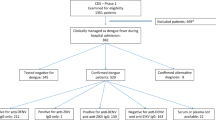
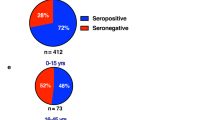
Abstract
Dengue is a global epidemic causing over 100 million cases annually. The clinical symptoms range from mild fever to severe hemorrhage and shock, including some fatalities. The current paradigm is that these severe dengue cases occur mostly during secondary infections due to antibody-dependent enhancement after infection with a different dengue virus serotype. India has the highest dengue burden worldwide, but little is known about disease severity and its association with primary and secondary dengue infections. To address this issue, we examined 619 children with febrile dengue-confirmed infection from three hospitals in different regions of India. We classified primary and secondary infections based on IgM:IgG ratios using a dengue-specific enzyme-linked immunosorbent assay according to the World Health Organization guidelines. We found that primary dengue infections accounted for more than half of total clinical cases (344 of 619), severe dengue cases (112 of 202) and fatalities (5 of 7). Consistent with the classification based on binding antibody data, dengue neutralizing antibody titers were also significantly lower in primary infections compared to secondary infections (P ≤ 0.0001). Our findings question the currently widely held belief that severe dengue is associated predominantly with secondary infections and emphasizes the importance of developing vaccines or treatments to protect dengue-naive populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All the raw data analyzed are provided as source files in the main text and in the extended data material. Individual de-identified data for age, sex and clinical disease classification are provided as source data in the supplementary information. Source data are provided with this paper.
References
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507 (2013).
World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control (WHO, 2009).
Farrar, J. J. et al. Dogma in classifying dengue disease. Am. J. Trop. Med. Hyg. 89, 198–201 (2013).
Srikiatkhachorn, A. et al. Dengue—how best to classify it. Clin. Infect. Dis. 53, 563–567 (2011).
Halstead, S. B. et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg. Infect. Dis. 8, 1474–1479 (2002).
Guzmán, M. G. et al. Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am. J. Epidemiol. 152, 793–799 (2000).
Halstead, S. B., Scanlon, J. E., Umpaivit, P. & Udomsakdi, S. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am. J. Trop. Med. Hyg. 18, 997–1021 (1969).
Winter, P. E. et al. Recurrence of epidemic dengue hemorrhagic fever in an insular setting. Am. J. Trop. Med. Hyg. 18, 573–579 (1969).
Nunes, P. C. G. et al. 30 years of dengue fatal cases in Brazil: a laboratorial-based investigation of 1047 cases. BMC Infect. Dis. 18, 346 (2018).
Rosen, L. The Emperor’s New Clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 26, 337–343 (1977).
Halstead, S. B., O’Rourke, E. J. & Allison, A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J. Exp. Med. 146, 218–229 (1977).
Ng, J. K. et al. First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS Pathog. 10, e1004031 (2014).
Katzelnick, L. C. et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932 (2017).
Cuzzubbo, A. J. et al. Comparison of PanBio Dengue Duo IgM and IgG Capture ELISA and Venture Technologies Dengue IgM and IgG Dot Blot. J. Clin. Virol. 16, 135–144 (2000).
Vaughn, D. W. et al. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 60, 693–698 (1999).
Vazquez, S., Hafner, G., Ruiz, D., Calzada, N. & Guzman, M. G. Evaluation of immunoglobulin M and G capture enzyme-linked immunosorbent assay Panbio kits for diagnostic dengue infections. J. Clin. Virol. 39, 194–198 (2007).
Murhekar, M. V. et al. Burden of dengue infection in India, 2017: a cross-sectional population based serosurvey. Lancet Glob. Health 7, e1065–e1073 (2019).
de Silva, A. Safety of dengue vaccine? Clin. Infect. Dis. 76, 371–372 (2023).
Clapham, H. E. & Wills, B. A. Implementing a dengue vaccination programme—who, where and how? Trans. R. Soc. Trop. Med. Hyg. 112, 367–368 (2018).
Thomas, S. J. Is new dengue vaccine efficacy data a relief or cause for concern? NPJ Vaccines 8, 55 (2023).
Chandele, A. et al. Characterization of human CD8 T cell responses in dengue virus-infected patients from India. J. Virol. 90, 11259–11278 (2016).
Gunisetty, S. et al. Analysis of dengue specific memory B cells, neutralizing antibodies and binding antibodies in healthy adults from India. Int. J. Infect. Dis. 84S, S57–S63 (2019).
Kar, M. et al. Isolation and molecular characterization of dengue virus clinical isolates from pediatric patients in New Delhi. Int. J. Infect. Dis. 84S, S25–S33 (2019).
Acknowledgements
This work was supported by National Institutes of Health grant no. ICIDR 1UO1A/115654; Department of Biotechnology (DBT), Government of India grant nos. BT/PR5132/MED/15/85/2012 and BT/PR8470/med/29/726/2013; and NIH-DBT Human Immunology Project Consortium grant no. AI090023. G. Medigeshi is supported by the Wellcome Trust-DBT India Alliance Intermediate fellowship (no. IA/S/14/1/501291). S. Kumar is supported by the DBT/Wellcome Trust India Alliance Early Career Fellowship grant no. IA/E/18/1/504307. The authors thank N. Khanna (International Centre for Genetic Engineering and Biotechnology (ICGEB)) for discussions, W. M. Orenstein (Emory Vaccine Center) for critical review of the manuscript, and S. Singh and A. Singh (ICGEB) for technical support.
Author information
Authors and Affiliations
Contributions
M.S., S.F.A., R.V., S.M., A.R., V.P.V., A.M.A., S.K.K., R.L. and A.S. carried out patient recruitment and follow-up. C.A., H.A., P. Sharma, H.P., K.N., R.C.R., D.M., S.G., L.P., S.K.B., S.F.A., R.V., E.S.R., Y.M.C., P. Bhatnagar, P. Singh, M.K., K.D., S.K., K.G., K.S., P. Bajpai, G.P.S., P. Shah, A.K., T.Y., C.W.D., R. Antia and G.R.M. performed the experiments, analysis and interpretation. J.W., A.A., A.M.A., S.K.K., R. Ahmed, R.L., A.S., A.C. and K.M-K. were involved in study design, analysis and interpretation. C.A., H.A., R. Ahmed, R.L., A.S., A.C. and K.M-K. prepared the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Eng Eong Ooi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Similar frequency of severe disease in primary versus secondary cases that were distinguished using stringent IgM/IgG ratios.
Pie charts show the frequency of Severe Dengue (SD), Dengue with warning signs (DW) and Dengue infection without warning signs (DI) cases in primary versus secondary dengue infections that were distinguished using more stringent IgM/IgG ratios indicated on left. The number of patients in each group is indicated below the pie chart. For all three classification methods, the proportion of severe disease was not significantly different between primary and secondary cases (p > 0.78, two-sided Fisher’s exact test). The 95% confidence interval for the percentages indicated in the pie charts are as below: IgM/IgG >1.32, primary: DI- 5.4-11.6, DW-53.4-64.4, SD-27.9-38.5, Secondary: DI- 6.7-13.1, DW-52.8-63.6, SD-27.4-37.6; IgM/IgG >1.4: primary: DI- 5.7-12.1, DW-52.2-63.5, SD-28.5-39.3, secondary: DI- 6.4-12.6, DW-53.8-64.4, SD-26.9-36.9; IgM/IgG >1.78: primary: DI- 5.8-13.0, DW-50.5-62.9, SD-28.7-40.6 and secondary: DI- 6.3-12.0, DW-54.8-64.6, SD-27.0-36.3 (Wilson CI).
Extended Data Fig. 2 Frequency of severe disease in primary versus secondary dengue infections using WHO 1997 and WHO 2009 disease classification.
Data from a subset of the patients from the AIIMS Delhi site where disease severity was classified using both WHO 2009 and WHO 1997 criteria. a, Data shown by WHO 1997 disease classification. Pie charts show the frequency of the cases with dengue shock syndrome (DSS), dengue hemorrhagic fever (DHF); or dengue fever (DF) among a subset of dengue confirmed children that are recruited from AIIMS site among all cases (n = 171), primary dengue cases (n = 66) and secondary dengue cases (n = 105). DSS case frequency is not significantly different between the primary and secondary dengue infections, (p = 0.106, two-sided Fisher’s exact test). b, Data shown by WHO 2009 disease classification among the same group of the patients from panel a. Pie charts show the frequency of the cases with severe dengue (SD), dengue with warning signs (DW); or dengue infection without warning signs (DI) among all cases, primary dengue cases or secondary dengue cases. Severe dengue case frequency was not significantly different between the primary and secondary dengue infections, (p = 0.344, two-sided Fisher’s exact test).
Extended Data Fig. 3 Dengue specific responses in infants (≤1-year-old).
a, Scatter plot shows dengue specific IgM and IgG index values by capture Elisa (Panbio) for dengue confirmed infants (n = 34). p values were calculated using two-sided Mann-Whitney U tests b, Neutralizing antibody titers to the indicated infecting virus serotype in dengue confirmed infants where the infecting serotype was determined (n = 26). c. Scatter plots show dengue specific IgM index values by Panbio Capture ELISA among the infants with different grades of disease severity. Severe dengue (SD, n = 22); Dengue with warning signs (DW, n = 12). Note that there are no Dengue infection without warning signs (DI) cases since all the hospitalized infants were either SD or DW cases. p values (p = 0.087) were calculated using two-sided Mann-Whitney U tests. Non-significant p values (>0.05) are indicated as n.s. d. Scatter plots show neutralizing activity against the indicated infecting dengue virus serotypes among the infants with different grades of disease severity. Severe dengue (SD, n = 15); Dengue with warning signs (DW, n = 11). Note that there are no DI cases since all of the hospitalized infants were either SD or DW cases. p values (p > 0.999) were calculated using two- sided Mann-Whitney U tests. Non-significant p values (>0.05) are indicated as n.s.
Extended Data Fig. 4 Neutralization responses were below detection or significantly lower for infecting serotype in the primary dengue cases compared to secondary dengue cases.
Neutralizing antibody titers against the infecting virus serotype in primary (n = 38) and secondary (n = 50) from a subset of the patients from 2b, where the infecting serotype was identified. p values were calculated using Mann-Whitney U test.
Supplementary information
Supplementary Information
Individual-level data for age, sex and clinical disease classification.
Supplementary Data
Source data for individual-level data in the Supplementary Information.
Source data
Source Data Fig. 1
Similar frequency of severe disease in pediatric patients with primary versus secondary dengue infections.
Source Data Fig. 2
Comparison of neutralizing antibody responses between cases with primary and secondary dengue infection.
Source Data Extended Data Fig. 1
Similar frequency of severe disease in primary versus secondary cases that were distinguished using stringent IgM/IgG ratios.
Source Data Extended Data Fig. 2
Frequency of severe disease in primary versus secondary dengue infections using WHO 1997 and WHO 2009 disease classification.
Source Data Extended Data Fig. 3
Dengue specific responses in infants (≤1 year old).
Source Data Extended Data Fig. 4
Neutralization responses were below detection or significantly lower for infecting serotype in the primary dengue cases compared to secondary dengue cases.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aggarwal, C., Ahmed, H., Sharma, P. et al. Severe disease during both primary and secondary dengue virus infections in pediatric populations. Nat Med 30, 670–674 (2024). https://doi.org/10.1038/s41591-024-02798-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-02798-x