Abstract
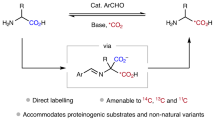
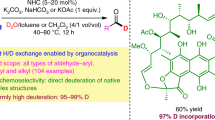
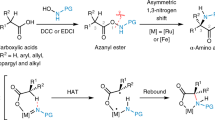
Isotopically carbon-labeled α-amino acids are valuable synthetic targets that are increasingly needed in pharmacology and medical imaging. Existing preparations rely on early stage introduction of the isotopic label, which leads to prohibitive synthetic costs and time-intensive preparations. Here we describe a protocol for the preparation of C1-labeled α-amino acids using simple aldehyde catalysts in conjunction with [*C]CO2 (* = 14, 13, 11). This late-stage labeling strategy is enabled by the one-pot carboxylate exchange of unprotected α-amino acids with [*C]CO2. The protocol consists of three separate procedures, describing the syntheses of (±)-[1-13C]phenylalanine, (±)-[1-11C]phenylalanine and (±)-[1-14C]phenylalanine from unlabeled phenylalanine. Although the delivery of [*C]CO2 is operationally distinct for each experiment, each procedure relies on the same fundamental chemistry and can be executed by heating the reaction components at 50–90 °C under basic conditions in dimethylsulfoxide. Performed on scales of up to 0.5 mmol, this methodology is amenable to C1-labeling of many proteinogenic α-amino acids and nonnatural derivatives, which is a breakthrough from existing methods. The synthesis of (±)-[1-13C]phenylalanine requires ~2 d, with product typically obtained in a 60–80% isolated yield (n = 3, μ = 71, σ = 8.3) with an isotopic incorporation of 70–88% (n = 18, μ = 72, σ = 9.0). Starting from the preformed imino acid (~3 h preparation time), rapid synthesis of (±)-[1-11C]phenylalanine can be completed in ~1 h with an isolated radiochemical yield of 13%. Finally, (±)-[1-14C]phenylalanine can be accessed in ~2 d with a 51% isolated yield and 11% radiochemical yield.
Key points
-
Amino acids can be labeled with carbon isotopes via carboxylate exchange using [*C]CO2 where *C is either 13C, 11C or 14C. Three procedures are described using phenylalanine as the example starting material; the main differences relate to the sources of [*C]CO2 and how it is delivered.
-
The products can be used immediately after a straightforward HPLC separation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All experimental data supporting the findings of this study can be found within the paper and its Supplementary Information. Additional data related to the protocol can be found in the key reference supporting this paper27.
References
Elmore, C. S. & Bragg, R. A. Isotope chemistry; a useful tool in the drug discovery arsenal. Bioorg. Med. Chem. Lett. 25, 167–171 (2015).
Derdau, V. et al. The future of (radio)-labeled compounds in research and development within the life science industry. Angew. Chem. Int. Ed. 62, e202306019 (2023).
Kopf, S. et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 122, 6634–6718 (2022).
Alauddin, M. M. Positron emission tomography (PET) imaging with 18F-based radiotracers. Am. J. Nucl. Med. Mol. Imaging 2, 55–76 (2012).
Han, J. et al. Chemical aspects of human and environmental overload with fluorine. Chem. Rev. 121, 4678–4742 (2021).
Krauser, J. A. A perspective on tritium versus carbon-14: ensuring optimal label selection in pharmaceutical research and development. J. Label. Compd. Radiopharm. 56, 441–446 (2013).
Jacobson, O., Kiesewetter, D. O. & Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjugate Chem. 26, 1–18 (2015).
Hughes, A. B. Amino Acids, Peptides and Proteins in Organic Chemistry (Wiley, 2009).
Voges, R., Heys, J. R. & Moenius, T. Preparation of Compounds Labeled with Tritium and Carbon-14 (John Wiley & Sons, 2009).
Dell’isola, A. et al. Synthesis of carbon-14–labelled peptides. J. Label. Compd. Radiopharm. 62, 713–717 (2019).
Mann, M. Functional and quantitative proteomics using SILAC. Nat. Rev. Mol. Cell Biol. 7, 952–958 (2006).
Lin, M. T. et al. A rapid and robust method for selective isotope labeling of proteins. Methods 55, 370–378 (2011).
Huang, C. & McConathy, J. Radiolabeled amino acids for oncologic imaging. J. Nucl. Med. 54, 1007–1010 (2013).
Galldiks, N. & Langen, K. J. Applications of PET imaging of neurological tumors with radiolabeled amino acids. Q. J. Nucl. Med. Mol. Imaging 59, 70–82 (2015).
Jager, P. L. et al. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J. Nucl. Med. 42, 432–445 (2001).
Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 20, 309–325 (2021).
Derdau, V. New trends and applications in cyanation isotope chemistry. J. Label. Compd. Radiopharm. 61, 1012–1023 (2018).
Bragg, R. A., Sardana, M., Artelsmair, M. & Elmore, C. S. New trends and applications in carboxylation for isotope chemistry. J. Label. Compd. Radiopharm. 61, 934–948 (2018).
Augustyniak, W., Kański, R. & Kańska, M. Synthesis of carbon-14 labeled [1-14C]-, and [2-14C]-L-tyrosine. J. Label. Compd. Radiopharm. 44, 553–560 (2001).
Pająk, M., Pałka, K., Winnicka, E. & Kańska, M. The chemo-enzymatic synthesis of labeled L-amino acids and some of their derivatives. J. Radioanal. Nucl. Chem. 317, 643–666 (2018).
Pekošak, A., Filp, U., Poot, A. J. & Windhorst, A. D. From carbon-11-labeled amino acids to peptides in positron emission tomography: the synthesis and clinical application. Mol. Imaging Biol. 20, 510–532 (2018).
Harding, J. R., Hughes, R. A., Kelly, N. M., Sutherland, A. & Willis, C. L. Syntheses of isotopically labelled L-α-amino acids with an asymmetric centre at C-3. J. Chem. Soc. Perkin Trans. 1 20, 3406–3416 (2000).
Song, F., Salter, R. & Weaner, L. E. A short synthesis of d-[1-14C]-serine of high enantiomeric purity. J. Label. Compd. Radiopharm. 58, 173–176 (2015).
Rees, D. O., Bushby, N., Harding, J. R., Song, C. & Willis, C. L. Synthesis of isotopically labelled amino acids. J. Label. Compd. Radiopharm. 50, 399–401 (2007).
Ling, J. R., Bronwen Cooper, P., Parker, S. J. & Armstead, I. P. Production and purification of mixed 14C-labelled peptides derived from plant biomass. J. Label. Compd. Radiopharm. 31, 417–426 (1992).
LeMaster, D. M. & Cronan, J. E. Biosynthetic production of 13C-labeled amino acids with site-specific enrichment. J. Biol. Chem. 257, 1224–1230 (1982).
Bsharat, O. et al. Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2. Nat. Chem. 14, 1367–1374 (2022).
Labiche, A., Malandain, A., Molins, M., Taran, F. & Audisio, D. Modern strategies for carbon isotope exchange. Angew. Chem. Int. Ed. 62, e202303535 (2023).
Destro, G. et al. Transition-metal-free carbon isotope exchange of phenyl acetic acids. Angew. Chem. Int. Ed. 59, 13490–13495 (2020).
Kong, D., Moon, P. J., Lui, E. K. J., Bsharat, O. & Lundgren, R. J. Direct reversible decarboxylation from stable organic acids in dimethylformamide solution. Science 369, 557–561 (2020).
Kong, D. et al. Fast carbon isotope exchange of carboxylic acids enabled by organic photoredox catalysis. J. Am. Chem. Soc. 143, 2200–2206 (2021).
Babin, V. et al. Photochemical strategy for carbon isotope exchange with CO2. ACS Catal. 11, 2968–2976 (2021).
Snider, M. J. & Wolfenden, R. The rate of spontaneous decarboxylation of amino acids. J. Am. Chem. Soc. 122, 11507–11508 (2000).
Silverman, R. B. (ed.) in Organic Chemistry of Enzyme-Catalyzed Reactions 2nd edn, Ch. 8, 321–357 (Academic, 2002).
Li, T., Huo, L., Pulley, C. & Liu, A. Decarboxylation mechanisms in biological system. Bioorg. Chem. 43, 2–14 (2012).
Pawelek, P. D. et al. The structure of l-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site. EMBO J. 19, 4204–4215 (2000).
Umhau, S. et al. The x-ray structure of d-amino acid oxidase at very high resolution identifies the chemical mechanism of flavin-dependent substrate dehydrogenation. Proc. Natl Acad. Sci. USA 97, 12463–12468 (2000).
Acknowledgements
Support was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) (RGPIN-2019-06050 and RGPAS-2019-00051 to R.J.L., RGPIN-2017-06167 to B.H.R., Canada Graduate Scholarship-Doctoral (CGS-D) fellowship to M.G.J.D., Postgraduate Scholarship-Doctoral (PGS-D) fellowship to B.A.M.), the Canadian Foundation for Innovation (IOF 32691 to R.J.L., JELF 36848 to B.H.R.), the Province of Alberta (AGES fellowship to M.G.J.D., AGES fellowship to O.B.), the Province of Ontario (ER17-13-119 to B.H.R.), the University of Ottawa Heart Institute (Endowed fellowship to M.M.) and the Killam trusts (Izaak Walton Killam (IWK) fellowship to M.G.J.D.). We thank M. Muñoz for HPLC recommendations as well as B. Reiz and A. Morales-Izquierdo for assistance with MS analyses.
Author information
Authors and Affiliations
Contributions
M.G.J.D. optimized the described procedure involving 13C. M.G.J.D., A.S. and V. D. carried out experiments and analyzed data involving 14C. B.A.M. optimized the described procedure involving 11C. O.B. optimized the initial reaction involving 13C. M.G.J.D. and O.B. carried out experiments and analyzed data related to studies involving the 13C procedure. B.A.M. and M.M. carried out experiments and analyzed data related to studies involving the 11C procedure. R.J.L. and B.H.R. directed the research. R.J.L. conceived the project. M.G.J.D. wrote the manuscript and supplementary information with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
A.S. and V.D. are employees of Sanofi and may hold shares or options of the company. The other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Sukwon Hong and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Bsharat, O. et al. Nat. Chem. 14, 1367–1374 (2022): https://doi.org/10.1038/s41557-022-01074-0
Supplementary information
Supplementary Information
Supplementary calculations, compound characterization data and discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Doyle, M.G.J., Mair, B.A., Sib, A. et al. A practical guide for the preparation of C1-labeled α-amino acids using aldehyde catalysis with isotopically labeled CO2. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-00974-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-00974-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.