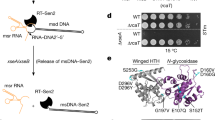
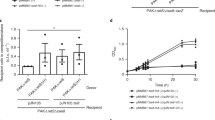
Abstract
Toxin–antitoxin systems (TAs) are abundant in bacterial chromosomes and can arrest growth under stress, but usually remain inactive. TAs have been increasingly implicated in halting the growth of infected bacteria from bacteriophages or foreign genetic elements1,2 to protect the population (abortive infection, Abi). The vast diversity and abundance of TAs and other Abi systems3 suggest they play an important immunity role, yet what allows them to sense attack remains largely enigmatic. Here, we describe a method called toxin activation–inhibition conjugation (TAC–TIC), which we used to identify gene products that trigger or block the toxicity of phage-defending tripartite retron-TAs4. TAC–TIC employs high-density arrayed mobilizable gene-overexpression libraries, which are transferred into cells carrying the full TA system or only its toxic component, on inducible vectors. The double-plasmid transconjugants are then pinned on inducer-containing agar plates and their colony fitness is quantified to identify gene products that trigger a TA to inhibit growth (TAC), or that block it from acting (TIC). TAC–TIC is optimized for the Singer ROTOR pinning robot, but can also be used with other robots or manual pinners, and allows screening tens of thousands of genes against any TA or Abi (with toxicity) within a week. Finally, we present a dual conjugation donor/cloning strain (Escherichia coli DATC), which accelerates the construction of TAC–TIC gene-donor libraries from phages, enabling the use of TAC–TIC for identifying TA triggers and antidefense mechanisms in phage genomes.
Key points
-
TAC–TIC employs gene-overexpression plasmid libraries conjugated into E. coli strains carrying the full toxin–antitoxin or phage defense system or only its toxin/effector. Colony fitness of double-plasmid transconjugants is quantified to reveal genes that trigger the system to inhibit growth or block its toxin from acting.
-
TAC–TIC can identify all potential phage genes that trigger/block a defense system, while phage escapee studies identify the strongest genetic elements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Code availability
Code used to analyze TAC–TIC data in ref. 4 can be found at https://git.embl.de/kritikos/tic-tac.
References
Song, S. & Wood, T. K. A primary physiological role of toxin/antitoxin systems is phage inhibition. Front. Microbiol. 11, 1895 (2020).
LeRoux, M. & Laub, M. T. Toxin–antitoxin systems as phage defense elements. Annu. Rev. Microbiol. 76, 21–43 (2022).
Tal, N. & Sorek, R. SnapShot: bacterial immunity. Cell 185, 578–578.e1 (2022).
Bobonis, J. et al. Bacterial retrons encode phage-defending tripartite toxin–antitoxin systems. Nature 609, 144–150 (2022).
Harms, A., Brodersen, D. E., Mitarai, N. & Gerdes, K. Toxins, targets, and triggers: an overview of toxin–antitoxin biology. Mol. Cell 70, 768–784 (2018).
Fraikin, N., Goormaghtigh, F. & Van Melderen, L. Type II toxin–antitoxin systems: evolution and revolutions. J. Bacteriol. 202, e00763–19 (2020).
Fineran, P. C. et al. The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc. Natl Acad. Sci. USA 106, 894–899 (2009).
Otsuka, Y. & Yonesaki, T. Dmd of bacteriophage T4 functions as an antitoxin against Escherichia coli LsoA and RnlA toxins. Mol. Microbiol. 83, 669–681 (2012).
Sberro, H. et al. Discovery of functional toxin/antitoxin systems in bacteria by shotgun cloning. Mol. Cell 50, 136–148 (2013).
Lopatina, A., Tal, N. & Sorek, R. Abortive infection: bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 7, 371–384 (2020).
Vassallo, C. N., Doering, C. R., Littlehale, M. L., Teodoro, G. I. C. & Laub, M. T. A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome. Nat. Microbiol. 7, 1568–1579 (2022).
Millman, A. et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 30, 1556–1569.e5 (2022).
Jaskólska, M., Adams, D. W. & Blokesch, M. Two defence systems eliminate plasmids from seventh pandemic Vibrio cholerae. Nature 604, 323–329 (2022).
Millman, A. et al. Bacterial retrons function in anti-phage defense. Cell 183, 1551–1561.e12 (2020).
Gao, L. et al. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 369, 1077–1084 (2020).
Samson, J. E., Magadán, A. H., Sabri, M. & Moineau, S. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 11, 675–687 (2013).
Jurėnas, D., Fraikin, N., Goormaghtigh, F. & Van Melderen, L. Biology and evolution of bacterial toxin–antitoxin systems. Nat. Rev. Microbiol. 20, 335–350 (2022).
Schubert, M. G. et al. High-throughput functional variant screens via in vivo production of single-stranded DNA. Proc. Natl Acad. Sci. USA 118, 1–10 (2021).
Farzadfard, F., Gharaei, N., Citorik, R. J. & Lu, T. K. Efficient retroelement-mediated DNA writing in bacteria. Cell Syst. 12, 860–872.e5 (2021).
Lopez, S. C., Crawford, K. D., Lear, S. K., Bhattarai-Kline, S. & Shipman, S. L. Precise genome editing across kingdoms of life using retron-derived DNA. Nat. Chem. Biol. 18, 199–206 (2022).
Mestre, M. R., González-Delgado, A., Gutiérrez-Rus, L. I., Martínez-Abarca, F. & Toro, N. Systematic prediction of genes functionally associated with bacterial retrons and classification of the encoded tripartite systems. Nucleic Acids Res. 48, 12632–12647 (2020).
Saka, K. A complete set of escherichia coli open reading frames in mobile plasmids facilitating genetic studies. DNA Res. 12, 63–68 (2005).
Otsuka, Y. et al. GenoBase: Comprehensive resource database of Escherichia coli K-12. Nucleic Acids Res. 43, D606–D617 (2015).
Depardieu, F. et al. A eukaryotic-like serine/threonine kinase protects Staphylococci against phages. Cell Host Microbe 20, 471–481 (2016).
Snyder, L. & McWilliams, K. The rex genes of bacteriophage lambda can inhibit cell function without phage superinfection. Gene 81, 17–24 (1989).
Meeske, A. J., Nakandakari-Higa, S. & Marraffini, L. A. Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature 570, 241–245 (2019).
Stokar-Avihail, A. et al. Discovery of phage determinants that confer sensitivity to bacterial immune systems. Cell 186, 1863–1876.e16 (2023).
Zhang, T. et al. Direct activation of a bacterial innate immune system by a viral capsid protein. Nature 612, 132–140 (2022).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Paradis-Bleau, C., Kritikos, G., Orlova, K., Typas, A. & Bernhardt, T. G. A genome-wide screen for bacterial envelope biogenesis mutants identifies a novel factor involved in cell wall precursor metabolism. PLoS Genet. 10, e1004056 (2014).
Gao, L. A. et al. Prokaryotic innate immunity through pattern recognition of conserved viral proteins. Science 377, eabm4096 (2022).
Amitsur, M., Levitz, R. & Kaufmann, G. Bacteriophage T4 anticodon nuclease, polynucleotide kinase and RNA ligase reprocess the host lysine tRNA. EMBO J. 6, 2499–2503 (1987).
Ferrières, L. et al. Silent mischief: bacteriophage Mu insertions contaminate products of Escherichia coli random mutagenesis performed using suicidal transposon delivery plasmids mobilized by broad-host-range RP4 conjugative machinery. J. Bacteriol. 192, 6418–6427 (2010).
Simon, R., Priefer, U. & Pühler, A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat. Biotechnol. 1, 784–791 (1983).
Thomason, L. C., Costantino, N. & Court, D. L. E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 79, 1–8 (2007).
Sharan, S. K., Thomason, L. C., Kuznetsov, S. G. & Court, D. L. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 4, 206–223 (2009).
Shiver, A. L., Culver, R., Deutschbauer, A. M. & Huang, K. C. Rapid ordering of barcoded transposon insertion libraries of anaerobic bacteria. Nat. Protoc. 16, 3049–3071 (2021).
Engler, C., Kandzia, R. & Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 3, e3647 (2008).
Yong, H. T. et al. Development of a system for discovery of genetic interactions for essential genes in Escherichia coli K-12. Genes Genet. Syst. 88, 233–240 (2013).
Babic, A., Guérout, A.-M. & Mazel, D. Construction of an improved RP4 (RK2)-based conjugative system. Res. Microbiol. 159, 545–549 (2008).
Haase, J., Lurz, R., Grahn, A. M., Bamford, D. H. & Lanka, E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J. Bacteriol. 177, 4779–4791 (1995).
Jahn, M., Vorpahl, C., Hübschmann, T., Harms, H. & Müller, S. Copy number variability of expression plasmids determined by cell sorting and droplet digital PCR. Microb. Cell Fact. 15, 211 (2016).
Guzman, L. M., Belin, D., Carson, M. J. & Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose P(BAD) promoter. J. Bacteriol. 177, 4121–4130 (1995).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006:2–2006.0008 (2006).
Kritikos, G. et al. A tool named Iris for versatile high-throughput phenotyping in microorganisms. Nat. Microbiol. 2, 17014 (2017).
Chung, C. T., Niemela, S. L. & Miller, R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc. Natl Acad. Sci. USA 86, 2172–2175 (1989).
Doherty, H. M., Kritikos, G., Galardini, M., Banzhaf, M. & Moradigaravand, D. ChemGAPP: a tool for chemical genomics analysis and phenotypic profiling. Bioinformatics 39, 2023.01.05.522861 (2023).
Acknowledgements
We thank the Typas laboratory for discussions and for advice when first establishing the automated steps of this protocol, especially M. Wartel and A. Koumoutsi. This work was funded by EMBL.
Author information
Authors and Affiliations
Contributions
J.B. and A.T. conceived the TAC–TIC protocol, J.B., A.L.J.Y. and C.G.P.V. optimized the protocol for constructing gene–donor phage libraries, A.L.J.Y. performed experiments for Supplementary Fig. 1a,b, J.B. designed figures with comments from all authors. J.B. and A.L.J.Y. wrote the manuscript, with extensive comments and edits from C.G.P.V. and A.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Kenn Gerdes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Bobonis, J. et al. Nature 609, 144–150 (2022): https://doi.org/10.1038/s41586-022-05091-4
Extended data
Supplementary information
Supplementary Information
Supplementary Methods, Table 1 and Figs. 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bobonis, J., Yang, A.L.J., Voogdt, C.G.P. et al. TAC–TIC, a high-throughput genetics method to identify triggers or blockers of bacterial toxin–antitoxin systems. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-00988-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-00988-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.