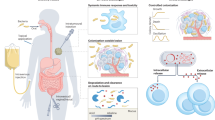
Abstract
Bacteria have been extensively utilized as living therapeutics for disease treatment due to their unique characteristics, such as genetic manipulability, rapid proliferation and specificity to target disease sites. Various in vivo insults can, however, decrease the vitality of dosed bacteria, leading to low overall bioavailability. Additionally, the innate antigens on the bacterial surface and the released toxins and metabolites may cause undesired safety issues. These limitations inevitably result in inadequate treatment outcomes, thereby hindering the clinical transformation of living bacterial therapeutics. Recently, we have developed a versatile platform to prepare advanced living bacterial therapeutics by nanocoating bacteria individually via either chemical decoration or physical encapsulation, which can improve bioavailability and reduce side effects for enhanced microbial therapy. Here we use interfacial self-assembly to prepare lipid membrane-coated bacteria (LCB), exhibiting increased resistance against a variety of harsh environmental conditions owing to the nanocoating’s protective capability. Meanwhile, we apply mechanical extrusion to generate cell membrane-coated bacteria (CMCB), displaying improved biocompatibility owing to the nanocoating’s shielding effect. We describe their detailed preparation procedures and demonstrate the expected functions of the coated bacteria. We also show that following oral delivery and intravenous injection in mouse models, LCB and CMCB present appealing potential for treating colitis and tumors, respectively. Compared with bioengineering that lacks versatile molecular tools for heterogeneous expression, the surface nanocoating technique is convenient to introduce functional components without restriction on bacterial strain types. Excluding bacterial culture, the fabrication of LCB takes ~2 h, while the preparation of CMCB takes ~5 h.
Key points
-
This protocol adds a surface nanocoating to bacteria via either chemical decoration or physical encapsulation to improve bioavailability and reduce side effects for enhanced microbial therapy.
-
Compared with biological engineering or genetic modification, surface nanocoating can easily introduce various functional components and can be applied to diverse bacterial strains. It can be achieved using either native or Food and Drug Administration-approved synthetic materials, ensuring satisfactory biocompatibility and safety.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The main data discussed in this protocol are available in the supporting primary research papers (refs. 21,39). Source data are provided with this paper.
References
Hou, K. et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 7, 135 (2022).
Chung, Y. et al. A synthetic probiotic engineered for colorectal cancer therapy modulates gut microbiota. Microbiome 9, 122 (2021).
Sepich-Poore, G. D. et al. The microbiome and human cancer. Science 371, eabc4552 (2021).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. 16, 143–155 (2018).
Chen, Y. E., Fischbach, M. A. & Belkaid, Y. Skin microbiota–host interactions. Nature 553, 427–436 (2018).
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Lavelle, A. & Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 223–237 (2020).
Lin, S. S. et al. Mucosal immunity-mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer’s patches. Sci. Adv. 7, eabf0677 (2021).
Wu, H.-J. & Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3, 4–14 (2012).
Roberts, N. J. et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 6, 249ra111 (2014).
Xu, W. et al. Attenuated Salmonella VNP20009 mutant (DeltahtrA) is a promising candidate for bacteria-mediated tumour therapy in hosts with TNFR1 deficiency. Lett. Appl. Microbiol. 67, 97–103 (2018).
Li, R. et al. Expressing cytotoxic compounds in Escherichia coli Nissle 1917 for tumor-targeting therapy. Res. Microbiol. 170, 74–79 (2019).
Jacouton, E. et al. Anti-tumoral effects of recombinant Lactococcus lactis strain secreting IL-17A cytokine. Front. Microbiol. 9, 3355 (2018).
Fan, J. X. et al. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-alpha expression via oral administration. Nano. Lett. 18, 2373–2380 (2018).
Agarwal, P., Khatri, P., Billack, B., Low, W.-K. & Shao, J. Oral delivery of glucagon like peptide-1 by a recombinant Lactococcus lactis. Pharm. Res. 31, 3404–3414 (2014).
Anselmo, A. C., McHugh, K. J., Webster, J., Langer, R. & Jaklenec, A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv. Mater. 28, 9486–9490 (2016).
Duran-Lobato, M., Niu, Z. & Alonso, M. J. Oral delivery of biologics for precision medicine. Adv. Mater. 32, e1901935 (2020).
Oka, A. & Sartor, R. B. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig. Dis. Sci. 65, 757–788 (2020).
Toso, J. F. et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 20, 142–152 (2002).
Fritz, S. E. et al. A phase I clinical study to evaluate safety of orally administered, genetically engineered Salmonella enterica serovar Typhimurium for canine osteosarcoma. Vet. Med. Sci. 2, 179–190 (2016).
Cao, Z., Cheng, S., Wang, X., Pang, Y. & Liu, J. Camouflaging bacteria by wrapping with cell membranes. Nat. Commun. 10, 3452 (2019).
Cao, Z., Lin, S. & Liu, J. Bacteria-based microdevices for the oral delivery of macromolecules. Pharmaceutics 13, 1610 (2021).
Feng, P., Cao, Z., Wang, X., Li, J. & Liu, J. On-demand bacterial reactivation by restraining within a triggerable nanocoating. Adv. Mater. 32, e2002406 (2020).
Luo, H. et al. Chemical reaction-mediated covalent localization of bacteria. Nat. Commun. 13, 7808 (2022).
Chowdhury, S. et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med. 25, 1057–1063 (2019).
Collins, J. H. & Young, E. M. Genetic engineering of host organisms for pharmaceutical synthesis. Curr. Opin. Biotechnol. 53, 191–200 (2018).
Danino, T. et al. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 7, 289ra284 (2015).
Hwang, I. Y. et al. Engineered probiotic Escherichia coli can eliminate and prevent Pseudomonas aeruginosa gut infection in animal models. Nat. Commun. 8, 15028 (2017).
Hyun, J. et al. Engineered attenuated Salmonella typhimurium expressing neoantigen has anticancer effects. ACS Synth. Biol. 10, 2478–2487 (2021).
He, L. et al. Intestinal probiotics E. coli Nissle 1917 as a targeted vehicle for delivery of p53 and Tum-5 to solid tumors for cancer therapy. J. Biol. Eng. 13, 58 (2019).
Geng, Z. et al. Aptamer-assisted tumor localization of bacteria for enhanced biotherapy. Nat. Commun. 12, 6584 (2021).
Guo, H. et al. Integrating bacteria with a ternary combination of photosensitizers for monochromatic irradiation-mediated photoacoustic imaging-guided synergistic photothermal therapy. ACS Nano 17, 5059–5071 (2023).
Hou, W. et al. Decorating bacteria with a therapeutic nanocoating for synergistically enhanced biotherapy. Small 17, e2101810 (2021).
Pan, C. et al. Polymerization-mediated multifunctionalization of living cells for enhanced cell-based therapy. Adv. Mater. 33, e2007379 (2021).
Wang, L., Cao, Z., Zhang, M., Lin, S. & Liu, J. Spatiotemporally controllable distribution of combination therapeutics in solid tumors by dually modified bacteria. Adv. Mater. 34, e2106669 (2022).
Wang, X. et al. Bioinspired oral delivery of gut microbiota by self-coating with biofilms. Sci. Adv. 6, eabb1952 (2020).
Wang, X. et al. Versatility of bacterial outer membrane vesicles in regulating intestinal homeostasis. Sci. Adv. 9, eade5079 (2023).
Zhou, J. et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 13, 3432 (2022).
Cao, Z., Wang, X., Pang, Y., Cheng, S. & Liu, J. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat. Commun. 10, 5783 (2019).
Hu, C. M. et al. ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale 5, 2664–2668 (2013).
Chen, J. et al. Oncolytic adenovirus complexes coated with lipids and calcium phosphate for cancer gene therapy. ACS Nano 10, 11548–11560 (2016).
Hu, C. M. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Tsai, R. K., Rodriguez, P. L. & Discher, D. E. Self inhibition of phagocytosis: the affinity of ‘marker of self’ CD47 for SIRPα dictates potency of inhibition but only at low expression levels. Blood Cells Mol. Dis. 45, 67–74 (2010).
Jiang, Q. et al. Red blood cell membrane-camouflaged melanin nanoparticles for enhanced photothermal therapy. Biomaterials 143, 29–45 (2017).
Rao, L. et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small 11, 6225–6236 (2015).
Romeis, E. et al. Genetic engineering of Treponema pallidum subsp. pallidum, the syphilis spirochete. PLoS Pathog. 17, e1009612 (2021).
Riley, L. A. & Guss, A. M. Approaches to genetic tool development for rapid domestication of non-model microorganisms. Biotechnol. Biofuels 14, 30 (2021).
Brito, I. L. Examining horizontal gene transfer in microbial communities. Nat. Rev. Microbiol. 19, 442–453 (2021).
Zhang, X., Goncalves, R. & Mosser, D. M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 83, 14.1.1–14.1.14 (2008).
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2021YFA0909400 to J.L.), the National Natural Science Foundation of China (32101218 to Z.C.), the Explorer Program of the Science and Technology Commission of Shanghai Municipality (21TS1400400 to J.L.), the Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20210900 to J.L.), the Foundation of National Infrastructures for Translational Medicine (Shanghai) (TMSK-2021-123 to J.L.), and the Two-hundred Talent (20181704 to J.L.).
Author information
Authors and Affiliations
Contributions
J.L. and Z.C. originated the methods of preparing LCB and CMCB. All authors wrote the manuscript and approved the contents of the protocol.
Corresponding author
Ethics declarations
Competing interests
J.L. and Z.C. are inventors on a filed provisional application China patent no. CN201911034435.7 (Surface modified bacteria, preparation method and application thereof), submitted by Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital that covers the potential therapeutic uses of coated bacteria for treating colitis and cancer.
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Fu-Gen Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Cao, Z. et al. Nat. Commun. 10, 5783 (2019): https://doi.org/10.1038/s41467-019-13727-9
Geng, Z. et al. Sci. Adv. 9, eade0997 (2023): https://doi.org/10.1126/sciadv.ade0997
Cao, Z. et al. Nat. Commun. 10, 3452 (2019): https://doi.org/10.1038/s41467-019-11390-8
Lin, S. et al. Sci. Adv. 7, eabf0677 (2021): https://doi.org/10.1126/sciadv.abf0677
Wang, X. et al. Sci. Adv. 6, eabb1952 (2020): https://doi.org/10.1126/sciadv.abb1952
Supplementary information
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blot.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, Z., Liu, J. Surface nanocoating of bacteria as a versatile platform to develop living therapeutics. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-01019-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-01019-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.