Abstract
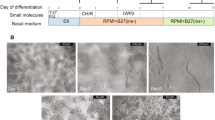
To fully realize the potential of human pluripotent stem cells (hPSCs) for both therapeutic and research purposes, it is critical to follow an efficient and reliable in vitro differentiation method that is based on optimal physical, chemical and developmental cues. This highly reproducible protocol describes how to grow hPSCs such as human induced pluripotent and embryonic stem cells in a physically confined area (‘spot’) and efficiently differentiate them into a highly enriched population of healthy and functional midbrain dopamine progenitors (mDAPs) and midbrain dopamine neurons (mDANs). The protocol takes 28 d, during which cells first grow and differentiate in spots for 14 d and then are replated and further differentiated for a further 14 d as a monolayer culture. We describe how to produce mDAPs, control the quality of cells and cryopreserve mDAPs without loss of viability. Previously we showed that mDANs generated by this ‘spotting’-based method exhibit gene expression and (electro)physiological properties typical of A9 mDANs lost in Parkinson’s disease brains and can rescue motor defects when transplanted into the striatum of 6-hydroxydopamine-lesioned rats. This protocol is scalable for production of mDAPs under good manufacturing practice conditions and was also previously successfully used to generate cells for the first autologous cell replacement therapy of a patient with Parkinson’s disease without the need for immune suppression. We anticipate this protocol could also be readily adapted to use spotting-based culture to further optimize the differentiation of hPSC to alternative differentiated cell types.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information files. Source data are provided with this paper.
References
de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535 (2006).
Kefalopoulou, Z. et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 71, 83–87 (2014).
Piccini, P. et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat. Neurosci. 2, 1137–1140 (1999).
Lindvall, O. Developing dopaminergic cell therapy for Parkinson’s disease—give up or move forward? Mov. Disord. 28, 268–273 (2013).
Barker, R. A., Barrett, J., Mason, S. L. & Bjorklund, A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 12, 84–91 (2013).
Sonntag, K. C. et al. Pluripotent stem cell-based therapy for Parkinson’s disease: current status and future prospects. Prog. Neurobiol. 168, 1–20 (2018).
Parmar, M., Grealish, S. & Henchcliffe, C. The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 21, 103–115 (2020).
Barker, R. A., Drouin-Ouellet, J. & Parmar, M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat. Rev. Neurol. 11, 492–503 (2015).
Lindvall, O. & Bjorklund, A. Cell therapeutics in Parkinson’s disease. Neurotherapeutics 8, 539–548 (2011).
Song, B. et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Invest. 130, 904–920 (2020).
Schweitzer, J. S. et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 382, 1926–1932 (2020).
Arenas, E., Denham, M. & Villaescusa, J. C. How to make a midbrain dopaminergic neuron. Development 142, 1918–1936 (2015).
Tao, Y. & Zhang, S. C. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell 19, 573–586 (2016).
Kittappa, R., Chang, W. W., Awatramani, R. B. & McKay, R. D. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 5, e325 (2007).
Chung, S. et al. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH–FoxA2 pathway. Cell Stem Cell 5, 646–658 (2009).
Joksimovic, M. et al. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat. Neurosci. 12, 125–131 (2009).
Bonilla, S. et al. Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia 56, 809–820 (2008).
Ono, Y. et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 134, 3213–3225 (2007).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480, 547–551 (2011).
Morizane, A., Doi, D., Kikuchi, T., Nishimura, K. & Takahashi, J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J. Neurosci. Res. 89, 117–126 (2011).
Doi, D. et al. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep. 2, 337–350 (2014).
Kirkeby, A. et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714 (2012).
Xi, J. et al. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells 30, 1655–1663 (2012).
Xiong, M. et al. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem Cell 28, 112–126 e116 (2021).
Kim, T. W. et al. Biphasic activation of WNT signaling facilitates the derivation of midbrain dopamine neurons from hESCs for translational use. Cell Stem Cell 28, 343–355 e345 (2021).
Gantner, C. W., Cota-Coronado, A., Thompson, L. H. & Parish, C. L. An optimized protocol for the generation of midbrain dopamine neurons under defined conditions. STAR Protoc. 1, 100065 (2020).
Knusel, B. et al. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc. Natl Acad. Sci. USA 88, 961–965 (1991).
Lin, L. F., Doherty, D. H., Lile, J. D., Bektesh, S. & Collins, F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 (1993).
Ye, W., Shimamura, K., Rubenstein, J. L., Hynes, M. A. & Rosenthal, A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93, 755–766 (1998).
Madl, C. M., Heilshorn, S. C. & Blau, H. M. Bioengineering strategies to accelerate stem cell therapeutics. Nature 557, 335–342 (2018).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Ko, J. Y., Lee, J. Y., Park, C. H. & Lee, S. H. Effect of cell-density on in-vitro dopaminergic differentiation of mesencephalic precursor cells. Neuroreport 16, 499–503 (2005).
Bolognin, S. et al. 3D Cultures of Parkinson’s disease–specific dopaminergic neurons for high content phenotyping and drug testing. Adv. Sci. 6, 1800927 (2019).
Jo, J. et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257 (2016).
Adil, M. M. & Schaffer, D. V. hPSC-derived midbrain dopaminergic neurons generated in a scalable 3-D biomaterial. Curr. Protoc. Stem Cell Biol. 44, 2D 21 21–22D 21 17 (2018).
Piao, J. et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell 28, 217–229 e217 (2021).
Nolbrant, S., Heuer, A., Parmar, M. & Kirkeby, A. Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat. Protoc. 12, 1962–1979 (2017).
Kirkeby, A. et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell 20, 135–148 (2017).
Doi, D. et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 11, 3369 (2020).
Lee, M. O. et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl Acad. Sci. USA 110, E3281–E3290 (2013).
Kirkeby, A., Parmar, M. & Barker, R. A. Strategies for bringing stem cell-derived dopamine neurons to the clinic: a European approach (STEM-PD). Prog. Brain Res. 230, 165–190 (2017).
Kikuchi, T. et al. Idiopathic Parkinson’s disease patient-derived induced pluripotent stem cells function as midbrain dopaminergic neurons in rodent brains. J. Neurosci. Res. 95, 1829–1837 (2017).
Kikuchi, T. et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 548, 592–596 (2017).
Chen, Y. et al. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson’s disease. Cell Stem Cell 18, 817–826 (2016).
Williams, G. T. Programmed cell death: apoptosis and oncogenesis. Cell 65, 1097–1098 (1991).
Fisher, D. E. Apoptosis in cancer therapy: crossing the threshold. Cell 78, 539–542 (1994).
Roos, W. P. & Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 12, 440–450 (2006).
Acknowledgements
This work was supported by NIH grants (NS070577 and OD024622) (to K.S.K.) and NRF grants 2017R1A2B4008456, 2020R1H1A2013386, IITP grant from MSIT 2020-0-01343 (to H.S.), as well as the Parkinson’s Cell Therapy Research Fund at McLean Hospital.
Author information
Authors and Affiliations
Contributions
K.S.K, H.S and J.K. designed the study; J.K., J.J., B.S., N.L., S.K. and Y.C. performed experiments; J.K., H.S. and J.J collected and analyzed data; J.K., J.J, P.L., H.S and K.S.K wrote the manuscript; H.S and K.S.K supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Asuka Morizane and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Song, B. et al. J. Clin. Invest. 130, 904–920 (2020): https://doi.org/10.1172/JCI130767
Schweitzer, J. S. et al. N. Engl. J. Med. 382, 1926–1932 (2020): https://doi.org/10.1056/NEJMoa1915872
Extended data
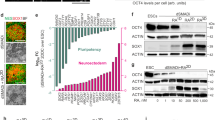
Extended Data Fig. 2 hPSC spotting condition optimization for H9 hESC cells.
The effects of cell number per spot and the number of spots per plate were determined for optimization of spotting condition. Cell loss, cell harvest, the percentage of dead cells in the final harvest and the ratio of cell loss per cell yield are shown.
Extended Data Fig. 4 Effects of C4 hiPSC spotting conditions on viability and gene expression.
a–c, The health of H9 hESCs and C4 hiPSCs were examined by TUNEL staining (a), expression of apoptosis marker cleaved caspase-3 (b) and expression of apoptosis marker genes (c), as described in ref. 10. Scale bars, 100 µm.
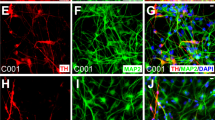
Extended Data Fig. 5 Characterization of H9-derived mDAPs by immunofluorescence on day 28 following spotting and monolayer culture.
The antibodies for FOXA2, LMX1A and TH were used to stain mDAPs, and the antibodies for cleaved caspase-3 were used to stain apoptotic cells. Scale bars, 100 µm.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 2b
Percent success rate of cells differentiating to Day 28.
Source Data Fig. 3
Source data for a) cell yield vs input; b) ratio of cell loss/Cell yield; c) dead cell percent in final harvested cell; d) percent of TUNEL-positive cells; e) percent of cleaved Caspase 3-positive cells; f) relative mRNA levels expression of BCL, BCL-XL, BAX, p53, and PUMA for H9 and C4 cells.
Source Data Fig. 5
Source data for: Cell loss; Total Cell yield; Dead Cell percent in final harvested cells; for H9 and C4 cells.
Source Data Extended Data Fig. 1
Source data for H9 cells spotting data using 5K, 10k, 20k, and monolayers looking at: Cell Loss; Cell yield/input; Cell harvest; percent dead cells in final harvest; ratio of cell loss/cell yield.
Source Data Extended Data Fig. 2
Source data for C4 cells spotting data using 5K, 10k, 20k, and monolayers looking at: Cell Loss; Cell yield/input; Cell harvest; percent dead cells in final harvest; ratio of cell loss/cell yield.
Source Data Extended Data Fig. 3
Source data for H9 cells a) TUNEL-positive cells using 10k and 20k cells per spot looking at: 4 spots, 6 spots, 9 spots, 12 spots, and monolayer. b) percent cleaved Caspase 3-positive cells using 10k and 20k cells per spot looking at: 4 spots, 6 spots, 9 spots, 12 spots, and monolayer.
Source Data Extended Data Fig. 4
Source data for C4 cells a) TUNEL-positive cells using 10k and 20k cells per spot looking at: 4 spots, 6 spots, 9 spots, 12 spots, and monolayer. b) percent cleaved Caspase 3-positive cells using 10k and 20k cells per spot looking at: 4 spots, 6 spots, 9 spots, 12 spots, and monolayer.
Rights and permissions
About this article
Cite this article
Kim, J., Jeon, J., Song, B. et al. Spotting-based differentiation of functional dopaminergic progenitors from human pluripotent stem cells. Nat Protoc 17, 890–909 (2022). https://doi.org/10.1038/s41596-021-00673-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00673-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.