Abstract
Exercise intolerance among the clinical symptoms in patients with atrial fibrillation (AF) has usually been masked by their adjusted life style. We sought to assess the role of CHA2DS2-VASc score to predict exercise intolerance in asymptomatic AF patients, and further examine whether the relationship differs by age and gender. Among the 6,275 participants of the prospective Korean registry of the Comparison study of Drugs for symptom control and complication prevention of Atrial Fibrillation (CODE-AF), 1,080 AF patients who underwent exercise treadmill testing were studied. Exercise intolerance was defined as a peak exercise capacity of 7 metabolic equivalents (METs) or less, and the patients were divided into two groups for the analysis: ≤7 METs (n = 131) and >7 METs (n = 949). Patients with exercise intolerance had a significantly higher CHA2DS2-VASc score than those without (3.1 ± 1.3 vs. 2.0 ± 1.5, p < 0.0001). In the multivariate analysis, a higher CHA2DS2-VASc score (OR 1.54, 95% CI 1.31–1.81, p < 0.0001), corrected QT interval (OR 1.01, 95% CI 1.00–1.02, p = 0.026), and increased left atrial volume index (OR 1.02, 95% CI 1.01–1.03, p = 0.001) were found to be independent predictors of exercise intolerance. The impact of the CHA2DS2-VASc score on exercise intolerance was significant only in male patients aged <65 years (OR 3.30, 95% CI 1.76–6.19, p < 0.0001). The CHA2DS2-VASc score may be a feasible risk assessment tool to predict exercise intolerance, especially in young and middle-aged male patients with asymptomatic AF.
Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, with a prevalence of 1–2% in the general population1,2. Many patients with AF experience various degrees of impaired exercise tolerance, which is associated with an increased morbidity and mortality, and poor quality of life3,4,5. However, some patients with AF are asymptomatic6 and their adjusted life style may also mask the exercise intolerance. Since asymptomatic AF patients are more likely to miss out on the appropriate treatment options, their prognosis is worse than that of symptomatic patients7,8,9. Therefore, early detection and treatment of exercise intolerance in these patients have important clinical implications. Exercise Treadmill testing (TMT) may be a useful tool to estimate exercise capacity in AF patients, however; it cannot be routinely performed in patients with mental or physical impairment, or conversely, in apparently healthy subjects without symptom10.
The CHA2DS2-VASc score (Congestive HF, hypertension, age ≥75 years [doubled], type 2 diabetes, previous stroke or transient ischemic attack (TIA) [doubled], vascular disease, age 65 to 74 years, and sex category) has been originally recommended for the stroke risk stratification in AF1. Recently, its usefulness as a risk assessment tool for adverse clinical outcomes other than thromboembolic events has also been explored beyond the AF field11,12,13. The aim of our study was to investigate the association between CHA2DS2-VASc score and exercise capacity in AF patients. In this study, we tested the hypothesis that the CHA2DS2-VASc score could predict exercise intolerance in asymptomatic AF patients. And we further examined whether the relationship differs by age and gender, because age and gender were very important risk factors which influence on exercise intolerance.
Methods
Study population
The COmparison study of Drugs for symptom control and complication prEvention of AF (CODE-AF) is a prospective, multicenter, ongoing observational study conducted in patients aged >18 years with AF at 10 tertiary hospitals in South Korea. The primary aim of the CODE-AF registry is to compare the outcomes according to the different medical treatment strategies such as anticoagulation, rate control, and rhythm control. The secondary aim of the study is to describe the clinical epidemiology of AF and to evaluate the diagnostic and therapeutic processes applied to patients with AF and their clinical outcomes. The registry was funded and designed by the Korea Ministry of Health & Welfare (HI15C1200), which provided national coordinators. And it was coordinated by the Korea Heart Rhythm Society, which provides support to related committees and participating centers. Data are entered into a common electronic database that limits inconsistencies and errors and provides online help for key variables. Each center has access not only to its own data, but also to data from all other participating centers. AF-related symptoms included palpitations, tachycardia, syncope or presyncope, dyspnea, orthopnea, shortness of breath on exertion, chest pain, fatigue, or malaise, which were assessed by a self-reported questionnaire and classified into three grades based on the EHRA symptom scale. The details of the study design has been reported previously14. This study was based on the first released database for enrolled patients between June 2016 and April 2017, who were >18 years with non-valvular AF, attended an outpatient clinic, and were hospitalized on the same day for AF. All patients were scheduled for clinically follow-up every 6 months, either through personal interview or telephone contact. This study was conducted in accordance with the Declaration of Helsinki and the relevant guidelines and regulations. The study protocol was approved by the research Ethics Committee of Ewha Womans University Mokdong Hospital (No. 216-02-056), and all patients gave their written informed consent prior to enrollment. This study was registered in the ClinicalTrials.gov (NCT02786095).
Among the 6,275 participants of the CODE-AF, 1,872 asymptomatic AF patients who underwent exercise TMT as a screening exam were selected. From those, 792 patients were excluded for the following reasons: a history of congestive HF or myocardial infarction (n = 190), structural or valvular heart disease (n = 142), or radiofrequency catheter ablation of AF (n = 417); presence of an intracardiac device such as a pacemaker or implantable cardioverter defibrillator (n = 89); left ventricular (LV) systolic dysfunction (ejection fraction [EF] <50%) (n = 105); the presence of stenosis (>50%) on coronary computed tomography (CT) or coronary angiography (n = 121); or missing data (n = 77). Finally, a total of 1,080 consecutive patients were eligible for our analysis. The CHA2DS2-VASc score was calculated for each patient based on their demographic and clinical information at the time of enrollment, and categorized into three groups (0–1 points, 2–3 points, and ≥4 points).
Exercise protocol
All patients underwent maximal, symptom-limited exercise TMT with electrographic monitoring using the Bruce or Naughton protocol. ST-segment depression was defined as a ≥1 mm horizontal or down-sloping at 80msec from the J point for 3 consecutive beats10. The exercise capacity was measured in peak metabolic equivalents (METs) estimated from the basis of the exercise speed and grade using the standardized equations. Exercise intolerance was defined as a peak exercise capacity of ≤7 METs15.
Statistical analysis
The continuous variables are presented as the mean ± SD, whereas categorical variables are presented as counts and percentages. Comparisons of the variables across the groups were performed using a Student t test or a one-way ANOVA combined with a Bonferroni post hoc analysis for continuous variables and Chi-square (χ2) or Fisher’s exact test for categorical variables, as appropriate. A multiple logistic regression analysis was performed to determine the independent predictors of exercise intolerance using covariates identified as significant in the univariate analysis or previously known to be important variables. A receiver operating characteristic (ROC) curve was constructed to assess the predictive ability of the CHA2DS2-VASc score for exercise intolerance. All statistical analyses were performed using the SPSS version 21.0 software package (IBM SPSS, New York, USA). A P < 0.05 was considered to be statistically significant.
Results
Patient demographic characteristics
The 1,080 patients had a mean age of 65 ± 11 years and consisted of 769 (71.2%) men. The mean CHA2DS2-VASc score was 2.1 ± 1.5, demonstrating a low CHA2DS2-VASc score (0–1) in 407 (37.7%) patients, intermediate score (2–3) in 492 (45.6%), and high score (≥4) in 181 (16.8%). The mean peak exercise capacity was 10.5 ± 2.7 METs, and the patients were divided into two groups according to the exercise capacity: ≤7 METs (n = 131) and >7 METs (n = 949).
The baseline characteristics are presented in Table 1. The patients with exercise intolerance were older, and had a higher prevalence of chronic kidney disease and higher body mass index (BMI). The prevalence of the components constituting the CHA2DS2-VASc score, including hypertension, diabetes mellitus, an age ≥75, an age 65–74, and a female gender were higher in the patients with exercise intolerance. However, there were no significant differences in the proportion of subjects with a stroke or TIA, peripheral artery disease, dyslipidemia, or smoking between the two groups. Persistent AF was more frequently found in patients with exercise intolerance, but the duration of AF was similar between the two groups. Angiotensin converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) and calcium channel blockers (CCBs) were more commonly used in patients with exercise intolerance, whereas the use of beta-blockers, digoxin, and antiarrhythmic drugs were similar between the two groups.
Difference in the clinical characteristics of the patients with and without exercise intolerance
Patients with exercise intolerance had a significantly higher CHA2DS2-VASc score (3.1 ± 1.3 vs. 2.0 ± 1.5, P < 0.0001) and larger proportion of intermediate or high-risk categories (Table 2). The peak exercise capacity according to the CHA2DS2-VASc score is shown in Fig. 1. As the CHA2DS2-VASc scores increased from 0 to 7, the peak exercise capacity significantly decreased from 12.1 to 6.5 METs (P < 0.0001) (Fig. 1A), and the categorical analysis also showed a significant difference among each risk group according to the CHA2DS2-VASc score (P < 0.0001) (Fig. 1B).
(A) Changes in the peak exercise capacity according to the CHA2DS2-VASc scores (n = 1,080). (B) Categorical analysis for changes in peak exercise capacity among each risk group according to the CHA2DS2-VASc scores (low vs. intermediate risk group, p < 0.0001; intermediate vs. high risk group, p < 0.0001; low vs. high risk group, p < 0.0001).
Patients with exercise intolerance had a significantly increased QTc interval and had higher E/E’ and left atrial volume index (LAVI) than those achieving a peak of more than 7 METs. The resting diastolic blood pressure (DBP) and peak exercise heart rate (HR) were significantly lower in the patients with exercise intolerance than in those without, as was the proportion of patients achieving ≥85% of their maximum age-predicted heart rate (MAPHR). There were no significant differences in the prevalence of exercise-induced ST-segment depression between the groups.
Predictors of exercise intolerance in the asymptomatic AF patients
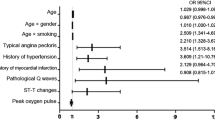
Logistic regression analyses for the association of the clinical variables to exercise intolerance are presented in Table 3. In the univariate analyses, the CHA2DS2-VASc score, BMI, chronic kidney disease, persistent AF, QTc interval, E/E’, LAVI, and resting DBP were related to exercise intolerance. After an adjustment for the significant covariates, a high CHA2DS2-VASc score (adjusted odds ratio [OR] 1.54, 95% confidence interval [CI] 1.31–1.81, P < 0.0001), prolonged QTc interval (adjusted OR 1.01, 95% CI 1.00–1.02, P = 0.026), and enlarged LAVI (adjusted OR 1.02, 95% CI 1.01–1.03, P = 0.001) remained as independent determinants of exercise intolerance.
Patients with a CHA2DS2-VASc score of 2–3 (16.5%) and CHA2DS2-VASc score of ≥4 (23.2%) were more likely to experience an impaired exercise capacity compared to those with a CHA2DS2-VASc score of 0–1 (2.0%) (P < 0.0001) (Fig. 2). Further, the association between CHA2DS2-VASc risk stratification and exercise intolerance was significant even after adjusting for the other confounding factors (Supplementary Table 1). In detail, the predictive value of the individual components of the CHA2DS2-VASc score were assessed, and we found that hypertension, diabetes mellitus, an age ≥75, an age 65 to 74, and a female gender were associated with exercise intolerance (Table 4).
Detection of exercise intolerance in young male AF patients
Finally, we performed subgroup analyses to evaluate whether there were age or gender-specific differences between the CHA2DS2-VASc score and exercise intolerance (Table 5). Interestingly, a significant association between the CHA2DS2-VASc score and exercise intolerance was found just in male patients aged <65 years (adjusted OR 3.15, 95% CI 1.84–5.38, P < 0.0001), whereas it was not in male patients aged ≥65 years and female patients. In the ROC analysis conducted among the male patients aged <65 years, a CHA2DS2-VASc score of ≥2 was identified as the optimal cut-off value for predicting exercise intolerance (AUC 0.805, 95% CI 0.68–0.93, P < 0.0001) with a sensitivity of 76.9% and specificity of 80.3% (Fig. 3).
Discussion
Although a reduced exercise capacity is commonly found in AF patients, the exact mechanisms remain uncertain. Recently, Haskiah F et al. documented the association between the CHA2DS2-VASc score and improvement in the functional capacity, however, their study population consisted of acute coronary syndrome patients referred for cardiac rehabilitation16. Approximately one third of patients with AF have no obvious AF-related symptoms and no noticeable deterioration in quality of life6. Exercise intolerance in these patients is often diagnosed later and, thus, results in worse outcomes compared with symptomatic patients7,8,9. We examined for the first time the clinical application of the CHA2DS2-VASc score for assessing the exercise capacity in asymptomatic AF patients. Further, our results showed that a high CHA2DS2-VASc score may predict exercise intolerance, especially in young and middle-aged male patients (<65 years).
Exercise intolerance in patients with normal LV systolic function is associated with diastolic heart failure (DHF), also described as HF with preserved EF (HFpEF)17. Epidemiological studies have documented that AF itself causes the LA and LV remodeling, which, in turn, contributes to diastolic dysfunction and HFpEF18,19,20,21. However, HFpEF is a challenging diagnosis since it requires typical symptoms or signs of HF and evidence of diastolic dysfunction, but a normal LV systolic function22. Exercise hemodynamics, especially in stable outpatients with normal EF is largely determined by LV diastolic filling during exercise. For this reason, exercise testing has been used not only to identify masked HFpEF, but also to predict the prognosis in patients with HFpEF23,24. Our results have important implications in that the CHA2DS2-VASc score may be used as a feasible risk assessment tool for the early detection of HFpEF in apparently asymptomatic AF patients. However, in our study, patients with exercise intolerance were more likely to be older and females. Moreover, among the CHA2DS2-VASc components, age and female were powerful determinants of exercise intolerance. Although old age and female gender are also risk factors for HFpEF22, the possibility of age- or sex-related declines in functional capacity cannot be excluded25. Actually, we found that a high CHA2DS2-VASc score was independently associated with exercise intolerance only in male patients younger than 65 years. Considering that CHA2DS2-VASc score among them is determined only by cardiovascular risk factors except for the age and gender, this finding may further support the clinical role of CHA2DS2-VASc score in predicting HFpEF.
Additionally, the QTc interval was an independent predictor of exercise intolerance in this study. The QTc interval has been reported as an electrocardiographic parameter of LV diastolic dysfunction, and a prolonged QTc interval is known to be a strong predictor of death due to worsening HF26. Unlike prior studies, our study did not show a significant relationship between the resting HR and exercise capacity. However, most data have been derived from studies in patients with sinus rhythm, and the prognostic impact of rate control in patients with AF is still controversial27,28. Moreover, the type of AF was not associated with the exercise capacity in the present study, which was inconsistent with the previously published report showing a higher prevalence of HF in patients with persistent or permanent AF than in those with paroxysmal AF29. The detrimental impact of AF on the exercise hemodynamics is well known, but most studies have been conducted in patients with HF or have not focused on the type of AF30,31,32. It is speculated that the presence of AF, rather than the type of AF may be a more important factor for determining the exercise capacity in asymptomatic AF patients with a normal LV global systolic function. We also found that an increased LAVI was associated with exercise intolerance, which is consistent finding with the previous reports33. On the other hand, E/E’ was not a significant factor related to exercise intolerance in our study. Some studies have been concerned about the limited clinical value of the E/E’ in predicting LV filling pressure in patients with normal LVEF, as well as in those with AF34. LAVI seems to be a more reliable echocardiographic parameter for the diagnosis of HFpEF as compared to the E/E’ in patients with AF.
Study limitations
This study had several limitations. First, our study included AF patients who underwent TMT as a screening exam. However, TMT was not routinely performed at all hospitals and thus, it was likely that patients with relatively more CV risk factors were referred for TMT at tertiary hospitals. Second, we arbitrarily defined exercise intolerance as ≤7 METs. Although various MET values have been used to predict adverse prognosis, there has been no widely accepted abnormal value for asymptomatic subjects15. Third, CODE-AF is an observational and multicenter registry conducted in patients with AF, so we lacked detailed information on the use of diuretics or whether medications were withheld before the exercise stress test. Moreover, the N-terminal pro B-type natriuretic peptide, the gold standard biomarker of HF, was not measured in the patients without symptoms or signs of HF. Finally, cardiopulmonary exercise testing that provides more comprehensive data on the exercise physiology than traditional exercise stress test was not available10.
Conclusion
A higher CHA2DS2-VASc score was independently associated with a lower exercise capacity. Our findings suggested that the CHA2DS2-VASc score may be used to predict exercise intolerance, especially in relatively young and middle-aged male patients with asymptomatic AF. Further prospective studies with a long-term follow-up might be needed to confirm the prognostic significance of this score for identifying AF patients at a higher risk of HF.
References
Camm, A. J. et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart. J. 31, 2369–2429 (2010).
January, C. T. et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 64, e1–76 (2014).
Singh, S. N. et al. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program Substudy. J. Am. Coll. Cardiol 48, 721–730 (2006).
Myers, J. et al. Exercise capacity and mortality among men referred for exercise testing. N. Engl. J. Med. 346, 93–801 (2002).
Mora, S. et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 290, 1600–1607 (2003).
Savelieva, I. et al. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J. Interv. Card. Electrophysiol. 4, 369–382 (2000).
Boriani, G. et al. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am. J. Med. 128, 509–518 (2015).
Vanassche, T. et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients iin ACTIVE-A and AVERROES. Eur. Heart. J. 36, 281–7a (2015).
Steinberg, B. A. et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur. Heart. J. 36, 288–296 (2015).
Fletcher, G. F. et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 128, 873–934 (2013).
Yoshihisa, A. et al. The CHA2DS2-VASc score as a predictor of high mortality in hospitalized heart failure patients. ESC. Heart Fail. 3, 261–269 (2016).
Orvin, K. et al. Usefulness of the CHA2DS2-VASC Score to Predict Adverse Outcomes in Patients Having Percutaneous Coronary Intervention. Am. J. Cardiol. 117, 1433–1438 (2016).
Rozenbaum, Z. et al. CHA2DS2-VASc score and clinical outcomes of patients with acute coronary syndrome. Eur. J. Intern. Med. 36, 57–61 (2016).
Kim, H. et al. A Prospective Survey of Atrial Fibrillation Management for Real-world Guideline Adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean. Circ. J. 47, 877–887 (2017).
Lauer, M. et al. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevension. Circulation. 112, 771–776 (2005).
Haskiah, F. et al. CHA2DS2-VASc score and exercise capacity of patients with coronary artery disease participating in cardiac rehabilitation programs. Coron Artery Dis. 28, 697–701 (2017).
Kitzman, D. W. et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. Jama. 288, 2144–2150 (2002).
Kotecha, D. et al. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 68, 2217–2228 (2016).
Vermond, R. A. et al. Incidence of Atrial Fibrillation and Relationship With Cardiovascular Events, Heart Failure, and Mortality: A Community-Based Study From the Netherlands. J. Am. Coll. Cardiol 66, 1000–1007 (2015).
Ho, J. E. et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ. Heart Fail. 6, 279–286 (2013).
Santhanakrishnan, R. et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 133, 484–492 (2016).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol 62, e147–239 (2013).
Kitzman, D. W. et al. Exercise intolerance. Heart Fail. Clin 4, 99–115 (2008).
Maor, E. et al. Exercise haemodynamics may unmask the diagnosis of diastolic dysfunction among patients with pulmonary hypertension. Eur. J. Heart Fail. 17, 151–158 (2015).
Sydó, N. et al. Relationship between exercise heart rate and age in men vs women. Mayo Clin. Proc. 89, 1664–1672 (2014).
Vrtovec, B. et al. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation 107, 1764–1769 (2003).
Kato, Y. et al. The relationship beween resting heart rate and peak VO2: A comparison of atrial fibrillation and sinus rhythm. Eur. J. Prev. Cardiol. 23, 1429–1436 (2016).
Levy, T. et al. Importance of rate control or rate regulation for improving exercise capacity and quality of life in patients with permanent atrial fibrillation and normal left ventricular function: a randomised controlled study. Heart. 85, 171–178 (2001).
Chiang, C. E. et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ. Arrhythm. Electrophysiol. 5, 632–639 (2012).
Pardaens, K. et al. Atrial fibrillation is associated with a lower exercise capacity in male chronic heart failure patients. Heart. 78, 564–568 (1997).
Zakeri, R. et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ. Heart Fail. 7, 123–130 (2014).
Atwood, J. E. et al. Exercise capacity in atrial fibrillation: a substudy of the Sotalol-Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T). Am. Heart. J. 153, 566–572 (2007).
Abhayaratna, W. P. et al. Left atrial size: physiologic determinants and clinical applications. J. Am. Coll. Cardiol. 47, 2357–2363 (2006).
Kotecha, D. et al. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. Europace. 19, 1427–1438 (2017).
Acknowledgements
This study was supported by a grant from the Korean Healthcare Technology R & D project funded by the Ministry of Health & Welfare (H15C1200), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1E1A1A01078382).
Author information
Authors and Affiliations
Contributions
Yi J.E.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data, Drafting the article or revising it critically for important intellectual content; Lee Y.S.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Choi E.K.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Cha M.J.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Kim T.H.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Park J.K.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Lee J.M.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Kang K.W.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Shim J.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Uhm J.S.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Kim J.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Kim C.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Kim J.B.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Park H.W.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data; Joung B.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data, Drafting the article or revising it critically for important intellectual content; Park J.: Conception and design of the study, or acquisition of data, or analysis and interpretation of data, Drafting the article or revising it critically for important intellectual content, Final approval of the version to be submitted.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yi, JE., Lee, Y.S., Choi, EK. et al. CHA2DS2-VASc score predicts exercise intolerance in young and middle-aged male patients with asymptomatic atrial fibrillation. Sci Rep 8, 18039 (2018). https://doi.org/10.1038/s41598-018-36185-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36185-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.