Abstract
The CRISPR-guided caspase (Craspase) complex is an assembly of the target-specific RNA nuclease known as Cas7-11 bound to CRISPR RNA (crRNA) and an ancillary protein known as TPR-CHAT (tetratricopeptide repeats (TPR) fused with a CHAT domain). The Craspase complex holds promise as a tool for gene therapy and biomedical research, but its regulation is poorly understood. TPR-CHAT regulates Cas7-11 nuclease activity via an unknown mechanism. In the present study, we use cryoelectron microscopy to determine structures of the Desulfonema magnum (Dm) Craspase complex to gain mechanistic insights into its regulation. We show that DmTPR-CHAT stabilizes crRNA-bound DmCas7-11 in a closed conformation via a network of interactions mediated by the DmTPR-CHAT N-terminal domain, the DmCas7-11 insertion finger and Cas11-like domain, resulting in reduced target RNA accessibility and cleavage.
Similar content being viewed by others
Main
CRISPR (clustered regularly interspaced short palindromic repeats)–Cas systems provide adaptive immunity for host prokaryotes via sequence-directed nucleic acid cleavage and are powerful genetic tools in biomedical research and gene therapy1,2. The newly discovered Cas7-11 system uses its bound crRNA as a guide to facilitate highly sequence-specific cleavage of target RNA at two sites separated by six nucleotides3. The discovery of this system provides a new tool for sequence-specific RNA targeting for knockdown and editing purposes with remarkably negligible nontarget effects and low cell toxicity3,4. CrRNA-bound Cas7-11 assembles into the Craspase complex with an ancillary protein known as TPR-CHAT5. TPR-CHAT is a caspase-related protein that regulates Cas7-11 activity via an unknown mechanism and could be involved in viral immunity in host prokaryotes3,4,5,6,7. Given the broad range of potential biomedical and therapeutic applications of the Cas7-11 system, understanding of its regulation is crucial for further development as a biotechnological tool.
To investigate the structural basis of regulation of the Craspase complex, we studied the CRISPR subtype III-E loci from D. magnum possessing a similar genetic structure to other described systems3,5 (Fig. 1a and Extended Data Fig. 1a,b). We purified the DmCas7-11 protein in complex with crRNA (DmCas7-11–crRNA) and confirmed its activity by demonstrating its sequence-specific target RNA cleavage in vitro (Fig. 1b and Extended Data Fig. 1c,d). DmCas7-11–crRNA is stably associated with DmTPR-CHAT to form the Craspase complex when copurified, as shown for other systems5 (Extended Data Fig. 2). We used cryoelectron microscopy (cryo-EM) to analyze the Craspase complex, which resulted in two structures, one showing clear density for crRNA and all protein domains of the complex (DmCas7-11–crRNA and DmTPR-CHATfull), whereas the second lacks density for the regions of DmTPR-CHAT (DmCas7-11–crRNA and DmTPR-CHATNTD). The maps were resolved to overall resolutions of 3.2 Å (0.32 nm) and 3.0 Å, respectively (Extended Data Figs. 3 and 4, Supplementary Fig. 1, Supplementary Videos 1 and 2 and Table 1).
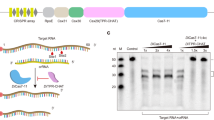
a, Domain organization of the Craspase complex. b, Denaturing urea–PAGE of DmCas7-11–crRNA incubated with 48-bp Cy5-labeled target and nontarget RNA. The red arrows indicate the cleavage products (n = 5). c,d, Cryo-EM density of the Craspase complex in two separate views rotated by 180°. e,f, Cartoon representation of the Craspase structure in two separate views rotated by 180°. The protein domains are indicated. Scale bar (c and d), 1 nm.
The obtained maps show the Craspase complex to be composed of an elongated structure resembling a ‘seahorse’, as also described for the structures of type I and type III CRISPR–Cas systems8,9,10 (Fig. 1c–f). The crRNA-bound DmCas7-11 forms the enzymatic core of the complex stably bound to DmTPR-CHAT which contacts multiple Cas7 repeat domains (Fig. 1c–f). The arrangement of DmCas7-11 domains starts with the amino-terminal Cas7.1 domain forming a cap at one end of the complex, followed by the interlocking Cas7.2, Cas7.3 and Cas7.4 repeat domains (Fig. 1e–f and Extended Data Fig. 5). The fold of these repeats shares similarities with previously determined Cas7 structures with a ‘right-hand’ morphology consisting of palm, thumb, web and finger regions8,9,10 (Extended Data Fig. 5). However, Cas7.4 is unique, containing a core Cas7 fold with the thumb replaced with the large insertion-1 and -2 subdomains (Fig. 1e–f and Extended Data Fig. 5). The Cas11-like domain (CLD) resides after a loop that extends from the Cas7.1 palm and docks between Cas7.3 and Cas7.4. The CLD forms a protrusion between these repeats and makes multiple interactions with Cas7.2, Cas7.3 and Cas7.4. Another extended loop connects the CLD to the Cas7.2 palm (Fig. 1e–f and Extended Data Fig. 5). The complex terminates at the other end with insertion-2, followed by insertion-1 of Cas7.4, which forms a ‘tail’ composed of coiled coils (Fig. 1e–f). Both proteins, insertion-1 and -2, are unique to Cas7.4 and are not present in previously published Cas7 structures from other systems, suggesting that they play a unique role in the function of Cas7-11.
The fully melted crRNA was stably bound to DmCas7-11, starting from Cas7.1 at its 5′-end, extending toward Cas7.2 and Cas7.3 and terminating in insertion-2 at its 3′-end (Fig. 1d–f and Extended Data Figs. 5 and 6). Given that we could unambiguously assign the nucleotide sequences to the crRNA in our structure, we could confidently determine the positions of the 5′-handle and spacer, revealing the molecular basis of crRNA recognition by DmCas7-11 (Extended Data Fig. 6). The amino acid side chains of Cas7.1 and Cas7.2 recognize the 13 nt of the 5′-handle mainly through sequence-specific interactions. Notably, nucleotides 2–8 of the crRNA 5′-handle adopt a canonical ‘hook-like’ structure stabilized by interactions involving the three nucleotides U6, G7 and A8 and charged amino acid residues from Cas7.1 (refs. 9,10) (Extended Data Fig. 6d–f). The crRNA spacer starts from the 5′-nucleotide C15 and terminates at nucleotide G40 at the 3′-end. The spacer divides into two segments that interact with Cas7-11 via nonsequence-specific interactions between the sugar–phosphate backbone of the spacer and amino acid side chains from Cas7.3 and Cas7.4 (Extended Data Fig. 6g–i). The absence of sequence-specific interactions allows for target RNA recognition by complementary base-pairing with the bound spacer, similar to structures of other type I and III systems8,9,10 (Extended Data Fig. 6c). During the manuscript revision of the present study, publications describing the cryo-EM structures of Cas7-11 from another two species were released11,12. These structures share overall similarity to the one in the present study, with striking differences in the organization of the insertion domains and the position of the CLD (Extended Data Fig. 7).
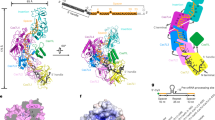
DmTPR-CHAT, the second protein component of the Craspase complex, is implicated in regulating or tuning Cas7-11 RNA nuclease activity via an elusive mechanism3. Our structure of the Craspase complex reveals the full-length structure of DmTPR-CHAT, the molecular basis of its interaction with Cas7-11 and molecular insights for its regulatory role in the Craspase complex (Figs. 1 and 2 and Extended Data Figs. 8, 9 and 10). DmTPR-CHAT organizes into three domains: the N-terminal domain (NTD), tetratricopeptide repeat (TPR) domain and the carboxy-terminal domain (CTD) (Fig. 1e–f and Extended Data Fig. 8a). The CTD contains a caspase-like subdomain (CLS) starting from β sheet-4 at its N terminus and terminating at β sheet-11 at its C terminus (Extended Data Fig. 8b). The CLS is of particular interest because predictions suggest that it acts as a trigger for cell death or dormancy via a caspase-related peptidase activity3,4,5,6,7. Structural alignments of DmTPR-CHAT with other caspases showed high similarity in the CTD and TPR with Human Separase13,14,15 (Extended Data Fig. 8c,d). The CLS was also similar to Cas7 (± substrate) and PIGK (phosphotidylinositol glycan anchor biosynthesis class K) with the root mean square deviation (r.m.s.d.) between the C-α atoms of the protein backbones of 1.1 and 1.4 Å (Extended Data Fig. 8e). In addition, we could also identify a putative catalytic cysteine residue (Cys728) and other residues involved in substrate recognition in the CLS from the structural alignments, supporting the predictions13,14,15 (Extended Data Fig. 8e,f). These findings show that the CTD harbors a putative caspase-related peptidase, with its substrate yet to be determined.
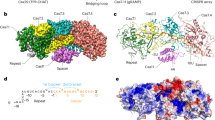
a, Left, the cryo-EM density of the Craspase complex, containing only density for the NTD from DmTPR-CHAT (DmCas7-11–crRNA–TPR-CHATNTD). Right, the positions of the CLD, NTD and insertion finger. b, Left, the ‘closed’ conformation of the Craspase complex highlighted by the red dashed box. Right, the details of the interactions in the box of CLD, insertion finger and NTD. c,e, The 2D classes from DmCas7-11–crRNA and the Craspase complex, with cartoon representations of these 2D classes shown in e. White arrows indicate the positions of the CLD. d, Denaturing urea–PAGE of WT and IF-del, DmCas7-11–crRNA incubated with labeled target RNA in the absence and presence of DmTPR-CHAT at equimolar (1×) or double (2×) concentrations (n = 3). The red arrows indicate the cleavage products. f, Denaturing urea–PAGE of DmCas7-11–crRNA incubated with labeled target RNA in the absence and presence of WT, CTD-del and CTD-alone DmTPR-CHAT (n = 3). g, Proposed model of DmCas7-11 regulation by DmTPR-CHAT.
DmTPR-CHAT makes multi-domain interactions with DmCas7-11–crRNA via its NTD and CTD, forming two major binding sites. The binding site of the NTD has a larger surface area than that of the CTD (Extended Data Fig. 9). The NTD interacts with the Cas7.4 palm, a loop extending from the Cas7.3 palm, and the insertion finger from Cas7.4 via salt bridges and hydrogen bonds (Extended Data Fig. 9a,d). At the other site, the CTD forms salt bridges with residues from the palms of Cas7.1 and Cas7.2 (Extended Data Fig. 9a–c). Cryo-EM data processing of the Craspase complex revealed flexibility of the TPR and CTD, in contrast to the NTD which is rigidly bound to the complex (Fig. 2a, Extended Data Fig. 3 and Supplementary Fig. 2). These findings show that the NTD is sufficient for the association of DmTPR-CHAT with DmCas7-11–crRNA.
The overall structure of D. magnum Craspase revealed that the complex adopts a ‘closed’ conformation characterized by the steric occlusion of the first segment of the spacer crRNA by the CLD (Fig. 2b). Notably, we observed from two-dimensional (2D) classes of DmCas7-11–crRNA that a feature corresponding to the CLD is not always present (Fig. 2c and Supplementary Fig. 3). This finding reveals that the CLD in DmCas7-11–crRNA can alternate between a rigid and a flexible state facilitated by its location between extended loops that form long linkers flanking the domain (Supplementary Video 3). In contrast, the CLD in the Craspase complex stably associates with the complex, showing that DmTPR-CHAT promotes the rigidity of the CLD (Fig. 2c,e). Asp310 or Asp307 (CLD) and Lys1526 (insertion finger) form a salt bridge facilitated by interactions between the insertion finger and the NTD (Fig. 2b and Supplementary Video 4). This network of interactions stabilizes the CLD. Strikingly, the highly conserved amino acid Asp682 implicated in nuclease activity is proximal to this region, indicating that target cleavage occurs nearby (Fig. 2b and Extended Data Fig. 6g). Published studies using the Cas7-11 system from Scalindua brodae (SbCas7-11) and Desulfonema ishitimonii (DiCas7-11) suggest an unclear role in the association between TPR-CHAT and DmCas7-11–crRNA3,5. The study using the DiCas7-11 showed that TPR-CHAT regulates Cas7-11 by reducing its nuclease activity, whereas a study using the SbCas7-11 showed that TPR-CHAT did not affect nuclease activity3,4,5. In the present study, we utilize in vitro assays to reveal that DmTPR-CHAT can stably inhibit the nuclease activity of DmCas7-11 by reducing target RNA binding (Fig. 2d,f and Extended Data Fig. 10a–c,e,f). Of note, we observed, in sequence alignments of Cas7-11 from different species, that the insertion finger is conserved in multiple species, including DmCas7-11 and DiCas7-11, but is absent in SbCas7-11 (Extended Data Fig. 10d and Supplementary Fig. 4). As the insertion finger is crucial for interacting with the CLD and stabilizing the closed conformation, we tested the effect of deletion of the insertion finger of DmCas7-11 (IF-del) on DmTPR-CHAT-mediated inhibition. Strikingly, we found that DmTPR-CHAT did not inhibit IF-del when compared with wild-type (WT) DmCas7-11 (Fig. 2d). We also show that the inhibitory role of DmTPR-CHAT is mediated by its NTD. Deletion of the CTD (CTD-del) does not affect inhibition in contrast to the CTD alone, which lacks the NTD and does not inhibit DmCas7-11 (Fig. 2f and Extended Data Fig. 10c,e).
Based on our structural and biochemical analyses, we propose a model of Cas7-11 regulation by TPR-CHAT via stabilizing interactions between the insertion finger and CLD of DmCas7-11 and the NTD of DmTPR-CHAT. The CLD then transiently blocks target RNA binding and reduces the Cas7-11 nuclease activity (Fig. 2g). The proposed mechanism is most likely to be conserved in other species, where the insertion finger and NTD are present in the Craspase complex, opening a path toward further engineering of the system to regulate Cas7-11 activity in future applications.
Methods
Plasmid constructs design and cloning
DmCas7-11 used in the present study was coexpressed together with crRNA in Escherichia coli BL21(DE3) from a single plasmid containing the kanamycin resistance gene. CrRNA is processed to shorter crRNA molecules on coexpression with Ca7-11. To construct the plasmid for coexpression, a coding sequence of full-length DmCas7-11 codon optimized for E. coli was synthesized and cloned (GenScript Biotech) into MCS-1 of a pRSFDuet-1 plasmid, using BamHI and SalI restriction enzyme sites. DmCas7-11 was inserted in-frame with an N-terminal 6× Histidine (6×His)-tag or N-terminal FLAG tag.
CrRNA from D. magnum, composed of an array of five 72-bp repeats of the native sequence, each containing the first native spacer sequence and a sixth shorter repeat without spacer, was cloned into MCS-2 using BglII and XhoI restriction enzyme sites. Both DmCas7-11 and crRNA were inserted downstream of a LacI-repressed T7 promoter with the coding sequence DmCas7-11, terminating via a stop codon, and crRNA terminating via the T7 terminator.
D. magnum TPR-CHAT used in the present study was expressed in E. coli BL21(DE3) from a plasmid containing the ampicillin resistance gene. The coding sequence of full-length D. magnum TPR-CHAT with N-terminal Twin-strep-tag was synthesized and cloned into pETDuet-1 using the NcoI and XhoI restriction enzyme sites (GenScript Biotech). In this way, the gene was inserted downstream of a LacI-repressed T7 promoter to facilitate inducible expression using isopropyl β-d-1-thiogalactopyranoside (IPTG).
Protein expression and purification
The D. magnum Craspase complex, composed of DmCas7-11–crRNA and DmTPR-CHAT, DmCas7-11–crRNA alone and DmTPR-CHAT alone, was expressed and purified from E. coli BL21(DE3). For expression of the Craspase complex, chemically competent E. coli BL21(DE3) was transformed with both the pRSFDuet-1 plasmid containing the kanamycin resistance gene for expression of DmCas7-11 together with crRNA, and the pETDuet-1 plasmid containing the ampicillin resistance gene for the expression of DmTPR-CHAT, and grown overnight at 37 °C on Luria broth (LB) agar plates containing both selection antibiotics (50 μg ml−1 of kanamycin and 100 μg ml−1 of carbenicillin). The colonies obtained were streaked from the plate and transferred to 50 ml of 2xYT medium containing the selection antibiotics and grown overnight at 37 °C with shaking. Then, 40 ml of the overnight culture was used to inoculate 4 l of 2xYT medium containing the selection antibiotics. The cultures were grown at 37 °C with shaking at 190 r.p.m. until they reached an absorbance at 600 nm of 0.5–0.7, then incubated on ice for 1 h, before inducing protein expression with 0.5 mM IPTG for 18 h at 20 °C. The overnight cultures were harvested by centrifugation at 3,000g for 30 min at 4 °C. The resulting supernatant was discarded and the pellet was resuspended in 100 ml of cold lysis buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol, 25 mM imidazole and 1 mM 2-mercaptoethanol) supplemented with two tablets of cOmplete EDTA-free Protease Inhibitor Cocktail (Roche) and 6 μg ml−1 of RNAseA (Thermo Fisher Scientific) before lysis by sonication. The lysate was clarified by centrifugation for 30 min at 70,560g and 4 °C in an Optima XPN Ultracentrifuge (Beckman Coulter) using a Ti-45 rotor. The supernatant, which contained soluble 6xHis-tagged DmCas7-11 in complex with crRNA and Twin-strep-tagged DmTPR-CHAT, was loaded on to 3 ml of HisPur Ni-NTA Resin (Thermo Fisher Scientific) pre-equilibrated with wash buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol, 35 mM imidazole and 1 mM 2-mercaptoethanol) in an XK16/20 column (Cytiva Life Sciences). After loading, the column was washed with 25 ml of wash buffer and eluted with 10 ml of elution buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol, 250 mM imidazole and 1 mM 2-mercaptoethanol). Pooled elution fractions were concentrated to ∼500 ul in 100K Amicon Ultra-15 concentrators (Millipore) and further purified by gel filtration chromatography on a 10/300 GL Superose 6 gel filtration column (Cytiva Life Sciences) in gel filtration buffer (25 mM Hepes-NaOH, pH7.5, 150 mM NaCl and 1 mM dithiothreitol (DTT)). Peak fractions (as determined by the chromatograms with ultraviolet light of 280 nm) generated from the Unicorn software (v.7.1) containing complete Craspase complexes of subunits DmCas7-11–crRNA and DmTPR-CHAT (as determined by sodium dodecylsulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) analysis), were pooled and concentrated to an absorbance at 280 nm of 0.8 to prepare cryo-EM grids. Craspase complex for use in biochemical experiments was purified in gel filtration buffer supplemented with 10% glycerol with peak fractions pooled, concentrated, flash-frozen and stored at −80 °C.
For the expression of full-length insertion finger and IF-del mutant of DmCas7-11–crRNA alone, chemically competent E. coli BL21(DE3) was transformed with a pRSFDuet-1 plasmid containing DmCas7-11 together with crRNA and kanamycin resistance gene, and grown overnight at 37 °C on LB agar plates supplemented with antibiotic (50 μg ml−1 of kanamycin). Downstream expression and purification were done using the same procedure described for the Craspase complex with the exception that RNAse A was omitted from the lysis buffer. However, for biochemical experiments, an N-terminal FLAG tag version of DmCas7-11 was used. Therefore purification was performed using anti-FLAG.
M2 affinity gel (Millipore): the beads were washed with 100 ml of wash buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol and 1 mM 2-mercaptoethanol) and eluted with 4 ml of elution buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol, 120 µg ml−1 of 3× FLAG peptide and 1 mM 2-mercaptoethanol), followed by buffer exchange and concentration on a 100K Amicon Ultra-15 concentrators (Millipore).
For the expression of full-length, CTD-del and CTD of DmTPR-CHAT alone, chemically competent E. coli BL21(DE3) was transformed with a pETDuet-1 plasmid containing DmTPR-CHAT and the ampicillin resistance gene and grown overnight at 37 °C on LB agar plates supplemented with antibiotic (50 μg ml−1 of kanamycin). The remaining procedure of protein expression was carried out as already described for the Craspase complex. For purification, the harvested cell pellet was resuspended in 100 ml of cold lysis buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol and 1 mM 2-mercaptoethanol) supplemented with two tablets of cOmplete EDTA-free Protease Inhibitor Cocktail before lysis by sonication. The lysate was clarified by centrifugation for 30 min at 70,560g and 4 °C in an Optima XPN Ultracentrifuge using a Ti-45 rotor. The supernatant, which contained soluble Twin-strep-tagged DmTPR-CHAT, was loaded on to 2 ml of Streptactin Sepharose High-Performance resin (Cytiva Life Sciences) pre-equilibrated with wash buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol and 1 mM 2-mercaptoethanol) on an XK16/20 column. After loading, the column was washed with 20 ml of wash buffer, 10 ml of high-salt wash buffer (25 mM Hepes-NaOH, pH 7.5, 800 mM KCl, 10% glycerol and 1 mM 2-mercaptoethanol) and another 10 ml of wash buffer before elution with 10 ml of elution buffer (25 mM Hepes-NaOH, pH 7.5, 200 mM NaCl, 10% glycerol, 5 mM desthiobiotin and 1 mM 2-mercaptoethanol). Pooled elution fractions were concentrated to ∼500 µl in 30K Amicon Ultra-15 concentrators and further purified by gel filtration chromatography on a 10/300 GL Superose 6 gel filtration column in gel filtration buffer (25 mM Hepes-NaOH pH7.5, 150 mM NaCl, 10% glycerol and 1 mM DTT). Peak fractions containing DmTPR-CHAT, as determined by SDS–PAGE analysis, were pooled, concentrated, flash-frozen and stored at −80 °C.
SDS–PAGE analysis
To assess the purity of purified proteins and the presence of individual subunits in the complex, an SDS–PAGE analysis was performed; 15 μl of protein sample was supplemented with 5 μl of 4× NuPAGE LDS Sample Buffer (Thermo Fisher Scientific). Samples were incubated at 95 °C for 10 min before loading on a 4–12% NuPAGE Bis–Tris Precast Gel (Thermo Fisher Scientific). PageRuler Prestained Protein Ladder (10–180 kDa) was also loaded on the gel to run as a size marker. Gels were run in 1× NuPAGE MES SDS running buffer (Thermo Fisher Scientific) at 200 V for 30 min, washed briefly in MilliQ water and stained for 2 h with QuickBlue Protein Stain (LuBioScience GmbH) with shaking. Gels were washed in MilliQ water before imaging on an iBright FL1500 Imaging System (Thermo Fisher Scientific). Gel images were processed and prepared on ImageJ (v.1.53k).
In vitro target RNA cleavage assay
To investigate the target RNA cleavage activity of DmCas7-11–crRNA in the presence and absence of DmTPR-CHAT, in vitro RNA cleavage assays were performed using 48 bp of synthesized 5′-Cy5-labeled single-stranded (ss)RNA (Microsynth) as a substrate. RNA cleavage reactions were performed with 50 nM labeled ssRNA incubated with 50–200 nM purified DmCas7-11–crRNA in nuclease assay buffer (32.5 mM Hepes-NaOH pH 7.5, 100 mM NaCl and 2.5 mM DTT) supplemented with 1 U µl−1 of RNAse inhibitor (New England Biolabs). Then, 200 nM purified DmTPR-CHAT was included in the reaction where indicated. Reactions were incubated at 37 °C for 1 h and quenched by adding urea and proteinase K (Thermo Fisher Scientific) at final concentrations of 0.1 M and 1 μg μl−1, respectively, and incubated at 50 °C for 15 min. The 2× loading dye (1× tris-borate–EDTA (TBE), 12% Ficoll, 7 M urea) was added to the reaction to a final 1× concentration, followed by heating at 95 °C for 5 min before loading on a 15% Novex PAGE TBE–urea gel (Thermo Fisher Scientific). The gel was run at 200 V for 45 min and imaged on an iBright FL1500 Imaging System. To investigate the inhibition of target RNA cleavage activity of DmCas7-11–crRNA, equimolar or twofold excess purified DmTPR-CHAT constructs were incubated with DmCas7-11–crRNA for 1 h on ice before the addition of labeled RNA. The reactions were incubated at 37 °C for 15 min or 1 h when indicated and further processed as already described. Gel images were processed and prepared on ImageJ (v.1.53k).
In vitro target RNA-binding assay
To investigate the target RNA binding of DmCas7-11–crRNA in the presence and absence of DmTPR-CHAT, electrophoresis mobility shift assays were performed with 50 nM 5′-Cy5-labeled ssRNA incubated with 1 µM purified DmCas7-11–crRNA in binding buffer (32.5 mM Hepes-NaOH pH 7.5, 100 mM NaCl and 2.5 mM DTT) supplemented with 1 U µl−1 of RNAse inhibitor. The reaction was incubated for 30 min on ice before the addition of 2× loading dye (15% sucrose) to a final 1× concentration. The sample was then directly loaded on Novex TBE gels, 4–12% (Thermo Fisher Scientific). The gel was run at 100 V for 90 min and imaged on an iBright FL1500 Imaging System. To investigate the effect on target RNA binding of DmCas7-11–crRNA, equimolar 200 nM purified DmTPR-CHAT constructs were incubated with DmCas7-11–crRNA for 1 h on ice before the addition of labeled RNA and the remaining steps of the assay were carried out. Gel images were processed and prepared on ImageJ (v.1.53k).
Negative stain analysis of D. magnum Craspase complex
The purified D. magnum Craspase complex and DmCas7-11–crRNA were first analyzed by negative staining to check for sample quality before cryo-EM sample preparation. Of the diluted sample at 80–100 nM, 3.5 μl was applied to glow-discharged 400-mesh copper grids coated with a continuous carbon film (Electron Microscopy Sciences). The grids were stained by incubating with 2% (w:v) uranyl acetate for a total of 30 s, then blotted and air-dried. Images were collected on a Philips CM100 Biotwin transmission electron microscope, operating at 80 kV, with a TVIPS F416 CMOS camera (4,000 × 4,000) at a physical pixel size of 0.6 nm at the sample level, with a total electron dose of approximately 4 e− Å−2 over a total exposure time of 400 ms.
Cryo-EM sample preparation and data collection
Cryo-EM grids were prepared by applying 3.5 µl of concentrated sample on to 400-mesh R1.2/1.3 UltrAuFoil grids (Quantifoil Micro Tools GmbH), which had been rendered hydrophilic by glow discharging at 15 mA for 60 s with a PELCO easiGlow device (Ted Pella, Inc.). The sample was adsorbed for 30 s on the grids, followed by blotting and plunge freezing into liquid ethane using a Vitrobot Mark IV plunge freezer (Thermo Fisher Scientific). Cryo-EM data were collected using the automated data acquisition software EPU (Thermo Fisher Scientific) on a Titan Krios G4 transmission electron microscope (Thermo Fisher Scientific), operating at 300 kV and equipped with a cold field emission gun electron source and a Falcon4 direct detection camera. Images were recorded in counting mode at a nominal magnification of ×96,000, corresponding to a physical pixel size of 0.83 Å at the sample level. Datasets were collected at a defocus range of 0.8–2.5 µm with a total electron dose of 60 e−/Å−2. Image data were saved as electron event recordings.
Cryo-EM image processing, model building and refinement
The cryo-EM image processing was performed using cryoSPARC v.3.3 (ref. 16).
The patch-based motion correction (cryoSPARC implementation) was used for aligning the EM video stacks, as well as applying dose-dependent resolution weighting to recorded videos. Contrast transfer function estimation was performed using the patch-based option as well. For the data of the Craspase (DmCas7-11–TPR-CHAT) complex, 2,000 particles were manually picked and used for one round of 2D classification for template creation. Template-based automated particle picking was then used on the recorded image data, which resulted in a set of 1,719,283 particles. Two rounds of 2D classification were performed for the initial step of particle cleaning. Multiple rounds of 2D classifications were performed for more specific 2D rebalancing, by deselecting the most abundant views manually, resulting in a particle set of 887,670 particles. Initial reconstruction and heterorefinement yielded multiple three-dimensional (3D) classes. Two classes accounting for 29% of the total particles were removed because they showed particles of a highly preferred orientation. The other 3D classes were grouped into two major classes for further processing, splitting the particles into one class with clear density for the entire TPR-CHAT (DmCas7-11 and DmTPR-CHATfull) and another class with less density in the TPR-CHAT-binding area (DmCas7-11 and DmTPR-CHATNTD). The signs of preferred orientation of the particles in the remaining DmCas7-11–TPR-CHATfull class were still noticeable after 3D refinement, so an additional step of 2D rebalancing was performed to further reduce this anisotropic effect. After this selection, 214,592 particles remained, which were subjected to 3D classification, resulting in 6 classes. The best 3D class consisting of 65,755 particles was refined and yielded a cryo-EM map at 3.20-Å overall resolution in C1 symmetry.
For the classes DmCas7-11 and TPR-CHATNTD, a total of 176,623 particles were classified by an additional heterorefinement and then subjected to 3D classification, resulting in another three classes. The best 3D class containing 53,969 particles produced a cryo-EM map in C1 symmetry at an overall resolution of 3.03 Å. Subsequently, local refinement was performed with an insertion region-specific mask volume. The resolution for the locally refined cryo-EM map was estimated at 3.43 Å in C1 symmetry. All resolution measures used the Fourier shell correlation (FSC) criterion of a 0.143 cutoff17.
Atomic models for both DmCas7-11–crRNA and DmTPR-CHATfull and DmCas7-11–crRNA and DmTPR-CHATNTD structures were mostly newly built manually in Coot 0.9.4 (ref. 18) For the regions of low density or low quality, an AlphaFold2 (ColabFold implementation) prediction was used as a guide for amino acid assignment and backbone tracing19. Real-space refinement for all built models was performed using Phenix v.1.19.2-4158 by applying a general restraints set-up20. Structural alignments and superpositions were performed with PyMOL (PyMOL Molecular Graphics System v.2.0, Schrödinger, LLC). Figures were created using PyMOL, UCSF Chimera, UCSF ChimeraX21 and Adobe Illustrator (https://adobe.com/products/illustrator).
Multiple sequence alignments
Multiple protein sequence alignments were performed using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo) and visualized in JALVIEW22. Phylogenetic trees were prepared using the neighbor-joining algorithm on Geneious prime (v.2022.0.2, Biomatters).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The reconstructed maps are available from the Electron Microscopy Data Bank (EMDB) database under accession nos. EMDB-14847 and EMDB-14848. The atomic models are available in the Protein Databank (PDB) database, accession nos. 7ZOL and 7ZOQ. The raw video data of this work are available in the Electron Microscopy Public Image Archive database, accession no. EMPIAR-11268 (https://doi.org/10.6019/EMPIAR-11268). Source data are provided with this paper.
References
Brouns, S. J. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Fellmann, C., Gowen, B. G., Lin, P.-C., Doudna, J. A. & Corn, J. E. Cornerstones of CRISPR–Cas in drug discovery and therapy. Nat. Rev. Drug Discov. 16, 89–100 (2017).
Özcan, A. et al. Programmable RNA targeting with the single-protein CRISPR effector Cas7-11. Nature 597, 720–725 (2021).
Catchpole, R. J. & Terns, M. P. New type III CRISPR variant and programmable RNA targeting tool: oh, thank heaven for Cas7-11. Mol. Cell. 81, 4354–4356 (2021).
van Beljouw, S. P. B. et al. The gRAMP CRISPR-Cas effector is an RNA endonuclease complexed with a caspase-like peptidase. Science 373, 1349–1353 (2021).
Hochstrasser, M. L. & Nuñez, J. K. CRISPR meets caspase. Nat. Microbiol. 6, 1481–1482 (2021).
Johnson, A. G. et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022).
Mulepati, S., Héroux, A. & Bailey, S. Structural biology. Crystal structure of a CRISPR RNA-guided surveillance complex bound to a ssDNA target. Science 345, 1479–1484 (2014).
Guo, T. W. et al. Cryo-EM structures reveal mechanism and inhibition of DNA targeting by a CRISPR–Cas surveillance complex. Cell 171, 414–426.e12 (2017).
Chowdhury, S. et al. Structure reveals mechanisms of viral suppressors that intercept a CRISPR RNA-guided surveillance complex. Cell 169, 47–57.e11 (2017).
Kato, K. et al. Structure and engineering of the type III-E CRISPR–Cas7-11 effector complex. Cell 185, 2324–2337.e16 (2022).
Hu, C. et al. Craspase is a CRISPR RNA-guided, RNA-activated protease. Science 377, 1278–1285 (2022).
Yu, J. et al. Structural basis of human separase regulation by securin and CDK1–cyclin B1. Nature 596, 138–142 (2021).
Zhang, H. et al. Structure of human glycosylphosphatidylinositol transamidase. Nat. Struct. Mol. Biol. 29, 203–209 (2022).
Chai, J. et al. Crystal structure of a procaspase-7 zymogen: mechanisms of activation and substrate binding. Cell 107, 399–407 (2001).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Scheres, S. H. W. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Acknowledgements
This work was supported by the Swiss National Science Foundation (grant no. CRSII5_177195) and the NCCR TransCure (grant no. 185544) awarded to H.S. We thank the Dubochet Center for Imaging from the EPFL, University of Lausanne and University of Geneva for their support.
Author information
Authors and Affiliations
Contributions
D.N. and B.E. conceived and designed the study. B.E., D.N. and D.T. performed experiments. B.B., S.N. and A.M collected cryo-EM data. D.N. and B.E. performed formal analysis. B.E. and D.N. prepared, reviewed and edited the manuscript. H.S. acquired funding and reviewed the manuscript. H.S. and D.N. supervised the study. All authors gave their input in the preparation of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Tian-Min Fu and Osamu Nureki for their contribution to the peer review of this work. Primary Handling Editor: Sara Osman in collaboration with the rest of the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Description and Purification of D. magnum Cas7-11-crRNA.
a) Phylogenetic tree of type III-E CRISPR effectors. b) Schematic representation of the Type−IIIE loci from D. magnum, D. ishimotonii and S. brodae. c) Schematic representation of DmCas7-11-crRNA recombinant expression in E. coli BL21 (DE3). d) The left panel shows an SDS-PAGE of DmCas7-11, and the right panel shows a Urea-PAGE of crRNA from purified DmCas7-11-crRNA (n = 6).
Extended Data Fig. 2 Purification of the D. magnum Craspase complex (DmCas7-11-crRNA and TPR-CHAT).
a) Schematic representation of DmCas7-11-crRNA and TPR-CHAT recombinant expression in E. coli BL21 (DE3). b) Size exclusion chromatogram from the purification of the D. magnum Craspase complex. Peak fractions pooled for structural studies are indicated by a pink box. c) The left panel shows an SDS-PAGE of DmCas7-11 and DmTPR-CHAT, and the right panel shows a Urea-PAGE of crRNA from the purified Craspase complex (n = 4). d) EM micrograph of a negatively stained preparation of purified Craspase complexes (n = 22).
Extended Data Fig. 3 Cryo-EM data processing workflow for D. magnum Craspase complex.
Processing workflow for DmCas7-11-crRNA-DmTPR-CHATfull and DmCas7-11-crRNA-DmTPR-CHATNTD showing 2D class averages of deselected views, 3D classification and refinement steps. Focused refinement with a mask for the insertion domains is also shown.
Extended Data Fig. 4 Zoom-in view of the cryo-EM maps of structural elements of crRNA (a), DmCas7-11 (b), and DmTPR-CHAT (c).
a) Cryo-EM map densities and atom displays for the crRNA 5‘ handle, crRNA spacer-Segment 1 and crRNA-Segment 2. b) Cryo-EM map densities and atom displays for a loop, alpha helices and beta sheets of DmCas7-11. c) Cryo-EM map densities and atom displays for regions of the NTD, TPR and CTD of DmTPR-CHAT.
Extended Data Fig. 5 Domain annotation of the Cas7 repeats.
Schematic representation of the domain organization of DmCas7-11-crRNA (center) surrounded by cartoon representations of the Insertion-1, Insertion-2, CLD, Cas7.1, Cas7.2, Cas7.3 and Cas7.4 domains. The thumb, web, fingers and palm of the Cas7 domains are indicated.
Extended Data Fig. 6 Structure of crRNA bound to Cas7-11.
a) Structure representation of crRNA bound to Cas7-11 indicating the positions of the 5’ handle and spacer RNA segments. b, c) Cartoon representation of target RNA bound to crRNA spacer. d-f) Detailed structural representation of the interaction between the ‘hook-like’ 5’ handle and Cas7.1 and Cas7.2 domains. g-i) Detailed structural representation of the interaction between the crRNA spacer and Cas7.1 and Cas7.2 domains. g,i) show the binding of crRNA spacer segment 1 to Cas7.3 and Cas7.4 domains. h) shows the binding of crRNA spacer segment 2 to the Cas7.4 Insertion-1 subdomain.
Extended Data Fig. 7 Structural alignment of Cas7-11 from different species.
a) Cartoon representations of Cas7-11 from D. magnum (DmCas7-11- this study), D. ishitimonii (DiCas7-11- PDB:7WAH) and S. brodae (SbgRAMP- PDB:8D8N)11,12. b) Comparison of the position of the CLD between DmCas7-11 and DiCas7-11 showing a 10 Å rotational movement. c) Structural comparison of the insertion domains between DmCas7-11 and DiCas7-11.
Extended Data Fig. 8 Structure of DmTPR-CHAT.
a) Ribbon representation of DmTPR-CHAT with domains distinctly coloured. b) Topology diagram of DmTPR-CHAT CTD. c, d) Structure alignment between DmTPR-CHAT and Human Separase (PDB ID: 7nj0). e) Structure alignment between the CLS (cyan), Seprase (green), Caspase7 (+/- substrate) (blue and purple), and PIGK (PDB IDs: 7nj0, 7w72, 1K88, and 1K86). The substrate binding site is shown as a red circle. f) Residues of the substrate−binding site in the CLS.
Extended Data Fig. 9 The interaction interfaces between DmCas7-11 and DmTPR-CHAT.
a) Surface representation of the interaction interface between DmCas7-11 (blue surface and cartoon representation) and DmTPR-CHAT (transparent cartoon representation) shown in pink. b–d) Detailed views of amino acid interactions at the interfaces corresponding to boxed areas in (a).
Extended Data Fig. 10 Inhibition of DmCas7-11 nuclease activity by DmTPR-CHAT.
a) Chromatogram at 280 nm and SDS-PAGE gel for the purification of DmTPR-CHAT (n = 3). b) Denaturing Urea-PAGE of DmCas7-11-crRNA incubated with 48bp-Cy5 labelled target and increasing concentrations of DmTPR-CHAT. Red arrows indicate the cleavage products (n = 3). c) Denaturing Urea-PAGE of DmCas7-11-crRNA incubated with 48bp-Cy5 labelled in the presence and absence of DmTPR-CHAT with 15 mins and 60 mins incubation times (n = 3). d) A sequence alignment of the Insertion-finger is shown in the top panel and represented as a ribbon bound to the NTD in the bottom panel. e) SDS-PAGE of CTD deletion (CTD-del) and CTD alone of DmTPR-CHAT (n = 3). f) EMSA comparing the binding of DmCas7-11-crRNA to 48bp-Cy5 labelled target RNA in the absence and presence of full-length, CTD deletion and CTD alone DmTPR-CHAT (n = 2).
Supplementary information
Supplementary Information
Supplementary Figs. 1–4
Supplementary Video 1
Structure of Cas7-11–TPR-CHAT.
Supplementary Video 2
Structure of Cas7-11–TPR-CHAT_NTD.
Supplementary Video 3
Potential opening state of Cas7-11.
Supplementary Video 4
TPR-CHAT complex formation.
Source data
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 2
Unprocessed gels.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 2
Unprocessed gels.
Source Data Extended Data Fig. 10
Unprocessed gels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ekundayo, B., Torre, D., Beckert, B. et al. Structural insights into the regulation of Cas7-11 by TPR-CHAT. Nat Struct Mol Biol 30, 135–139 (2023). https://doi.org/10.1038/s41594-022-00894-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-022-00894-5
This article is cited by
-
Structural basis of negative regulation of CRISPR-Cas7-11 by TPR-CHAT
Signal Transduction and Targeted Therapy (2024)
-
CRISPR–Cas has a new juggling act: interplay between nuclease and protease
Nature Structural & Molecular Biology (2023)