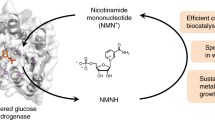
Abstract
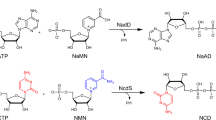
Nature’s two redox cofactors, nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+), are held at different reduction potentials, driving catabolism and anabolism in opposite directions. In biomanufacturing, there is a need to flexibly control redox reaction direction decoupled from catabolism and anabolism. We established nicotinamide mononucleotide (NMN+) as a noncanonical cofactor orthogonal to NAD(P)+. Here we present the development of Nox Ortho, a reduced NMN+ (NMNH)-specific oxidase, that completes the toolkit to modulate NMNH:NMN+ ratio together with an NMN+-specific glucose dehydrogenase (GDH Ortho). The design principle discovered from Nox Ortho engineering and modeling is facilely translated onto six different enzymes to create NMN(H)-orthogonal biocatalysts with a consistent ~103–106-fold cofactor specificity switch from NAD(P)+ to NMN+. We assemble these enzymes to produce stereo-pure 2,3-butanediol in cell-free systems and in Escherichia coli, enabled by NMN(H)’s distinct redox ratio firmly set by its designated driving forces, decoupled from both NAD(H) and NADP(H).

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Information on the strains and plasmids used in this study is available in Supplementary Data. Accession codes for genes investigated in this study can be found in Supplementary Tables 4 and 5. All DNA sequence and structural information used is publicly available (Ser Bdh2, UniProt A0A7U3Z3T2; Kp Dar, UniProt D7RP28; Bs BdhA, UniProt O34788; Cs Bdh, UniProt M1MWX5; Bs Gdh, UniProt P12310; Ll Nox, UniProt A2RIB7; Lb Nox, UniProt Q03Q85, PDB 5VN0; Tp Nox (Lb Nox G159A;D177A;A178R;M179S;P184R); Ecl Dar, GenBank JN035909; Kp BudC, PDB 1GEG; Ka BudC, National Center for Biotechnology Information (NCBI) Ref WP_015366942; Ka Dar, GenBank VEC79507; Ko BudC, GenBank AEX06195; As Bdh, GenBank NLH91458; Cr Bdh, GenBank AEI90716; Ca Bdh, NCBI Ref WP_073006451; Pb Bdh, NCBI Ref WP_148550966; Cbo Bdh, NCBI Ref WP_075141790; Km Bdh, NCBI Ref WP_102401827; Cbu Bdh, NCBI Ref WP_104675707; Zp Bdh, NCBI Ref WP_027704711). All data generated in this study are provided in the Supplementary Information. Source data are provided with this paper.
Code availability
The input files and source code for the Rosetta molecular simulation and MD simulations are publicly accessible on Zenodo (https://doi.org/10.5281/zenodo.11478967)80.
References
Liu, Y. et al. Biofuels for a sustainable future. Cell 184, 1636–1647 (2021).
Liao, J. C., Mi, L., Pontrelli, S. & Luo, S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 14, 288–304 (2016).
Opgenorth, P. H., Korman, T. P. & Bowie, J. U. A synthetic biochemistry molecular purge valve module that maintains redox balance. Nat. Commun. 5, 4113 (2014).
Cresko, J. et al. Industrial Decarbonization Roadmap (US Department of Energy, 2022).
Lee, S. Y. & Kim, H. U. Systems strategies for developing industrial microbial strains. Nat. Biotechnol. 33, 1061–1072 (2015).
Walsh, C. T., Tu, B. P. & Tang, Y. Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chem. Rev. 118, 1460–1494 (2018).
Zachos, I., Döring, M., Tafertshofer, G., Simon, R. C. & Sieber, V. carba nicotinamide adenine dinucleotide phosphate: robust cofactor for redox biocatalysis. Angew. Chem. Int. Ed. Engl. 60, 14701–14706 (2021).
Beier, A. et al. Switch in cofactor specificity of a Baeyer–Villiger monooxygenase. ChemBioChem 17, 2312–2315 (2016).
Chánique, A. M. & Parra, L. P. Protein engineering for nicotinamide coenzyme specificity in oxidoreductases: attempts and challenges. Front. Microbiol. 9, 194 (2018).
Rollin, J. A., Tam, T. K. & Zhang, Y.-H. P. New biotechnology paradigm: cell-free biosystems for biomanufacturing. Green Chem. 15, 1708 (2013).
Mampel, J., Buescher, J. M., Meurer, G. & Eck, J. Coping with complexity in metabolic engineering. Trends Biotechnol. 31, 52–60 (2013).
Black, W. B. et al. Engineering a nicotinamide mononucleotide redox cofactor system for biocatalysis. Nat. Chem. Biol. 16, 87–94 (2020).
Pandit, A. V., Srinivasan, S. & Mahadevan, R. Redesigning metabolism based on orthogonality principles. Nat. Commun. 8, 1–11 (2017).
Wang, X. et al. Creating enzymes and self-sufficient cells for biosynthesis of the non-natural cofactor nicotinamide cytosine dinucleotide. Nat. Commun. 12, 2116 (2021).
Richardson, K. N., Black, W. B. & Li, H. Aldehyde production in crude lysate- and whole cell-based biotransformation using a noncanonical redox cofactor system. ACS Catal. 10, 8898–8903 (2020).
Bat-Erdene, U. et al. Cell-free total biosynthesis of plant terpene natural products using an orthogonal cofactor regeneration system. ACS Catal. 11, 9898–9903 (2021).
Milo, R., Jorgensen, P., Moran, U., Weber, G. & Springer, M. BioNumbers—the database of key numbers in molecular and cell biology. Nucleic Acids Res. 38, D750–D753 (2010).
Pan, X. et al. A genetically encoded tool to increase cellular NADH/NAD+ ratio in living cells. Nat. Chem. Biol. 20, 594–604 (2024).
Voss, C. V. et al. Orchestration of concurrent oxidation and reduction cycles for stereoinversion and deracemisation of sec-alcohols. J. Am. Chem. Soc. 130, 13969–13972 (2008).
Liu, H. & Bowie, J. U. Cell-free synthetic biochemistry upgrading of ethanol to 1,3 butanediol. Sci. Rep. 11, 9449 (2021).
Knaus, T., Cariati, L., Masman, M. F. & Mutti, F. G. In vitro biocatalytic pathway design: orthogonal network for the quantitative and stereospecific amination of alcohols. Org. Biomol. Chem. 15, 8313–8325 (2017).
Drenth, J., Yang, G., Paul, C. E. & Fraaije, M. W. A tailor-made deazaflavin-mediated recycling system for artificial nicotinamide cofactor biomimetics. ACS Catal. 11, 11561–11569 (2021).
Knaus, T. et al. Better than nature: nicotinamide biomimetics that outperform natural coenzymes. J. Am. Chem. Soc. 138, 1033–1039 (2016).
Zachos, I. et al. Hot flows: evolving an archaeal glucose dehydrogenase for ultrastable carba-NADP+ using microfluidics at elevated temperatures. ACS Catal. 12, 1841–1846 (2022).
Campbell, E., Meredith, M., Minteer, S. D. & Banta, S. Enzymatic biofuel cells utilizing a biomimetic cofactor. Chem. Commun. 48, 1898 (2012).
Guarneri, A. et al. Flavoenzyme‐mediated regioselective aromatic hydroxylation with coenzyme biomimetics. ChemCatChem 12, 1368–1375 (2020).
Meng, D. et al. Coenzyme engineering of glucose-6-phosphate dehydrogenase on a nicotinamide-based biomimic and its application as a glucose biosensor. ACS Catal. 13, 1983–1998 (2023).
King, E. et al. Orthogonal glycolytic pathway enables directed evolution of noncanonical cofactor oxidase. Nat. Commun. 13, 7282 (2022).
Zhang, L. et al. Directed evolution of phosphite dehydrogenase to cycle noncanonical redox cofactors via universal growth selection platform. Nat. Commun. 13, 5021 (2022).
King, E. et al. Engineering Embden–Meyerhof–Parnas glycolysis to generate noncanonical reducing power. ACS Catal. 12, 8582–8592 (2022).
Huang, R., Chen, H., Upp, D. M., Lewis, J. C. & Zhang, Y.-H. P. J. A high-throughput method for directed evolution of NAD(P)+-dependent dehydrogenases for the reduction of biomimetic nicotinamide analogues. ACS Catal. 9, 11709–11719 (2019).
Liang, K. & Shen, C. R. Selection of an endogenous 2,3-butanediol pathway in Escherichia coli by fermentative redox balance. Metab. Eng. 39, 181–191 (2017).
Maina, S. et al. Prospects on bio-based 2,3-butanediol and acetoin production: recent progress and advances. Biotechnol. Adv. 54, 107783 (2022).
Xiao, Z. et al. A novel whole-cell biocatalyst with NAD+ regeneration for production of chiral chemicals. PLoS ONE 5, e8860 (2010).
He, Y. et al. Efficient (3S)-acetoin and (2S,3S)-2,3-butanediol production from meso-2,3-butanediol using whole-cell biocatalysis. Molecules 23, 691 (2018).
Zu, H. et al. Highly enantioselective synthesis of (R)-1,3-butanediol via deracemization of the corresponding racemate by a whole-cell stereoinverting cascade system. Microb. Cell Fact. 19, 125 (2020).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Sellés Vidal, L., Kelly, C. L., Mordaka, P. M. & Heap, J. T. Review of NAD(P)H-dependent oxidoreductases: properties, engineering and application. Biochim. Biophys. Acta Proteins Proteom. 1866, 327–347 (2018).
Jan, J., Martinez, I., Wang, Y., Bennett, G. N. & San, K. Y. Metabolic engineering and transhydrogenase effects on NADPH availability in Escherichia coli. Biotechnol. Prog. 29, 1124–1130 (2013).
Weckbecker, A. & Hummel, W. Improved synthesis of chiral alcohols with Escherichia coli cells co-expressing pyridine nucleotide transhydrogenase, NADP+-dependent alcohol dehydrogenase and NAD+-dependent formate dehydrogenase. Biotechnol. Lett. 26, 1739–1744 (2004).
Cracan, V., Titov, D. V., Shen, H., Grabarek, Z. & Mootha, V. K. A genetically encoded tool for manipulation of NADP+/NADPH in living cells. Nat. Chem. Biol. 13, 1088–1095 (2017).
Krebs, H. A. & Veech, R. L. Equilibrium relations between pyridine nucleotides and adenine nucleotides and their roles in the regulation of metabolic processes. Adv. Enzym. Regul. 7, 397–413 (1969).
Wu, J. T., Wu, L. H. & Knight, J. A. Stability of NADPH: effect of various factors on the kinetics of degradation. Clin. Chem. 32, 314–319 (1986).
Guarneri, A., van Berkel, W. J. & Paul, C. E. Alternative coenzymes for biocatalysis. Curr. Opin. Biotechnol. 60, 63–71 (2019).
Nowak, C., Pick, A., Csepei, L.-I. & Sieber, V. Characterization of biomimetic cofactors according to stability, redox potentials, and enzymatic conversion by NADH oxidase from Lactobacillus pentosus. ChemBioChem 18, 1944–1949 (2017).
Nowak, C. et al. A water-forming NADH oxidase from Lactobacillus pentosus suitable for the regeneration of synthetic biomimetic cofactors. Front. Microbiol. 6, 957 (2015).
Zachos, I., Nowak, C. & Sieber, V. Biomimetic cofactors and methods for their recycling. Curr. Opin. Chem. Biol. 49, 59–66 (2019).
Rasor, B. J., Yi, X., Brown, H., Alper, H. S. & Jewett, M. C. An integrated in vivo/in vitro framework to enhance cell-free biosynthesis with metabolically rewired yeast extracts. Nat. Commun. 12, 5139 (2021).
Sudar, M., Findrik, Z., Domanovac, M. V. & Vasić-Rački, D. Coenzyme regeneration catalyzed by NADH oxidase from Lactococcus lactis. Biochem. Eng. J. 88, 12–18 (2014).
Kim, S. & Hahn, J. S. Efficient production of 2,3-butanediol in Saccharomyces cerevisiae by eliminating ethanol and glycerol production and redox rebalancing. Metab. Eng. 31, 94–101 (2015).
Richter, F., Leaver-Fay, A., Khare, S. D., Bjelic, S. & Baker, D. De novo enzyme design using Rosetta3. PLoS ONE 6, e19230 (2011).
Bennett, B. D. et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009).
Wang, J. et al. Engineering formaldehyde dehydrogenase from Pseudomonas putida to favor nicotinamide cytosine dinucleotide. ChemBioChem 23, 6–11 (2022).
Liu, Y. et al. Structural insights into phosphite dehydrogenase variants favoring a non-natural redox cofactor. ACS Catal. 9, 1883–1887 (2019).
Li, J. X. et al. Enhanced production of optical (S)-acetoin by a recombinant Escherichia coli whole-cell biocatalyst with NADH regeneration. RSC Adv. 8, 30512–30519 (2018).
Otagiri, M. et al. Crystal structure of meso-2,3-butanediol dehydrogenase in a complex with NAD+ and inhibitor mercaptoethanol at 1.7 Å resolution for understanding of chiral substrate recognition mechanisms. J. Biochem. 129, 205–208 (2001).
Li, L. et al. Biocatalytic production of (2S,3S)-2,3-butanediol from diacetyl using whole cells of engineered Escherichia coli. Bioresour. Technol. 115, 111–116 (2012).
Zhang, L. et al. Mechanism of 2,3-butanediol stereoisomers formation in a newly isolated Serratia sp. T241. Sci. Rep. 6, 19257 (2016).
Yan, Y., Lee, C.-C. & Liao, J. C. Enantioselective synthesis of pure (R,R)-2,3-butanediol in Escherichia coli with stereospecific secondary alcohol dehydrogenases. Org. Biomol. Chem. 7, 3914 (2009).
Jones, D. P. & Sies, H. The redox code. Antioxid. Redox Signal. 23, 734–746 (2015).
Nielsen, D. R., Yoon, S.-H., Yuan, C. J. & Prather, K. L. J. Metabolic engineering of acetoin and meso-2,3-butanediol biosynthesis in E. coli. Biotechnol. J. 5, 274–284 (2010).
Liang, K. & Shen, C. R. Engineering cofactor flexibility enhanced 2,3-butanediol production in Escherichia coli. J. Ind. Microbiol. Biotechnol. 44, 1605–1612 (2017).
Black, W. B. et al. Metabolic engineering of Escherichia coli for optimized biosynthesis of nicotinamide mononucleotide, a noncanonical redox cofactor. Microb. Cell Fact. 19, 1–10 (2020).
Toledo-Patiño, S., Pascarelli, S., Uechi, G. & Laurino, P. Insertions and deletions mediated functional divergence of Rossmann fold enzymes. Proc. Natl Acad. Sci. USA 119, e2207965119 (2022).
Rao, S. T. & Rossmann, M. G. Comparison of super-secondary structures in proteins. J. Mol. Biol. 76, 241–256 (1973).
Medvedev, K. E., Kinch, L. N., Dustin Schaeffer, R., Pei, J. & Grishin, N. V. A fifth of the protein world: Rossmann-like proteins as an evolutionarily successful structural unit. J. Mol. Biol. 433, 166788 (2021).
Sillitoe, I. et al. CATH: increased structural coverage of functional space. Nucleic Acids Res. 49, D266–D273 (2021).
Bücher, T. & Klingenberg, M. Wege des Wasserstoffs in der lebendigen organisation. Angew. Chem. 70, 552–570 (1958).
Agapakis, C. M. & Silver, P. A. Modular electron transfer circuits for synthetic biology. Bioeng. Bugs 1, 413–418 (2010).
Rutter, J., Reick, M., Wu, L. C. & McKnight, S. L. Regulation of crock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293, 510–514 (2001).
Atkinson, J. T. et al. Metalloprotein switches that display chemical-dependent electron transfer in cells. Nat. Chem. Biol. 15, 189–195 (2019).
Huang, G. et al. Circadian oscillations of NADH redox state using a heterologous metabolic sensor in mammalian cells. J. Biol. Chem. 291, 23906–23914 (2016).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Maxel, S. et al. A growth-based, high-throughput selection platform enables remodeling of 4-hydroxybenzoate hydroxylase active site. ACS Catal. 10, 6969–6974 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Tyka, M. D. et al. Alternate states of proteins revealed by detailed energy landscape mapping. J. Mol. Biol. 405, 607–618 (2011).
Wallen, J. R. et al. Structural analysis of Streptococcus pyogenes NADH oxidase: conformational dynamics involved in formation of the C(4a)-peroxyflavin intermediate. Biochemistry 54, 6815–6829 (2015).
Otagiri, M. et al. Structural basis for chiral substrate recognition by two 2,3-butanediol dehydrogenases. FEBS Lett. 584, 219–223 (2010).
Fleishman, S. J. et al. RosettaScripts: a scripting language interface to the Rosetta macromolecular modeling suite. PLoS ONE 6, e20161 (2011).
Zhu, Q. Rosetta files related to the engineering of Nox and Bdh enzymes. Zenodo https://doi.org/10.5281/zenodo.11478967 (2024).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug‐like molecules compatible with the CHARMM all‐atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Feller, S. E., Zhang, Y., Pastor, R. W. & Brooks, B. R. Constant pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Allen, M. P. & Tildesley, D. J. Computer Simulation of Liquids 2nd edn (Oxford University Press, 2017).
Phillips, J. C. et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 153, 044130 (2020).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Zhang, Y. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Acknowledgements
H.L. acknowledges support from the University of California, Irvine, the National Science Foundation (NSF) (award nos. 1847705 and MCB-2328145), the National Institutes of Health (NIH) (award no. DP2 GM137427), an Alfred Sloan research fellowship and the Advanced Research Projects Agency—Energy (award no. DE-AR0001508). Y.C., E.L. and J.B.S. acknowledge the funding of the National Institute of Environmental Health Sciences (grant no. P42ES004699), the NIH (grant no. R01 GM 076324-11) and the NSF (grant nos. 1627539, 1805510 and 1827246). R.L. acknowledges support from the NIH (award no. R35 GM130367). D.A acknowledges support from the NSF Graduate Research Fellowship Program (grant no. DGE1839285). We acknowledge valuable technical support provided by AAT Bioquest for the redox ratio kits.
Author information
Authors and Affiliations
Contributions
D.A., Y.Z., E.K. and H.L. designed the experiments. Y.C. and E.L. performed Rosetta modeling. E.L. and D.A. performed bioinformatic analysis of the NAD(P)-binding Rossmann-like domain superfamily. E.K. and Y.Z. performed rational protein engineering experiments. Y.Z. performed growth-based selection of Nox. Q.Z., Y.W. and R.L. performed MD simulations and analyzed the results. D.A. and E.K. bioprospected Bdh homologs. D.A. performed the Michaelis–Menten kinetic experiments for Bdhs. Y.Z. performed the Michaelis–Menten kinetic experiments for Nox. D.A. and S.P. performed cell-free biotransformation experiments. D.A. performed the resting cell biotransformation experiments. W.B.B. and D.A. reformulated the colorimetric redox ratio assay. D.A. performed the colorimetric redox ratio assays. Y.C., E.L. and J.B.S. analyzed the modeling results. All authors analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Zhen Chen, Steffen Lindner and Carine Vergne-Vaxelaire for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–5 and Figs. 1–12.
Supplementary Data
Key resources table.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aspacio, D., Zhang, Y., Cui, Y. et al. Shifting redox reaction equilibria on demand using an orthogonal redox cofactor. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01702-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01702-5