Abstract
NAD metabolism is essential for all forms of life. Compartmental regulation of NAD+ consumption, especially between the nucleus and the mitochondria, is required for energy homeostasis. However, how compartmental regulation evolved remains unclear. In the present study, we investigated the evolution of the macrodomain-containing histone variant macroH2A1.1, an integral chromatin component that limits nuclear NAD+ consumption by inhibiting poly(ADP-ribose) polymerase 1 in vertebrate cells. We found that macroH2A originated in premetazoan protists. The crystal structure of the macroH2A macrodomain from the protist Capsaspora owczarzaki allowed us to identify highly conserved principles of ligand binding and pinpoint key residue substitutions, selected for during the evolution of the vertebrate stem lineage. Metabolic characterization of the Capsaspora lifecycle suggested that the metabolic function of macroH2A was associated with nonproliferative stages. Taken together, we provide insight into the evolution of a chromatin element involved in compartmental NAD regulation, relevant for understanding its metabolism and potential therapeutic applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The reported protein structures are deposited in the Protein Data Bank with PDB accession nos. 7NY6 (unliganded Capsaspora macroH2A macrodomain) and 7NY7 (ADP-ribose bound Capsaspora macroH2A macrodomain). Source data are provided with this paper.
Code availability
We have exclusively used publicly available packages for bioinformatic analysis and provide their references in Methods. If not stated otherwise, we have used default parameters. Specific scripts are available on request.
Change history
21 December 2021
In the PDF version of this article initially published online, Extended Data Figs. 1,2 were duplicates of Extended Data Figs. 7,8, respectively, and have been restored to the file as of 21 December 2021.
References
Covarrubias, A. J., Perrone, R., Grozio, A. & Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119–141 (2021).
Rajman, L., Chwalek, K. & Sinclair, D. A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 27, 529–547 (2018).
Xiao, W., Wang, R. S., Handy, D. E. & Loscalzo, J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid. Redox Signal 28, 251–272 (2018).
Palazzo, L., Mikolčević, P., Mikoč, A. & Ahel, I. ADP-ribosylation signalling and human disease. Open Biol. https://doi.org/10.1098/rsob.190041 (2019).
Cambronne, X. A. & Kraus, W. L. Location, location, location: compartmentalization of NAD+ synthesis and functions in mammalian cells. Trends Biochem. Sci. 45, 858–873 (2020).
Strømland, Ø. et al. Keeping the balance in NAD metabolism. Biochem. Soc. Trans. 47, 119–130 (2019).
Cantó, C., Menzies, K. J. & Auwerx, J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 (2015).
Altmeyer, M. & Hottiger, M. O. Poly(ADP-ribose) polymerase 1 at the crossroad of metabolic stress and inflammation in aging. Aging 1, 458–469 (2009).
Bai, P. et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011).
Pirinen, E. et al. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 19, 1034–1041 (2014).
Posavec-Marjanović, M. et al. MacroH2A1.1 regulates mitochondrial respiration by limiting nuclear NAD+ consumption. Nat. Struct. Mol. Biol. 24, 902–910 (2017).
Luo, X. et al. PARP-1 controls the adipogenic transcriptional program by PARylating C/EBPβ and modulating its transcriptional activity. Mol. Cell 65, 260–271 (2017).
Ryu, K. W. et al. Metabolic regulation of transcription through compartmentalized NAD+ biosynthesis. Science 360, eaan5780 (2018).
Hurtado-Bagès, S. et al. The histone variant macroH2A1 regulates key genes for myogenic cell fusion in a splice-isoform dependent manner. Cells 9, 1109 (2020).
Oláh, G. et al. Differentiation-associated downregulation of poly(ADP-ribose) polymerase-1 expression in myoblasts serves to increase their resistance to oxidative stress. PLoS ONE 10, e0134227 (2015).
Rack, J. G. M., Perina, D. & Ahel, I. Macrodomains: structure, function, evolution and catalytic activities. Annu. Rev. Biochem. 85, 431–54 (2016).
Karras, G. I. et al. The macro domain is an ADP-ribose binding module. EMBO J. 24, 1911–1920 (2005).
Singh, H. R. et al. A poly-ADP-ribose trigger releases the auto-inhibition of a chromatin remodeling oncogene. Mol. Cell 68, 860–871.e7 (2017).
Timinszky, G. et al. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat. Struct. Mol. Biol. 16, 923–929 (2009).
Jankevicius, G. et al. A family of macrodomain proteins reverses cellular mono-ADP-ribosylation. Nat. Struct. Mol. Biol. 20, 508–514 (2013).
Rosenthal, F. et al. Macrodomain-containing proteins are new mono-ADP-ribosylhydrolases. Nat. Struct. Mol. Biol. 20, 502–507 (2013).
Buschbeck, M. & Hake, S. B. Variants of core histones and their roles in development, stem cells and cancer. Nat. Publ. Gr. https://doi.org/10.1038/nrm.2016.166 (2017).
Kustatscher, G., Hothorn, M., Pugieux, C., Scheffzek, K. & Ladurner, A. G. Splicing regulates NAD metabolite binding to histone macroH2A. Nat. Struct. Mol. Biol. 12, 624–625 (2005).
Kozlowski, M. et al. MacroH2A histone variants limit chromatin plasticity through two distinct mechanisms. EMBO Rep. 19, e44445 (2018).
Chen, H. et al. MacroH2A1.1 and PARP-1 cooperate to regulate transcription by promoting CBP-mediated H2B acetylation. Nat. Struct. Mol. Biol. 21, 981–989 (2014).
Ouararhni, K. et al. The histone variant mH2A1.1 interferes with transcription by down-regulating PARP-1 enzymatic activity. Genes Dev. 20, 3324–3336 (2006).
Rivera-Casas, C., Gonzalez-Romero, R., Cheema, M. S., Ausió, J. & Eirín-López, J. M. The characterization of macroH2A beyond vertebrates supports an ancestral origin and conserved role for histone variants in chromatin. Epigenetics 11, 415–425 (2016).
Torruella, G. et al. Phylogenomics reveals convergent evolution of lifestyles in close relatives of animals and fungi. Curr. Biol. 25, 2404–2410 (2015).
Sebé-Pedrós, A. et al. Regulated aggregative multicellularity in a close unicellular relative of metazoa. eLife 2013, e01287 (2013).
Allen, M. D., Buckle, A. M., Cordell, S. C., Löwe, J. & Bycroft, M. The crystal structure of AF1521 a protein from Archaeoglobus fulgidus with homology to the non-histone domain of macroH2A. J. Mol. Biol. 330, 503–511 (2003).
Sebé-Pedrós, A. et al. High-throughput proteomics reveals the unicellular roots of animal phosphosignaling and cell differentiation. Dev. Cell 39, 186–197 (2016).
Douet, J. et al. MacroH2A histone variants maintain nuclear organization and heterochromatin architecture. J. Cell Sci. 130, 1570–1582 (2017).
Catara, G., Corteggio, A., Valente, C., Grimaldi, G. & Palazzo, L. Targeting ADP-ribosylation as an antimicrobial strategy. Biochem. Pharmacol. 167, 13–26 (2019).
Chen, W., Smeekens, J. M. & Wu, R. Systematic study of the dynamics and half-lives of newly synthesized proteins in human cells. Chem. Sci. 7, 1393–1400 (2016).
Commerford, S. L., Carsten, A. L. & Cronkite, E. P. Histone turnover within nonproliferating cells. Proc. Natl Acad. Sci. USA 79, 1163–1165 (1982).
Fornasiero, E. F. et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. https://doi.org/10.1038/s41467-018-06519-0 (2018).
Mathieson, T. et al. Systematic analysis of protein turnover in primary cells. Nat. Commun. 9, 689 (2018).
Pillai, A. S. et al. Origin of complexity in haemoglobin evolution. Nature 581, 480–485 (2020).
Huh, J. W., Shima, J. & Ochi, K. ADP-ribosylation of proteins in Bacillus subtilis and its possible importance in sporulation. J. Bacteriol. 178, 4935–4941 (1996).
Setlow, P. & Kornberg, A. Biochemical studies of bacterial sporulation and germination. J. Biol. Chem. 245, 3637–3644 (1970).
Setlow, R. & Setlow, P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J. Bacteriol. 129, 857–865 (1977).
Berger, F., Lau, C., Dahlmann, M. & Ziegler, M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 280, 36334–36341 (2005).
Cambronne, X. A. et al. Biosensor reveals multiple sources for mitochondrial NAD+. Science 352, 1474–1477 (2016).
Simonet, N. G. et al. SirT7 auto-ADP-ribosylation regulates glucose starvation response through mH2A1. Sci. Adv. https://doi.org/10.1101/719559 (2020).
Creppe, C. et al. MacroH2A1 regulates the balance between self-renewal and differentiation commitment in embryonic and adult stem cells. Mol. Cell. Biol. 32, 1442–1452 (2012).
Sporn, J. C. & Jung, B. Differential regulation and predictive potential of macroH2A1 isoforms in colon cancer. Am. J. Pathol. 180, 2516–2526 (2012).
Sebé-Pedrós, A. et al. The dynamic regulatory genome of capsaspora and the origin of animal multicellularity. Cell 165, 1224–1237 (2016).
Bockwoldt, M. et al. Identification of evolutionary and kinetic drivers of NAD-dependent signaling. Proc. Natl Acad. Sci. USA 116, 15957–15966 (2019).
Morowitz, H. J. A theory of biochemical organization, metabolic pathways, and evolution. Complexity 4, 39–53 (1999).
Pehrson, J. R., Changolkar, L. N., Costanzi, C. & Leu, N. A. Mice without macroH2A histone variants. Mol. Cell. Biol. 34, 4523–4533 (2014).
Sebastian, R. et al. Epigenetic regulation of DNA repair pathway choice by MacroH2A1 splice variants ensures genome stability. Mol. Cell 79, 836–845.e7 (2020).
Lavigne, M. D. et al. Composite macroH2A/NRF-1 nucleosomes suppress noise and generate robustness in gene expression. Cell Rep. https://doi.org/10.1016/j.celrep.2015.04.022 (2015).
Afgan, E. et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 44, W3–W10 (2016).
Brown, M. W. et al. Phylogenomics places orphan protistan lineages in a novel eukaryotic super-group. Genome Biol. Evol. 10, 427–433 (2018).
de Mendoza, A., Suga, H., Permanyer, J., Irimia, M. & Ruiz-Trillo, I. Complex transcriptional regulation and independent evolution of fungal-like traits in a relative of animals. eLife 4, e08904 (2015).
Dudin, O. et al. A unicellular relative of animals generates a layer of polarized cells by actomyosin-dependent cellularization. eLife 8, e49801 (2019).
Grau-Bové, X. et al. Dynamics of genomic innovation in the unicellular ancestry of animals. eLife 6, e26036 (2017).
Hehenberger, E. et al. Novel predators reshape holozoan phylogeny and reveal the presence of a two-component signaling system in the ancestor of animals. Curr. Biol. 27, 2043–2050.e6 (2017).
Richter, D., Berney, C., Strassert, J., Burki, F. & de Vargas, C. EukProt: a database of genome-scale predicted proteins across the diversity of eukaryotic life. Preprint at bioRxiv https://doi.org/10.1101/2020.06.30.180687 (2020).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Crooks, G. E., Hon, G., Chandonia, J. M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res 14, 1188–1190 (2004).
Bawono, P. & Heringa, J. PRALINE: a versatile multiple sequence alignment toolkit. Methods Mol. Biol. 1079, 245–262 (2014).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Le, S. Q. & Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320 (2008).
Hedges, S. B., Dudley, J. & Kumar, S. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 22, 2971–2972 (2006).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Suga, H. et al. The Capsaspora genome reveals a complex unicellular prehistory of animals. Nat. Commun. 4, 2325 (2013).
Liao, Y., Smyth, G. K. & Shi, W. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Huerta-Cepas, J. et al. EggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M., Sato, Y. & Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 428, 726–731 (2016).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 236 (2019).
Ruiz, P. D. & Gamble, M. J. MacroH2A1 chromatin specification requires its docking domain and acetylation of H2B lysine 20. Nat. Commun. 9, 5143 (2018).
Mayer, M. & Meyer, B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. 38, 1784–1788 (1999).
Aretz, J. et al. Allosteric inhibition of a mammalian lectin. J. Am. Chem. Soc. 140, 14915–14925 (2018).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. Electronic ligand builder and optimization workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65, 1074–1080 (2009).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. Ligplot: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng. Des. Sel. 8, 127–134 (1995).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Sporn, J. C. et al. Histone macroH2A isoforms predict the risk of lung cancer recurrence. Oncogene 28, 3423–3428 (2009).
Fackelmayer, F., Dahm, K., Renz, A., Ramsperger, U. & Richter, A. Nucleic-acid-binding properties of hnRNP-U/SAF-A, a nuclear-matrix protein which binds DNA and RNA in vivo and in vitro. Eur. J. Biochem. 221, 749–757 (1994).
Acknowledgements
We thank D. Corujo for help and advice on many occasions and the members of the MSCA Innovative Training Network ‘ChroMe’, the Buschbeck and Ladurner laboratories for constructive discussions throughout the development of this interdisciplinary project. For technical support, we thank the research service facilities of IJC and IGTP, the Crystallization Facility of the Max Planck Institute of Biochemistry, the ICTS NMR facility from the Scientific and Technological Centres of the University of Barcelona and Biophysics Core Facility of BMC-LMU. I.G. was a fellow of the Marie Skłodowska Curie Training network ‘ChroMe’ (H2020-MSCA-ITN-2015-675610, awarded to M.B. and A.G.L.). The project was further supported by national grants (nos. RTI2018-094005-B-I00 and BFU2015-66559-P from FEDER/Ministerio de Ciencia e Innovación—Agencia Estatal de Investigación to M.B.). Research in the participating labs was further supported by the following grants: the Marie Skłodowska Curie Training network ‘INTERCEPT-MDS’ no. H2020-MSCA-ITN-2020-953407 (to M.B.), MINECO-ISCIII no. PIE16/00011 (to M.B.); the Deutsche José Carreras Leukämie Stiftung DJCLS (no. 14R/2018 to M.B.), AGAUR (no. 2017-SGR-305 to M.B.), Fundació La Marató de TV3 (no. 257/C/2019 to M.B.), German Research Foundation Project (ID 213249687—SFB 1064 and Project ID 325871075—SFB 1309 to A.G.L.), the Spanish Ministry of Science (PID2019-110183RB-C21 to A.R.M.), Community of Madrid (P2018/BAA-4343-ALIBIRD2020-CM to A.R.M), Ramón Areces Foundation (to A.R.M.), National Science Foundation (EF-1921402 to J.M.E.L.), 2015 International Doctoral Fellowship La Caixa-Severo Ochoa (to M.F.V.), Marie Skłodowska-Curie Individual Fellowship (no. 747789 to M.M.L.), Juan de la Cierva-Incorporación (IJC2018-036657-I to M.M.L., ERC-2012-CoG-616960 to I.R.T.), MINECO (BFU2017-90114-P to I.R.T.), AGAUR (2017-SGR-324 to X.S.) and MINECO (BIO2015-70092-R and ERC-2014-CoG-648201 to X.S.). Research at the IJC is supported by the ‘La Caixa’ Foundation, Fundació Internacional Josep Carreras, Celgene Spain and the CERCA Programme/Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
I.G., S.H.B. and M.B. conceived the study. I.G., C.R.C., M.M.L. and R.M. curated the data. C.R.C., G.K., J.B., R.M. and M.G.C. performed the formal analysis. M.B. and A.G.L. acquired the funding. I.G., S.H.B., V.V., M.G.C., C.R.C., J.M.E.L., M.M.L. and J.G. performed the investigations. I.G., S.H.B. and M.B. administered the project. I.G., C.R.C., J.M.E.L., M.G.C. and M.F.V. provided the methodology. M.S.C., J.A., X.S., I.R.T. and A.R.M. organized the resources. M.B., A.G.L. and G.K. supervised the study. I.G., S.H.B., V.V., J.G., M.G.C. and A.P. validated the project. I.G., C.R.C., G.K. and R.M. visualized the project. I.G. and M.B. wrote the original draft of the manuscript. C.R.C., G.K., M.M.L., A.G.L., J.M.E.L., I.R.T. and X.S. wrote, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.K. is an employee and A.G.L. a cofounder and managing director of Eisbach Bio GmbH, a biotechnology company. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Structural and Molecular Biology thanks Matthew Gamble and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Beth Moorefield was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The Capsaspora macroH2A macrodomain specifically binds ADP-ribose (accompanying Fig. 2).
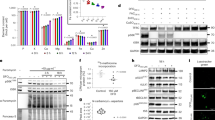
a, Scheme of the saturation transfer difference nuclear magnetic resonance (STD-NMR) experiment, which involves recording two analogous 1D NMR spectra: the reference spectrum of a ligand without saturation of protein signals (irradiating at a position far enough from any signal in the 1H spectrum to avoid saturation), and a spectrum recorded after selective saturation of the protein and bound molecules. The difference spectrum shows only the signals of the binders. b, STD-NMR experiments indicate the presence of interaction between Capsaspora macroH2A macrodomain (mH2A) and ADP-ribose (ADPR) comparable to the interaction with mouse macroH2A1.1 macrodomain (mH2A1.1) and ADPR. No interaction is observed between ADPR and the loss-of-function mutant mH2A1.1G224E that we included as a control. STD spectrum for ADPR in the absence of protein is shown as a reference. The STD effect (%) is quantified as ISTD = 100 * (I0 - Isat) / I0. c, d, e, Representative plots of raw heat evolution in isothermal titration calorimetry (ITC) experiments. ADPR was titrated into a solution with either purified Capsaspora mH2A (c), mouse mH2A1.1 (d) macrodomain, or into the buffer alone (e) as a control. The integration of the raw heat evolution results in a curve representing the equilibrium binding isotherm, represented below the raw heat evolution plots (c, d). f, Table showing the results of ITC binding affinity measurements (Kd, N, ∆H and -T∆S) of Capsaspora mH2A macrodomain and a range of nucleotides (ATP, ADP, AMP, GDP) and ribose. Kd values for ATP, AMP, GDP and ribose are estimated to be higher than 100 µM, as their ITC binding curves show weak or absence of binding. If no binding occurred or binding was below ITC detection limit, reaction stoichiometries and thermodynamics could not be determined (ND).
Extended Data Fig. 2 The apo and ADPR-bound-structure of the Capsaspora macrodomain (accompanying Fig. 2).
a, Topology diagram of apo-form of Capsaspora mH2A macrodomain. The topology diagram shows β-sheets in a conserved 1276354 order, surrounded by 6 α-helices. b, Topology diagram of Capsaspora mH2A macrodomain in complex with ADP-ribose (ADPR). The topology diagram shows β-sheets in a conserved 1276354 order, surrounded by 5 α-helices. The C-terminal α-helix observed in the apo-structure (a) was not detected in the ADPR-bound structure. This prevented the modelling of the C-terminal 13 amino acids, while the apo structure could be fully refined all the way to the C-terminus of the macrodomain. Whether this is the result of a conformational change upon ligand binding or an artefact from the crystal packing remains to be determined. However, we previously observed that ADP-ribose binding induces a conformational change in the human macroH2A1.1 macrodomain, specifically by a 30° rotation of the C-terminal α-helix away from the globular macrodomain fold (Timinszky et al., 2009, NSMB). c, The schematic representation of ADPR interaction with Capsaspora macroH2A macrodomain. All non-covalent interactions between ADPR and the macrodomain are summarized in the LigPlot diagram. ADPR ligand is represented in thick purple line and the amino acid residues of Capsaspora macroH2A macrodomain in thin orange lines. Hydrogen bonds are represented by the dashed lines between atoms involved, while the circles or semicircles with radiating lines represent atoms or residues involved in hydrophobic contacts between protein and ligand.
Extended Data Fig. 3 Conservation of the macrodomain and bonds established with ADP-ribose (accompanying Fig. 3).
a, Pairwise sequence identity (%) of macrodomains from representative macroH2A sequences of 9 different phyla. For the vertebrates, amino acid sequences corresponding to macroH2A1.1 have been used. b, Table indicating the amino acids establishing H-bond or other bonds with ADP-ribose for Capsaspora macroH2A (see also Extended Data Fig. 2c) and human macroH2A1.1 (Timinszky et al., 2009). c, Snapshots of the apo structure of Capsaspora macroH2A macrodomain starting from the front perspective of the binding pocket and subsequently rotated by 90°, 180° and 270° around y-axis. The level of conservation is calculated by Consurf server and is projected on the protein surface. See also the movie provided as Supplementary Data File S5. Please note that in the movie conservation degree is color-coded from low (turquoise), intermediate (white) to high (magenta).
Extended Data Fig. 4 Inferred ancestral amino acid states at sites 225 and 316 (accompanying Fig. 4).
Simplified version of the maximum likelihood tree shown in Supplementary Data File S3. The tree shows a set of possible amino acid (states) at each ancestral node based on their inferred likelihood at sites 225 (in bold) and 316 (in italics). Protein numeration is based on the full-length Capsaspora macroH2A. Probability cutoff is 0.95 and the ambiguous states are not shown. Collapsed branches in choral represent the vertebrate-characteristic pair E225-R316 (diffused when only one was inferred), in turquoise the Capsaspora-characteristic pair Q225-N316 (diffused when only one was inferred), and in brown the species with E225 (vertebrate-like) and N316 (Capsaspora-like) residues.
Extended Data Fig. 5 The identity and biophysical consequences of evolution of residues 225 and 316 closing the binding pocket (accompanying Fig. 4).
a, The pie charts showing the identity of other residues at positions 225 and 316. Residue colors are based on their biochemical properties: positively charged in blue, negatively charged in red, polar in gray, and non-polar in black. b, The pie charts showing the identity of the residues at the position 316 which pair with either protist-characteristic Q225, or vertebrate-characteristic E225. The numbering of the residues is based on the Capsaspora mH2A. In particular, the protist-characteristic residue Q225 was paired with varying residues at 316. c, The pie charts showing the identity of the residues at the position 225 which pair with either protist-characteristic N316, or vertebrate-characteristic R316. The numbering of the residues is based on the Capsaspora mH2A. Strikingly, R316 was paired with E225 or the biochemically similar D225 in more than 95% of the species, while its pairing with Q225 was rare. d, Thermal shift assay profile of melting temperatures of wild type, Q225E and N316R mutants of Capsaspora macroH2A macrodomains (mH2AWT, mH2AQ225E and mH2AN316R, respectively) show that the folding of the mutant proteins is unaffected. The subtle shift in the melting temperature of Q225E mutant macrodomain to higher temperatures might be indicative of an increase in stability. e, Representative plots of raw heat evolution in isothermal titration calorimetry (ITC) experiments. ADP-ribose was titrated into a solution with either purified Capsaspora Q225E (left panel) or N316R (right panel) mutant macroH2A macrodomain. The integration of the raw heat evolution resulted in a curve representing the equilibrium binding isotherm, represented in the inset. f, Table showing the thermodynamics of the binding interaction calculated on the basis of ITC data shown in Fig. 4b.
Extended Data Fig. 6 Alternative splicing of exon 7 in molluscs (accompanying Fig. 4).
a, The organization of the human MACROH2A1 gene and the corresponding gene from the mollusc Crassostrea gigas (Gene ID: 105347164) are represented on an arbitrary scale for comparison purposes. For simplification only the alternative exon 5 defining macroH2A1.1 is represented for human MACROH2A1. Both alternative exons 7 of the Mollusca macroH2A gene are represented. b, Transcriptomic data was available for 50 Mollusca species from three of eight different classes. The number of species per class is indicated (left panel). The percentage of species per Mollusca class containing alternatively spliced macroH2A isoforms are shown (right panel). c, Alignment of C-terminal alternative exons from the mollusc species in which we detected the 2 alternative splice isoforms. Almost all species have both R316 and N316-containing isoforms.
Extended Data Fig. 7 Metabolic dynamics of Capsaspora life stages (accompanying Fig. 5).
a, Heatmap of 349 metabolic genes differentially expressed across the three life stages of Capsaspora. b, Table showing the ‘other’ genes in the Cys high cluster (Fig. 5c) grouped according to their physiological role. c, Antibody specific for Capsaspora PARP1. Rabbits were immunized with three different peptides corresponding to the N-terminal part of PARP1. The antibody specificity was corroborated by immunoblotting bacterial lysates: non-transformed (non-transf.), transformed with a plasmid encoding for N-terminal tagged fragment of PARP1 corresponding to amino acids 1-350 but not induced (non-ind.), and bacteria producing His-tagged PARP1 (His-N-PARP1). Representative immunoblot is shown (n = 3). d, Antibody specific for Capsaspora macroH2A. Rabbits were immunized with purified His-tagged macroH2A macrodomain (AA: 182-368). The specificity was corroborated by immunoblotting using stable cell lines expressing GFP-tagged constructs of mouse macroH2A1.1 (mH2A1.1) and Capsaspora macroH2A (mH2A) and purified His-tagged macrodomain of Capsaspora mH2A. Representative immunoblot is shown (n = 3).
Extended Data Fig. 8 Characterization of stable DKD cell lines (accompanying Fig. 6).
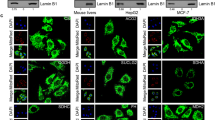
a, Fluorescence intensity of stable DKD cell lines stably expression either GFP or GFP-tagged constructs of mouse macroH2A1.1 (mH2A1.1) or chimeric wild type or G224E proteins containing the Capsaspora macrodomain as illustrated in Fig. 6c. Scale represents 50 µm. Cells were checked daily for GFP expression, and the representative images are shown. b, Flow cytometric detection of GFP in the same cells lines as A. Bar plot to the left compares mean fluorescence intensities of all four stable cell lines. Bar plot in the center shows GFP fluorescence in cell lines stably expressing macroH2A constructs. The bar plot to the right shows high percentage (≥ 99%) of GFP positive cells. c, Stable cell lines expressing the GFP-tagged mouse mH2A1.1 were compared to a similar cell line expressing GFP-tagged mouse macroH2A1.2 (mH2A1.2), control DKD cells and the parental HepG2 cell line. Immunoblotting using anti-GFP and anti-mH2A1.2 specific antibodies allows evaluating the expression level of the exogenous mH2A1.1 relative to endogenous mH2A1.2. Comparison to Extended Data Fig. 7b indicates that the chimeric proteins are expressed at a similar range or slightly higher than endogenous macroH2A proteins. Representative immunoblot is shown (n = 3). d, Immunoblot analysis after cell fractionation shows that all macroH2A constructs but not GFP alone are incorporated in chromatin. Representative immunoblot is shown (n = 3). e, The chimeric Capsaspora-mouse macroH2A construct interacts with PARP1 as detected by immunoblotting after co-immunoprecipitation. Representative immunoblot is shown (n = 4).
Supplementary information
Supplementary Information
File containing Supplementary Fig. 1 (related to Fig. 4 and Extended data Fig. 4), and Tables 3 and 4.
Supplementary Tables
Xls workbook with Supplementary Tables 1, 2, 5, 6 and 7.
Supplementary Video
Movie illustrating the conservation of Capsaspora macroH2A macrodomain in nine levels from low (turquoise), intermediate (white) to high (magenta).
Source data
Source Data Fig. 2
Statistical source data for Fig. 2b,c.
Source Data Fig. 4
Statistical source data for Fig. 4b.
Source Data Fig. 5
Uncropped western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Uncropped western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Uncropped western blots.
Source Data Extended Data Fig. 8
Uncropped western blots.
Rights and permissions
About this article
Cite this article
Guberovic, I., Hurtado-Bagès, S., Rivera-Casas, C. et al. Evolution of a histone variant involved in compartmental regulation of NAD metabolism. Nat Struct Mol Biol 28, 1009–1019 (2021). https://doi.org/10.1038/s41594-021-00692-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-021-00692-5
This article is cited by
-
The Depletion of NAMPT Disturbs Mitochondrial Homeostasis and Causes Neuronal Degeneration in Mouse Hippocampus
Molecular Neurobiology (2023)
-
MacroH2A1.1 has evolved to let PARP1 do more by loosening its grip on PAR
Nature Structural & Molecular Biology (2021)