens that were expected to adapt to low H2 pressures, which finally resulted in the isolation of phylogenetically novel methanogens such as Methanocella spp. and Methanolinea spp.12,13. The coculture method has also been employed to analyze physiological responses of methanogens to low H2 pressures14,15. Although these methods yielded laboratory cultures under low H2 pressures, several issues still need to be addressed. The continuous gas influx process cannot be carried out in parallel with a large number of cultures because it requires relatively complex systems, including large- or small-scale reactors and gas supply devices. Although the coculture method only requires simple systems, which makes it suitable for enrichment cultures, it cannot be directly utilized for isolation of H2-utilizing microorganisms because it relies on coexistence with fermentative bacteria. Furthermore, it cannot be excluded that metabolites other than H2 (e.g., organic acids such as acetate) affect the growth of H2-utilizing microorganisms.
In this study, we aimed to develop a simple method capable of selective culture of microorganisms under low H2 pressures. The reaction on which we focused was the corrosion of metallic iron in anoxic solution: Fe0 + 2H+ ↔ Fe2+ + H2. The concept of culturing microorganisms using H2 derived from iron corrosion has already been reported16,17. The authors demonstrated that hydrogenotrophic methanogens can be cultured using H2 derived from metallic iron as sole energy source. However, this method has not been applied to culture microorganisms adapted to low H2 environments. Considering that iron corrosion proceeds very slowly in anoxic and circumneutral solution because of the small difference in the standard redox potentials of Fe0 oxidation (E0ʹ ≈ − 0.47 V) and the reduction of protons to generate H2 (E0ʹ ≈ − 0.41 V), we can expect that H2 supply via iron corrosion is suitable to culture hydrogenotrophic microorganisms under low H2 pressures. Here we report that a culture system based on iron corrosion reactions has been successfully used for the selective enrichment of hydrogenotrophic methanogens and acetogens, which have the potential to adapt to environments with very low H2 content.
Results and discussion
Validation of continuous H2 supply by iron corrosion
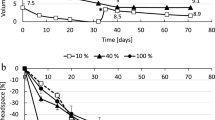
Because the growth of microorganisms is considered to be very slow under low H2 pressures, the culture system requires a continuous supply of H2 over a long period of time. Hence, we first determined whether H2 can be continuously supplied for a long time by the iron corrosion reaction. Furthermore, to develop a culture system capable of regulating the H2 supply rate, we added various amounts of Fe0 with different particle sizes (Fe0 granules with a diameter of 1–2 mm or Fe0 powder with a diameter < 45 µm) to the anoxic buffer solution and determined the H2 production rates (Fig. 1).
We observed continuous H2 production for more than 6 months in the vials supplemented with Fe0 granules (Fig. 1A). The H2 production rates were almost proportional to the amount of Fe0 granules added (0.03 to 3 g vial−1) and were in the range of 0.12 to 4.4 μmol vial−1 day−1 (Fig. 1C). With Fe0 powder, H2 production ceased after approximately 2 weeks, 1 month, and 3 months in the vials supplemented with Fe0 at 3, 1, and 0.3 g vial−1, respectively (Fig. 1B). The highest H2 accumulation was approximately 1000 μmol vial−1, corresponding to approximately 50 kPa. We assume that H2 production ceased for thermodynamic reasons (increase in H2 partial pressure and decrease in proton concentration).The H2 production rates were also proportional to the amount of Fe0 powder added, with a range of 2.0 to 93 μmol vial−1 day−1 (Fig. 1C). Since the specific surface area of the spherical material is inversely proportional to the diameter, the Fe0 powder (< 45 µm) has a surface 20 times larger than the Fe0 granule (1–2 mm), if used particles are assumed to be spherical. The H2 production from Fe0 powder was 16–22 times greater than from Fe0 granule (Fig. 1C), suggesting that surface area is the major determinant of H2 production rate. These results demonstrate that it is possible to supply H2 over a long period of time by utilizing the iron corrosion reaction, and also that it is possible to regulate the rate of H2 supply by altering the size and amount (i.e., total surface area) of Fe0 particles.
Development of a culture system with low H2 pressures
It has been reported that H2-utilizing anaerobic microorganisms grow on H2 derived from iron corrosion16,17,18,19,20. If microorganisms are cultured in the coexistence with Fe0, however, some undesirable phenomena can occur. For example, the iron corrosion reaction produces a high concentration of ferrous iron and induces an increase in pH due to the consumption of protons. Furthermore, it can promote growth of anaerobic microorganisms that use Fe0 itself as the energy source18,21,22. These phenomena likely hamper the culture of target microorganisms. We therefore constructed a culture system in which the gas phases in two vials (one for the culture of microorganisms and the other for the iron corrosion reaction) are connected by a stainless-steel tube (Fig. 2), in reference to the system developed by Daniels et al.16. This system, hereafter referred to as the “iron corrosion-assisted H2-supplying (iCH) system”, was expected to allow the culture of microorganisms under low H2 pressures while avoiding the unfavorable effects of the iron corrosion reaction.
The iron corrosion-assisted H2-supplying (iCH) system. The vial on the left is for culture of microorganisms and that on the right is for the iron corrosion (i.e., H2 production) reaction. The gas phases of the two vials are connected by a stainless steel tube to allow diffusion of H2 produced in the corrosion vial.
Pure culture of a hydrogenotrophic methanogen in the iCH system
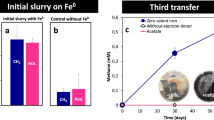
We evaluated the capability of the iCH system to culture microorganisms under low H2 pressures using a hydrogenotrophic methanogen Methanobacterium formicicum, which is known to be able to grow under both high and low H2 partial pressures23, as a model strain. We inoculated the iCH system with M. formicicum using Fe0 powder at 1 g vial–1 as the H2 source, and monitored the amounts of CH4 and H2 in the gas phase (Fig. 3). We observed continuous CH4 production by M. formicicum in the iCH system for almost 1 month. Although there was accumulation of H2 at the beginning of the culture (around 190 Pa at day 3, corresponding to 7.4 µmol vial–1), the partial pressure of H2 subsequently decreased and remained extremely low (30 to 50 Pa after day 14). The observed H2 partial pressures are comparable to those observed in natural anaerobic environments and laboratory cocultures of methanogens with fermentative bacteria4,24,25. These results demonstrated that the iCH system is capable of long-term culture of H2-utilizing microorganisms under low H2 pressures.
The amount of CH4 and the partial pressure of H2 during the culture of Methanobacterium formicicum with the iron corrosion-assisted H2-supplying (iCH) system supplemented with 1 g Fe0 powder. Data are presented as the means of three independent cultures, and error bars represent standard deviations.
Enrichment cultures of hydrogenotrophic methanogens using the iCH system
We performed enrichment cultures of methanogens to demonstrate the capability of the iCH system to selectively culture microorganisms adapted to low H2 pressures. We used rice paddy field soil as the microbial source, because it is known as a low H2 environment4 and was the isolation source of Methanocella spp. that is known to adapt to low H2 environments12,26,27. In addition to the iCH system (with Fe0 powder at 1 g vial–1 as the H2 source), we set up conventional cultures under high H2 pressures (160 kPa of H2 in the gas phase, hereafter referred to as “high H2 enrichments”) and cultures under iron corrosion conditions (microorganisms cultured in the same vial with 1 g vial–1 of Fe0 powder, hereafter referred to as “Fe0 enrichments”) as control experiments.
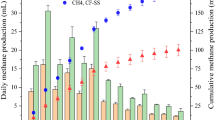
Hydrogenotrophic methanogens were enriched in inorganic medium supplemented with rifampicin to suppress growth of bacteria. After three successive subcultures, the metabolic products (CH4 and H2) were analyzed during incubation (Fig. 4A–C). In the high H2 enrichments, we observed CH4 production almost equal to the theoretical value calculated from the consumption of H2 (Fig. 4A). In the Fe0 enrichments, we observed CH4 production comparable to the theoretical value (broken line in Fig. 4B) calculated from the H2 production via iron corrosion in the early phase of the incubation (day 0–14). However, CH4 production levelled off in the later phase (after day 14). The pH of the culture solution of Fe0 enrichments increased from 7.0 to around 8.1 during the incubation, whereas the pH of the culture solution remained around 7.0 in the other enrichments. The increase in pH, and possibly the increase in concentration of ferrous iron, might have inhibited the growth of methanogens in the Fe0 enrichments. In the iCH enrichments, CH4 was produced almost proportional to the theoretical value (broken line in Fig. 4C) without levelling off. We observed accumulation of H2 at the beginning of culture (around day 10), which then decreased below the detection limit (< 10 Pa). These results indicate that enrichment cultures of methanogens under low H2 pressures were achieved in the iCH system.
Production and consumption of H2, CH4, and acetate in enrichment cultures. (A–C) Enrichment cultures for methanogens (supplemented with rifampicin). (D–F) Enrichment cultures for acetogens (supplemented with 2-bromoethanesulphonate [BES]). (A,D) “High H2 enrichments” supplemented with 160 kPa of H2 in the gas phase. (B,E) “Fe0 enrichments” in which microorganisms were cultured in the same vial with 1 g vial−1 of Fe0 powder. (C,F) Enrichment cultures with the iron corrosion-assisted H2-supplying (iCH) system, in which H2 was continuously supplied by the iron corrosion reaction. For ease of comparison, the amounts of all metabolites are represented as electron equivalents in units of mmol e– per culture vial, using the respective half reaction formulas; 2H+ + 2e− ↔ H2, HCO3− + 9H+ + 8e− ↔ CH4 + 3H2O, and 2HCO3− + 9H+ + 8e− ↔ CH3COO− + 4H2O. The volumes of the liquid and gaseous headspace were 20 and 48 ml, respectively. The broken lines represent the rate of H2 production via the iron corrosion reaction calculated from the data shown in Fig. 1C (35 µmol vial−1 day−1).
Microbial community analysis of the methanogenic enrichment cultures
To confirm the capability of the iCH system to specifically enrich microorganisms adapting to low H2 pressures, we assessed the microbial community structures of the enrichment cultures and the inoculum soil by high throughput sequencing analysis of 16S rRNA gene amplicons. A total of 56,388 16S rRNA gene reads (3744–5531 reads per sample) were retrieved and classified into 2949 operational taxonomic units (OTUs) using a 97% sequence identity cut-off. The microbial composition is displayed in Fig. S1A. All OTUs dominant in the enrichment cultures (relative abundance > 3% in at least one enrichment culture) had low abundance in the inoculum soil (~ 1.2%), suggesting that H2-utilizing microorganisms were sufficiently enriched. Principal component analysis was performed to quantitatively evaluate the similarity of microbial community structures of each sample (Fig. S1B). The results showed that the microbial community patterns of the duplicate enrichment cultures were very similar, and that the microbial community composition differs between the different experimental setups. Therefore, for further analysis we used the average values of community analysis data of the duplicate cultures.
We plotted the relative abundances of the dominant archaea (> 3% under at least one set of culture conditions) in the methanogenic enrichments (Fig. 5A). Different types of methanogens were enriched depending on the culture conditions. In the high H2 enrichments, two OTUs closely related to Methanobacterium spp. (OTU183, with 99% identity to Methanobacterium oryzae and OTU192, with 100% identity to Methanobacterium lacus) predominated. By contrast, OTU173 and OTU1307 (100% identity to Methanocella arvoryzae and Methanoculleus chikugoensis, respectively) were specifically enriched in the iCH cultures. The genera Methanocella and Methanoculleus have been frequently detected as dominant hydrogenotrophic methanogens in various anaerobic environments with low H2 concentrations, including rice paddy fields, peat bogs, marine and freshwater sediments, and subsurface environments28,29,30,31,32,33. Furthermore, methanogens closely related to these genera have been selectively enriched from rice paddy field soils and marine/freshwater sediments under the low H2 pressures achieved by the coculture method11. These findings suggest that the iCH system can selectively culture hydrogenotrophic methanogens adapting to low H2 pressures. By contrast, a different type of methanogen (OTU153, with 100% identity to Methanobacterium flexile) was enriched in the Fe0 enrichment cultures. This suggests that factors other than H2 concentration (e.g., increase in pH and/or high concentration of ferrous iron) were the main selective pressures in the Fe0 enrichments.
Relative abundance of the OTUs recovered from the enrichment cultures for methanogens (A) and acetogens (B). The dominant OTUs (> 3% in at least one enrichment) and their closest relatives (sequence identity, %) are shown in the legends. The OTUs specifically enriched in the iron corrosion-assisted H2-supplying (iCH) system are highlighted in red. Minor OTUs, ≤ 3% in all enrichments.
Acetogen enrichment culture and its microbial community analysis
In addition to methanogenic archaea, acetogenic bacteria are also one of the important H2-utilizing microorganisms in anaerobic environments34,35. Hence, we set up enrichment cultures of H2-utilizing acetogens with inorganic medium supplemented with 2-bromoethanesulphonate (BES) to inhibit methanogens. We followed the transitions of metabolites during the incubations of enrichment cultures of acetogens in the high H2, Fe0, and iCH cultures (Fig. 4D–F). As described below, the trends were similar to those observed with the enrichment of methanogens. In the high H2 enrichments, we observed acetate production with concomitant consumption of H2, and the H2 consumption rate was much higher than in enrichment cultures of methanogens (Fig. 4D). In the Fe0 enrichments, we observed acetate production comparable to the theoretical value from day 0 to day 14, after which acetate production ceased (Fig. 4E), suggesting that the Fe0 cultures have inhibitory effects on acetogens as observed in the methanogenic enrichments. In the iCH enrichments, acetate was produced at a rate comparable with theoretical values (Fig. 4F). Although H2 initially accumulated in the iCH enrichments, it was below the detection limit (< 10 Pa) after day 14. These results indicate that acetogens adapting to low H2 pressures could be enriched in the iCH system.
We plotted the relative abundances of the dominant bacteria (> 3% under at least one set of conditions) in the enrichments for acetogens (Fig. 5B). As with the methanogen enrichments, the acetogen community structures were completely different for each culture condition. Only one phylotype (OTU898) closely related to Clostridium magnum, which is well known as an acetogen33, was enriched in the high H2 enrichments. In contrast, two phylotypes closely related to other acetogen species (OTU2305 and OTU2514, with 100% identities to Acetobacterium carbinolicum and Sporomusa sphaeroides, respectively) were selectively enriched in the iCH cultures. The phylotypes closely related to Clostridium glycolicum and Romboutsia lituseburensis (OTU316 and OTU1895, respectively) predominated in the Fe0 enrichments, in addition to OTU898 (C. magnum) and OTU2514 (S. sphaeroides), which were also detected in other enrichments. Clostridium glycolicum is a well-known acetogen species34. Romboutsia spp. have often been detected in enrichment cultures of H2-utilizing acetogens36, although there is no report of their acetogenic metabolism.
It has been reported that affinities for H2 and kinetics of H2 consumption differ depending on the species of acetogens, mainly due to the differences in their energy acquisition efficiencies37. In contrast to methanogens, however, there are only few studies of the response of acetogens to low H2 pressures. Generally, acetogens have lower affinity for H2 than methanogens for thermodynamic reasons38, which may be one of the reasons that the ecophysiology of acetogens under low H2 pressures has not attracted much attention. Our result shows that different types of acetogens can be selectively enriched under conditions with different H2 availability. This suggests that environmental H2 concentration provides an ecological niche not only for methanogens but also for acetogens.
Conclusion
We constructed a simple system for culturing anaerobic microorganisms under low H2 pressures by using the iron corrosion reaction as the source of H2. The system, which we call the iCH system, can continuously supply H2 for several months, and it is possible to control the H2 supply rate by changing the amount and size of Fe0 particles. We demonstrated that the iCH system can selectively enrich anaerobic microorganisms adapting to low H2 pressures. Although this study focused only on methanogens and acetogens, the iCH system is applicable to cultures of other H2-utilizing microorganisms such as nitrate, iron, and sulfate reducers. The iCH system is also applicable to colony isolation using agar-solidified media (e.g., a roll-tube method), which is an on-going study in our research group. This culture method should enable selective enrichment and isolation of unidentified microorganisms adapting to or even specialized for low H2 pressures from anaerobic environments with low H2 availability, such as subsurface environments, peat soils, and deep-sea sediments, which would shed light on the novel ecophysiology of hydrogenotrophic microorganisms in anaerobic environments.
Materials and methods
Bacterial strains and culture conditions
To culture microorganisms we used a freshwater basal medium containing (per liter) 0.3 g KH2PO4, 1 g NH4Cl, 0.1 g MgCl2·6H2O, 0.08 g CaCl2·2H2O, 0.6 g NaCl, 2 g KHCO3, 0.02 g MgSO4·7H2O, 9.52 g 4-(2-hydroxyethyl)-1-piperazineethanesulfonate (HEPES), 0.1 g yeast extract, and 10 ml each of trace metal and vitamin solutions39. The pH of the medium was adjusted to 7.0 by adding 6 N KOH solution. Methanobacterium formicicum (DSM1535T) was cultured in the freshwater basal medium supplemented with 0.1 g l–1 of sodium acetate and reducing agents (0.3 g l–1 each of cysteine·HCl·H2O and Na2S·9H2O) at 37 °C without shaking under an atmosphere of 200 kPa of H2:CO2 (80:20). CH4 and H2 in the gas phases were measured using a gas chromatograph (GC-2014; Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector (for quantification of H2) and a flame ionization detector (for quantification of CH4) as described previously40. The concentration of acetate was determined using high-performance liquid chromatography (D-2000 LaChrom Elite HPLC system; Hitachi, Tokyo, Japan) equipped with an ion exclusion column (Aminex HPX-87H; Bio-Rad Laboratories, Hercules, CA, USA) and UV detector (L2400; Hitachi). The culture experiments were conducted in triplicate.
The iron corrosion-assisted H2-supplying (iCH) system
The iCH system consists of two vials (68 ml in capacity). One vial (“corrosion vial” in Fig. 2) was filled with 20 ml of the freshwater basal medium and supplemented with Fe0 granules (1–2 mm, 99.98% purity; Alfa Aesar, Ward Hill, MA, USA) or Fe0 powder (< 45 µm, 99.9% purity; Wako Pure Chemical, Osaka, Japan). The second vial (“culture vial” in Fig. 2) was also filled with 20 ml of the freshwater basal medium. After removing the air from the medium by bubbling with N2:CO2 (80:20) gas for 5 min, the vials were sealed with butyl rubber stoppers and aluminum seals, and sterilized by autoclaving. After the cultivation vials were supplemented with reducing agents, inhibitor chemicals, and/or microorganisms, the gas phases of the two vials were connected by sterile, stainless-steel tube with an inner diameter of 1.8 mm (Swagelok, Solon, OH, USA), which was separately sterilized by autoclaving, through a guiding hole made by a gauge 18 syringe needle. Before incubation, the vials were again purged with N2:CO2 (80:20) gas for 5 min to remove trace oxygen. To confirm that the gases were uniformly diffused, CH4 and H2 in the gas phases of the two vials were measured during incubation by gas chromatography as described above.
Enrichment cultures from rice paddy field soil
High-H2 and Fe0 enrichment cultures were performed in vials (not connected to the corrosion vial) filled with 20 ml of the freshwater basal medium supplemented with 200 kPa of H2:CO2 (80:20) gas and 1 g of Fe0 powder as the sole energy source, respectively. Rifampicin (final concentration, 10 µg l–1) and BES (final concentration, 10 mM) were supplemented to the enrichment cultures for methanogens and acetogens, respectively, from filter-sterilized stock solutions. Approximately 50 mg (wet weight) of rice paddy field soil was suspended in 200 µl of the freshwater basal medium, inoculated into the culture vial using a syringe and incubated at 30 °C without shaking. After sufficient microbial growth, 0.4 ml of culture solution was transferred to fresh media in a culture vial connected to a fresh corrosion vial. After three transfers, the enrichment cultures were subjected to chemical and phylogenetic analyses. The enrichment culture experiments were conducted in duplicate.
Microbial community analysis
Microbial DNA was extracted with the FAST DNA Spin Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. Partial 16S rRNA gene fragments were amplified by PCR using Phusion Hot Start II High-Fidelity DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA) with the primer pair 515ʹF/805R41 prolonged by adaptor and index sequence tags42. PCR products were purified using a QIAquick PCR Purification Kit (Qiagen, Hilden, Germany), and were qualified and quantified using a spectrophotometer (DS-11; Denovix, Wilmington, DE, USA) and Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific), respectively. The 16S rRNA gene amplicon libraries were analyzed by Illumina paired-end (2 × 301 bp) sequencing on MiSeq platform (Illumina, San Diego, CA, USA) by Hokkaido System Science Co. Ltd. (Sapporo, Japan). Raw 16S rRNA sequence data were adaptor trimmed at the 3ʹ end to remove adaptor sequences (cutadapt 1.1)43, quality trimmed (Trimmomatic v. 0.32; TRAILING:20 MINLEN:50), and the individual read pairs were overlapped to form single synthetic reads (fastq-join v. 1.1.2–537; 8 percent maximum difference, 6 minimum overlap; https://github.com/brwnj/fastq-join). The obtained reads were clustered with the UCLUST algorithm using a ≥ 97% sequence identity cut-off44 with MacQIIME 1.9.145. Representative sequences of each OTU were aligned using PyNAST46 and chimeric sequences were removed using ChimeraSlayer47. Taxonomic assignment of each OTU was carried out with a dataset obtained from the Greengenes website (gg_13_8_otus; https://greengenes.secondgenome.com/)48. Principal component analysis was performed using MacQIIME 1.9.145. The sequence data obtained in this study have been deposited in DDBJ/EMBL/GenBank under the accession numbers DRA007911 and DRA007912.
References
Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 61, 262–280 (1997).
McInerney, M. J., Sieber, J. R. & Gunsalus, R. P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 20, 623–632 (2009).
Kato, S. & Watanabe, K. Ecological and evolutionary interactions in syntrophic methanogenic consortia. Microbes Environ. 25, 145–151 (2010).
Conrad, R., Schink, B. & Phelps, T. J. Thermodynamics of H2-consuming and H2-producing metabolic reactions in diverse methanogenic environments under in situ conditions. FEMS Microbiol. Lett. 38, 353–360 (1986).
Utsumi, M., Belova, S. E., King, G. M. & Uchiyama, H. Phylogenetic comparison of methanogen diversity in different wetland soils. J. Gen. Appl. Microbiol. 49, 75–83 (2003).
Takami, H. et al. A deeply branching thermophilic bacterium with an ancient acetyl-CoA pathway dominates a subsurface ecosystem. PLoS ONE 7, e30559 (2012).
Adam, P. S., Borrel, G., Brochier-Armanet, C. & Gribaldo, S. The growing tree of Archaea: new perspectives on their diversity, evolution and ecology. ISME J. 11, 2407–2425 (2017).
Morgan, R. M., Pihl, T. D., Nölling, J. & Reeve, J. N. Hydrogen regulation of growth, growth yields, and methane gene transcription in Methanobacterium thermoautotrophicum deltaH. J. Bacteriol. 179, 889–898 (1997).
Mukhopadhyay, B., Johnson, E. F. & Wolfe, R. S. A novel pH2 control on the expression of flagella in the hyperthermophilic strictly hydrogenotrophic methanarchaeaon Methanococcus jannaschii. Proc. Natl. Acad. Sci. USA 97, 11522–11527 (2000).
Hendrickson, E. L., Haydock, A. K., Moore, B. C., Whitman, W. B. & Leigh, J. A. Functionally distinct genes regulated by hydrogen limitation and growth rate in methanogenic Archaea. Proc. Natl. Acad. Sci. USA 104, 8930–8934 (2007).
Sakai, S. et al. Cultivation of methanogens under low-hydrogen conditions by using the coculture method. Appl. Environ. Microbiol. 75, 4892–4896 (2009).
Sakai, S. et al. Methanocella paludicola gen. nov., sp. nov., a methane-producing archaeon, the first isolate of the lineage “Rice Cluster I”, and proposal of the new archaeal order Methanocellales ord. nov. Int. J. Syst. Evol. Microbiol. 58, 929–936 (2008).
Sakai, S. et al. Methanolinea mesophila sp. nov., a hydrogenotrophic methanogen isolated from rice field soil, and proposal of the archaeal family Methanoregulaceae fam nov. within the order Methanomicrobiales. Int. J. Syst. Evol. Microbiol. 62, 1389–1395 (2012).
Luo, H. W., Zhang, H., Suzuki, T., Hattori, S. & Kamagata, Y. Differential expression of methanogenesis genes of Methanothermobacter thermoautotrophicus (formerly Methanobacterium thermoautotrophicum) in pure culture and in cocultures with fatty acid-oxidizing syntrophs. Appl. Environ. Microbiol. 68, 1173–1179 (2002).
Enoki, M., Shinzato, N., Sato, H., Nakamura, K. & Kamagata, Y. Comparative proteomic analysis of Methanothermobacter themautotrophicus ΔH in pure culture and in co-culture with a butyrate-oxidizing bacterium. PLoS ONE 6, e24309 (2011).
Daniels, L., Belay, N., Rajagopal, B. S. & Weimer, P. J. Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 237, 509–511 (1987).
Belay, N. & Daniels, L. Elemental metals as electron sources for biological methane formation from CO2. Antonie Van Leeuwenhoek 57, 1–7 (1990).
Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 9, 141–148 (2016).
Mand, J., Park, H. S., Jack, T. R. & Voordouw, G. The role of acetogens in microbially influenced corrosion of steel. Front. Microbiol. 5, 268 (2014).
Enning, D. & Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl. Environ. Microbiol. 80, 1226–1236 (2014).
Dinh, H. T. et al. Iron corrosion by novel anaerobic microorganisms. Nature 427, 829–832 (2004).
Kato, S., Yumoto, I. & Kamagata, Y. Isolation of acetogenic bacteria that induce biocorrosion by utilizing metallic iron as the sole electron donor. Appl. Environ. Microbiol. 81, 67–73 (2015).
Sedano-Núñez, V. T., Boeren, S., Stams, A. J. M. & Plugge, C. M. Comparative proteome analysis of propionate degradation by Syntrophobacter fumaroxidans in pure culture and in coculture with methanogens. Environ. Microbiol. 20, 1842–1856 (2018).
Ishii, S., Kosaka, T., Hori, K., Hotta, Y. & Watanabe, K. Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl. Environ. Microbiol. 71, 7838–7845 (2005).
Kato, S., Kosaka, T. & Watanabe, K. Substrate-dependent transcriptomic shifts in Pelotomaculum thermopropionicum grown in syntrophic co-culture with Methanothermobacter thermautotrophicus. Microb. Biotechnol. 2, 575–584 (2009).
Sakai, S., Conrad, R., Liesack, W. & Imachi, H. Methanocella arvoryzae sp. nov., a hydrogenotrophic methanogen isolated from rice field soil. Int. J. Syst. Evol. Microbiol. 60, 2918–2923 (2010).
Lü, Z. & Lu, Y. Methanocella conradii sp. nov., a thermophilic, obligate hydrogenotrophic methanogen, isolated from Chinese rice field soil. PLoS ONE 7, e35279 (2012).
Nüsslein, B., Chin, K. J., Eckert, W. & Conrad, R. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ. Microbiol. 3, 460–470 (2001).
Juottonen, H. et al. Methanogen communities and Bacteria along an ecohydrological gradient in a northern raised bog complex. Environ. Microbiol. 7, 1547–1557 (2005).
Conrad, R., Erkel, C. & Liesack, W. Rice Cluster I methanogens, an important group of Archaea producing greenhouse gas in soil. Curr. Opin. Biotechnol. 17, 262–267 (2006).
Parkes, R. J. et al. Biogeochemistry and biodiversity of methane cycling in subsurface marine sediments (Skagerrak, Denmark). Environ. Microbiol. 9, 1146–1161 (2008).
Cadillo-Quiroz, H., Yashiro, E., Yavitt, J. B. & Zinder, S. H. Characterization of the archaeal community in a minerotrophic fen and terminal restriction fragment length polymorphism-directed isolation of a novel hydrogenotrophic methanogen. Appl. Environ. Microbiol. 74, 2059–2068 (2008).
Schmidt, O., Hink, L., Horn, M. A. & Drake, H. L. Peat: home to novel syntrophic species that feed acetate- and hydrogen-scavenging methanogens. ISME J. 10, 1954–1966 (2016).
Drake, H. L., Gössner, A. S. & Daniel, S. L. Old acetogens, new light. Ann. N. Y. Acad. Sci. 1125, 100–128 (2008).
Balch, W. E., Schoberth, S., Tanner, R. S. & Wolfe, R. S. Acetobacterium, a new genus of hydrogenoxidizing, carbon dixoide-reducing, anaerobic bacteria. Int. J. Syst. Bacteriol. 27, 355–361 (1977).
Xu, S., Fu, B., Zhang, L. & Liu, H. Bioconversion of H2/CO2 by acetogen enriched cultures for acetate and ethanol production: the impact of pH. World. J. Microbiol. Biotechnol. 31, 941–950 (2015).
Philips, J. Extracellular electron uptake by acetogenic bacteria: Does H2 consumption favor the H2 evolution reaction on a cathode or metallic iron?. Front. Microbiol. 10, 2997 (2020).
Kotsyurbenko, O. R., Glagolev, M. V., Nozhevnikova, A. N. & Conrad, R. Competition between homoacetogenic bacteria and methanogenic archaea for hydrogen at low temperature. FEMS Microbiol. Ecol. 38, 153–159 (2001).
Kato, S. et al. Enrichment and isolation of Flavobacterium strains with tolerance to high concentrations of cesium ion. Sci. Rep. 6, 20041 (2016).
Kato, S., Sasaki, K., Watanabe, K., Yumoto, I. & Kamagata, Y. Physiological and transcriptomic analyses of the thermophilic, aceticlastic methanogen Methanosaeta thermophila responding to ammonia stress. Microbes Environ. 29, 162–167 (2014).
Hugerth, L. W. et al. DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies. Appl. Environ. Microbiol. 80, 5116–5123 (2014).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Caporaso, J. G. et al. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26, 266–267 (2010).
Haas, B. J. et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21, 494–504 (2011).
McDonald, D. et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6, 610–618 (2012).
Acknowledgements
This research was supported by the Japan Society for the Promotion of Science KAKENHI Grant Numbers 15H01071, 16KK0154, 16K14895, 17H03800, and 19K22296.
Author information
Authors and Affiliations
Contributions
S.K. designed the experiments, analyzed the data, and wrote the paper. M.T. and K.I. carried out the culture experiments and microbial community analysis, and analyzed the data. H.M., D.M. and H.T. were involved in the design of the experiments and helped the data interpretation. All authors reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kato, S., Takashino, M., Igarashi, K. et al. An iron corrosion-assisted H2-supplying system: a culture method for methanogens and acetogens under low H2 pressures. Sci Rep 10, 19124 (2020). https://doi.org/10.1038/s41598-020-76267-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76267-z
This article is cited by
-
Insights into the various mechanisms by which Shewanella spp. induce and inhibit steel corrosion
npj Materials Degradation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.