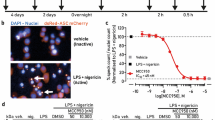
Abstract
Inflammasomes are multiprotein complexes that sense intracellular danger signals and induce pyroptosis. CARD8 and NLRP1 are related inflammasomes that are repressed by the enzymatic activities and protein structures of the dipeptidyl peptidases 8 and 9 (DPP8/9). Potent DPP8/9 inhibitors such as Val-boroPro (VbP) activate both NLRP1 and CARD8, but chemical probes that selectively activate only one have not been identified. Here we report a small molecule called CQ31 that selectively activates CARD8. CQ31 inhibits the M24B aminopeptidases prolidase (PEPD) and Xaa-Pro aminopeptidase 1 (XPNPEP1), leading to the accumulation of proline-containing peptides that inhibit DPP8/9 and thereby activate CARD8. NLRP1 is distinct from CARD8 in that it directly contacts DPP8/9’s active site; these proline-containing peptides, unlike VbP, do not disrupt this repressive interaction and thus do not activate NLRP1. We expect that CQ31 will now become a valuable tool to study CARD8 biology.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The proteomics dataset is available in the PRIDE database: accession code, PXD027707; project name, ‘Target identification of CQ31, a selective activator of the CARD8 inflammasome’ (https://www.ebi.ac.uk/pride/archive/projects/PXD027707). All other data in this study are available within the paper, the Supplementary Information and the Extended Data, and/or from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Rathinam, V. A. & Fitzgerald, K. A. Inflammasome complexes: emerging mechanisms and effector functions. Cell 165, 792–800 (2016).
D’Osualdo, A. et al. CARD8 and NLRP1 undergo autoproteolytic processing through a ZU5-like domain. PLoS ONE 6, e27396 (2011).
Finger, J. N. et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 287, 25030–25037 (2012).
Frew, B. C., Joag, V. R. & Mogridge, J. Proteolytic processing of Nlrp1b is required for inflammasome activity. PLoS Pathog. 8, e1002659 (2012).
Chui, A. J. et al. N-terminal degradation activates the NLRP1B inflammasome. Science 364, 82–85 (2019).
Sandstrom, A. et al. Functional degradation: a mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science 364, eaau1330 (2019).
Taabazuing, C. Y., Griswold, A. R. & Bachovchin, D. A. The NLRP1 and CARD8 inflammasomes. Immunol. Rev. 297, 13–25 (2020).
Bachovchin, D. A. NLRP1: a jack of all trades, or a master of one? Mol. Cell 81, 423–425 (2021).
Okondo, M. C. et al. DPP8 and DPP9 inhibition induces pro-caspase-1-dependent monocyte and macrophage pyroptosis. Nat. Chem. Biol. 13, 46–53 (2017).
Okondo, M. C. et al. Inhibition of Dpp8/9 activates the Nlrp1b inflammasome. Cell Chem. Biol. 25, 262–267 (2018).
Johnson, D. C. et al. DPP8/DPP9 inhibitor-induced pyroptosis for treatment of acute myeloid leukemia. Nat. Med. 24, 1151–1156 (2018).
Zhong, F. L. et al. Human DPP9 represses NLRP1 inflammasome and protects against autoinflammatory diseases via both peptidase activity and FIIND domain binding. J. Biol. Chem. 293, 18864–18878 (2018).
Gai, K. et al. DPP8/9 inhibitors are universal activators of functional NLRP1 alleles. Cell Death Dis. 10, 587 (2019).
Griswold, A. R. et al. DPP9’s enzymatic activity and not its binding to CARD8 inhibits inflammasome activation. ACS Chem. Biol. 14, 2424–2429 (2019).
Chui, A. J. et al. Activation of the CARD8 inflammasome requires a disordered region. Cell Rep. 33, 108264 (2020).
Hollingsworth, L. R. et al. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature 592, 778–783 (2021).
Sharif, H. et al. Dipeptidyl peptidase 9 sets a threshold for CARD8 inflammasome formation by sequestering its active C-terminal fragment. Immunity 54, 1392–1404 (2021).
Huang, M. et al. Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature 592, 773–777 (2021).
Van Goethem, S. et al. Inhibitors of dipeptidyl peptidase 8 and dipeptidyl peptidase 9. Part 2: isoindoline containing inhibitors. Bioorg. Med. Chem. Lett. 18, 4159–4162 (2008).
Linder, A. et al. CARD8 inflammasome activation triggers pyroptosis in human T cells. EMBO J. 39, e105071 (2020).
Johnson, D. C. et al. DPP8/9 inhibitors activate the CARD8 inflammasome in resting lymphocytes. Cell Death Dis. 11, 628 (2020).
Zhong, F. L. et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell 167, 187–202 (2016).
Robinson, K. S. et al. Enteroviral 3C protease activates the human NLRP1 inflammasome in airway epithelia. Science 370, eaay2002 (2020).
Drutman, S. B. et al. Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc. Natl Acad. Sci. USA 116, 19055–19063 (2019).
Grandemange, S. et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis). Ann. Rheum. Dis. 76, 1191–1198 (2017).
Ball, D. P. et al. Caspase-1 interdomain linker cleavage is required for pyroptosis. Life Sci. Alliance 3, e202000664 (2020).
Hollingsworth, L. R. et al. Mechanism of filament formation in UPA-promoted CARD8 and NLRP1 inflammasomes. Nat. Commun. 12, 189 (2021).
Gong, Q. et al. Structural basis for distinct inflammasome complex assembly by human NLRP1 and CARD8. Nat. Commun. 12, 188 (2021).
Tang, H. K. et al. Biochemical properties and expression profile of human prolyl dipeptidase DPP9. Arch. Biochem. Biophys. 485, 120–127 (2009).
Lee, H. J. et al. Investigation of the dimer interface and substrate specificity of prolyl dipeptidase DPP8. J. Biol. Chem. 281, 38653–38662 (2006).
Geiss-Friedlander, R. et al. The cytoplasmic peptidase DPP9 is rate-limiting for degradation of proline-containing peptides. J. Biol. Chem. 284, 27211–27219 (2009).
Griswold, A. R. et al. A chemical strategy for protease substrate profiling. Cell Chem. Biol. 26, 901–907 (2019).
Brandt, W. et al. A model of the active site of dipeptidyl peptidase IV predicted by comparative molecular field analysis and molecular modelling simulations. Int. J. Pept. Protein Res. 46, 494–507 (1995).
Hikida, A., Ito, K., Motoyama, T., Kato, R. & Kawarasaki, Y. Systematic analysis of a dipeptide library for inhibitor development using human dipeptidyl peptidase IV produced by a Saccharomyces cerevisiae expression system. Biochem. Biophys. Res. Commun. 430, 1217–1222 (2013).
Lan, V. T. et al. Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem. 175, 66–73 (2015).
Sekine, K., Fujii, H., Abe, F. & Nishikawa, K. Augmentation of death ligand-induced apoptosis by aminopeptidase inhibitors in human solid tumor cell lines. Int. J. Cancer 94, 485–491 (2001).
Krige, D. et al. CHR-2797: an antiproliferative aminopeptidase inhibitor that leads to amino acid deprivation in human leukemic cells. Cancer Res. 68, 6669–6679 (2008).
Lupi, A., Tenni, R., Rossi, A., Cetta, G. & Forlino, A. Human prolidase and prolidase deficiency: an overview on the characterization of the enzyme involved in proline recycling and on the effects of its mutations. Amino Acids 35, 739–752 (2008).
Wilk, P. et al. Substrate specificity and reaction mechanism of human prolidase. FEBS J. 284, 2870–2885 (2017).
Maggiora, L. L., Orawski, A. T. & Simmons, W. H. Apstatin analogue inhibitors of aminopeptidase P, a bradykinin-degrading enzyme. J. Med. Chem. 42, 2394–2402 (1999).
Singh, R. et al. Structure of the human aminopeptidase XPNPEP3 and comparison of its in vitro activity with Icp55 orthologs: insights into diverse cellular processes. J. Biol. Chem. 292, 10035–10047 (2017).
Bissonnette, R. et al. Prolidase deficiency: a multisystemic hereditary disorder. J. Am. Acad. Dermatol. 29, 818–821 (1993).
Dickson, M. A. et al. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell Biol. 20, 1436–1447 (2000).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Lupi, A. et al. Characterization of a new PEPD allele causing prolidase deficiency in two unrelated patients: natural-occurrent mutations as a tool to investigate structure–function relationship. J. Hum. Genet. 49, 500–506 (2004).
Duhrkop, K. et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 16, 299–302 (2019).
Perez-Riverol, Y. et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019).
Acknowledgements
This work was supported by the Pew Charitable Trusts (D.A.B. is a Pew-Stewart Scholar in Cancer Research), the NIH (R01 AI137168 and R01 AI163170 to D.A.B.; T32 GM007739-Andersen to A.R.G.; NIH T32 GM115327-Tan to E.L.O.-H.; F30 CA008748 to A.R.G.; the MSKCC Core Grant P30 CA008748; R25AI140472 to J.R.C.), Gabrielle’s Angel Foundation (D.A.B.), Mr William H. and Mrs Alice Goodwin, the Commonwealth Foundation for Cancer Research, and The Center for Experimental Therapeutics of Memorial Sloan Kettering Cancer Center (D.A.B.), the Emerson Collective (D.A.B.) and a Marie-Josée Kravis Women in Science Endeavor (WISE) fellowship (S.D.R.).
Author information
Authors and Affiliations
Contributions
D.A.B. conceived and directed the project. S.D.R., E.L.O.-H., Q.C., Q.W., A.R.G., D.P.B., H.-C.H., A.J.C., S.Y., and D.J.C. performed cloning, gene editing, biochemistry and cell biology experiments. A.B. synthesized peptides. Q.C. synthesized all other small molecules and performed chemoproteomics experiments. E.L.O.-H., J.R.C. and M.S. performed and analyzed metabolomics experiments. D.A.B. and S.D.R. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Rebecca Coll and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Activation of the NLRP1 and CARD8 inflammasomes.
The proteasome-mediated degradation of the NT fragments of NLRP1 and CARD8 release the CT fragments from autoinhibition. DPP8/9 inhibitors and several other danger signals (for example, pathogen proteases) accelerate the rate of NT degradation. If the rate of degradation is slow (top), CT fragments are restrained in ternary complexes consisting of the CT fragment, a full-length (FL) PRR, and DPP9. Disruption of the ternary complex (for example, by VbP) can release the CT fragments to form an inflammasome. If the rate of degradation is fast (bottom), sufficient CT fragments are released to overwhelm the DPP9 ternary complex checkpoint and to form an inflammasome. NLRP1 is activated by a similar overall mechanism, but only CARD8 is shown for clarity. Key differences between NLRP1 and CARD8 are discussed in the text.
Extended Data Fig. 2 Certain Xaa-Pro dipeptides inhibit DPP9 and activate CARD8.
(a) Inhibition of recombinant DPP9 by the twenty XP dipeptides in an AP-AMC cleavage assay. (b) Inhibition of AP-AMC cleavage activity in HEK 293 T cell lysates (pretreated with 10 μM sitagliptin to inhibit any DPP4 activity) by indicated compounds. (c) The indicated cell types were immunoblotted for proteins involved in VbP-induced pyroptosis. MV4;11 and THP-1 cells express CARD8, whereas N/TERT-1 and HEKa cells express NLRP1. Asterisks indicate background bands. (d,e) The indicated MV4;11 cells were treated with compounds (1 mM) and monitored for PI uptake over 12 h (d) or analyzed by CTG and CTF after 24 h (e). (f) MV4;11 cells were treated with XP-OMes (1 mM) for 14 h before CTG and CTF analyses. (g) The indicated AML cell lines were treated with VP-OMe (dose range = 5mM-19.5 μM, 2-fold dilution) for 24 h before CTG analysis. (h) Primary resting CD3+ T-cells were treated with VP-OMe (1 mM), IP-OMe (1 mM) or VbP (10 μM) for 18 h before immunoblot analysis. Data is representative of two independent experiments. (i) J774.1 macrophages were treated with VP-OMe (1 mM) or VbP (2 μM) for 24 h before assaying for LDH release. (j) The indicated RAW264.7 cells were treated with VP-OMe or IP-Ome (5 mM, 24 h) before CTF and CTG analyses. Data in d, e, and g (n = 4) and a,b, f, i, and j (n = 3) are means ± SEM of replicates. All data except where indicated, including immunoblots, are representative of three or more independent experiments.
Extended Data Fig. 3 CQ31 releases CQ04 in cells and causes pyroptosis.
(a) The indicated MV4;11 cells were treated with CQ04 or CQ31 for 24 h before assessing cell viability by CTG. Data are means ± SEM of 3 biological replicates. (b) The indicated MV4;11 cells were treated with CQ31 (16 μM) or VbP (16 μM), incubated for 4 h, and stained with PI. PI uptake was recorded for 12 h. Data are means ± SEM of 10 replicates. a and b are representative of three or more independent experiments. (c) HEK 293 T cells were treated with vehicle control (DMSO) or CQ31(10 μM) for 24 h before intracellular metabolites were extracted. Methyl ester and free acid of CQ31 were measured by LC-MS and confirmed against pure standards.
Extended Data Fig. 4 CQ31 selectively activates the CARD8 inflammasome.
(a) Viability of cell lines after treatment with CQ31 for 24 h as assessed by CTF. (b) Human resting T-cells were treated with CQ31 (10 μM) or VbP (10 μM) for 24 h before assessing cell viability by CTG and pyroptotic and apoptotic markers by immunoblotting. Data is representative of two independent experiments. (c) N/TERT-1 immortalized keratinocyte cells were treated with CQ31 (10 μM) or VbP (10 μM), incubated for 1 h, and stained with PI. PI uptake was assessed over 14 h. (d) HEKa immortalized keratinocyte cells were treated with CQ31 (10 μM) or VbP (10 μM), before assaying for LDH release. (e) HEK 293 T cells stably expressing CASP1 and GSDMD were transfected with plasmids expressing NLRP1 and ASC and treated with CQ31 (10 μM) or VbP (10 μM) for 24 h. GSDMD cleavage was assessed by immunoblotting. (f) RAW264.7 cells were treated with CQ31 (100 μM) or VbP (2 μM) for 24 h, before assaying for LDH release. (n = 3) (g) J774.1 cells were treated with various doses of CQ31 before cell viability was measured by CTG and CTF. Data in a (n = 4), b,d,f (n = 3), and c,g (n = 6) are means ± SEM of the indicated replicates. All data except where indicated, including immunoblots, are representative of three or more independent experiments.
Extended Data Fig. 5 CQ31-induced pyroptosis is DPP8/9 dependent.
(a,b) Indicated THP-1 cells were treated with VP-OMe (1 mM, 14 h) (a) or varying doses of CQ31 or VbP (b) before assaying cell viability by CTG and CTF assays. (c) HEK 293T cells were treated with vehicle control (DMSO), CQ31 (10 μM), or VbP (10 μM) for 6 h before intracellular metabolites were extracted, and dipeptide concentrations were measured by LC-MS. Data in a (n = 4) and b,c (n = 3) are means ± SEM of the indicated replicates. Data in a and b are representative of three or more independent experiments.
Extended Data Fig. 6 PEPD knockout cells are sensitive to CQ31.
(a) The indicated MV4;11 cells were treated with compounds (16 μM) for 4 h before monitoring for PI uptake. Data is mean ± SEM of 10 biological replicates. (b) The indicated MV4;11 cells were treated with varying doses of CQ31 before assessing cell viability by CTG and CTF assays. (c) The indicated MV4;11 cells were all treated with VX-765 (50 μM) to prevent pyroptosis. Cells were then co-treated with DMSO (control) or CQ31 (10 μM). Intracellular metabolites were extracted and dipeptide concentrations were measured by LC-MS. Data are means ± SEM of the 3 biological replicates, unless indicated otherwise. Data in a and b are representative of three or more independent experiments.
Extended Data Fig. 7 CQ31 also targets XPNPEP1.
(a) Representative TMT-labeled peptides used to quantify the enrichment of the indicated proteins by CQ73. (b) CETSA analysis of CQ04 (0.5, 5 μM) and CQ31 (0.5, 5 μM) in THP-1Cas9 lysates is representative of three or more independent experiments.
Extended Data Fig. 8 Dual PEPD and XPNPEP1 inhibition induces pyroptosis.
(a) XPNPEP1/PEPD knockout THP-1 cells were treated with the indicated concentrations of CQ31 for 48 h prior to CTG analysis. (b) The indicated THP-1 cells were treated with VbP (10 μM) or CQ31 (20 μM) for 48 h prior to evaluating supernatants for IL-1β levels by ELISA and evaluating lysates and supernatants for IL-1β cleavage by immunoblotting. (c) Evaluation of XPNPEP1 and PEPD levels in MV4;11 cells stably expressing Cas9 and treated with sgRNAs targeting XPNPEP1. (d) The indicated cells were treated with CQ31 for 24 h before LDH release was evaluated. (e) The indicated MV4;11 cells were treated with VbP (10 μM) or CQ31 (20 μM) for 48 h before LDH release and immunoblot analyses. PEPD/XPNPEP1 knockout MV4;11 cells were generated with sgPEPD_2 and sgXPNPEP1. Data are means ± SEM of biological replicates. *** p < 0.001, ** p < 0.01 by two-sided Students t-test. (f) The indicated MV4;11 cells were treated with CQ31 for 48 h before assessing cell viability by CTF. (g) Inhibition of AP-AMC cleavage in HEK 293 T lysates by methyl esters of IPI or VPI tripeptides. (h) The indicated MV4;11 cells were treated with methyl esters of IPI or VPI for 24 h before CTG analysis. Data in b-h are means ± SEM of 3 replicates. All data, including immunoblots, are representative of three or more independent experiments.
Extended Data Fig. 9 Schematic comparing CQ31-induced and VbP-induced activation inflammasome activation.
(a) Weak DPP8/9 inhibition selectively activates CARD8, whereas strong DPP8/9 inhibition activates both NLRP1 and CARD8. (b) Strong DPP8/9 inhibitors (for example, VbP) are required to compete with the neo-N-terminus of the NLRP1CT fragment for the DPP8/9 active site and destabilize the ternary complex. (c) CARD8 does not directly interact with the DPP8/9 active site, and thus strong inhibitors are not needed to disrupt that interaction. It has not yet been established precisely how DPP8/9 inhibitors impact the CARD8-DPP9 ternary complex in cells. Red dots in b and c represent the DPP9 active site serine.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and note.
41589_2021_964_MOESM3_ESM.xlsx
Supplementary Data 1 Supplementary dataset. List of proteins enriched by CQ73 analyzed by TMT-mass spectrometry. Rank-ordered list of proteins enriched by CQ73 and whose binding was competed off by CQ31. No probe treatment corresponds to TMT labels 126 and 127, CQ73 treatment corresponds to TMT labels 128 and 129, and CQ73 treatment in competition with CQ31 corresponds to TMT labels 130 and 131.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Figure 7
Unprocessed western blots.
Source Data Extended Data Figure 8
Unprocessed western blots.
Source Data Extended Data Figure 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Rao, S.D., Chen, Q., Wang, Q. et al. M24B aminopeptidase inhibitors selectively activate the CARD8 inflammasome. Nat Chem Biol 18, 565–574 (2022). https://doi.org/10.1038/s41589-021-00964-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00964-7
This article is cited by
-
NLRP inflammasomes in health and disease
Molecular Biomedicine (2024)
-
Activation of Inflammasomes and Relevant Modulators for the Treatment of Microglia-mediated Neuroinflammation in Ischemic Stroke
Molecular Neurobiology (2024)
-
Chemical inhibition of DPP9 sensitizes the CARD8 inflammasome in HIV-1-infected cells
Nature Chemical Biology (2023)