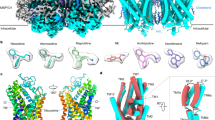
Abstract
Sphingosine-1-phosphate receptor 1 (S1PR1) is a master regulator of lymphocyte egress from the lymph node and an established drug target for multiple sclerosis (MS). Mechanistically, therapeutic S1PR1 modulators activate the receptor yet induce sustained internalization through a potent association with β-arrestin. However, a structural basis of biased agonism remains elusive. Here, we report the cryo-electron microscopy (cryo-EM) structures of Gi-bound S1PR1 in complex with S1P, fingolimod-phosphate (FTY720-P) and siponimod (BAF312). In combination with functional assays and molecular dynamics (MD) studies, we reveal that the β-arrestin-biased ligands direct a distinct activation path in S1PR1 through the extensive interplay between the PIF and the NPxxY motifs. Specifically, the intermediate flipping of W2696.48 and the retained interaction between F2656.44 and N3077.49 are the key features of the β-arrestin bias. We further identify ligand–receptor interactions accounting for the S1PR subtype specificity of BAF312. These structural insights provide a rational basis for designing novel signaling-biased S1PR modulators.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data produced or analyzed in this study are included in the main text or the Supplementary Materials. A reporting summary for this article is available as a Supplementary Information file. The cryo-EM density maps and atomic coordinates have been deposited in the Electron Microscopy Data Bank (EMDB) and PDB under accession numbers EMD-32461 and 7WF7 for the S1P-bound S1PR1–Gi complex, EMD-31225 and 7EO2 for the FTY720-P-bound S1PR1–Gi complex and EMD-31226 and 7EO4 for the BAF312-bound S1PR1–Gi complex. All other data relating to this study are available from the corresponding author on reasonable request. All other published PDB codes cited in this paper are 3V2Y and 6VMS. Source data are provided with this paper.
Change history
10 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41589-022-00968-x
References
Cartier, A. & Hla, T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366, eaar5551 (2019).
Spiegel, S. Sphingosine-1-phosphate: from insipid lipid to a key regulator. J. Biol. Chem. 295, 3371–3384 (2020).
Bryan, A. M. & Del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol. 20, e12836 (2018).
Brinkmann, V. et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9, 883–897 (2010).
Chun, J., Kihara, Y., Jonnalagadda, D. & Blaho, V. A. Fingolimod: lessons learned and new opportunities for treating multiple sclerosis and other disorders. Annu. Rev. Pharmacol. Toxicol. 59, 149–170 (2019).
Kawahara, A. et al. The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 (2009).
Vu, T. M. et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550, 524–528 (2017).
Oo, M. L. et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282, 9082–9089 (2007).
Sykes, D. A. et al. Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalization. Br. J. Pharmacol. 171, 4797–4807 (2014).
Murakami, A. et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Mol. Pharmacol. 77, 704–713 (2010).
Scott, L. J. Siponimod: a review in secondary progressive multiple sclerosis. CNS Drugs 34, 1191–1200 (2020).
Hanson, M. A. et al. Crystal structure of a lipid G protein-coupled receptor. Science 335, 851–855 (2012).
Roth, C. B., Hanson, M. A. & Stevens, R. C. Stabilization of the human β2-adrenergic receptor TM4–TM3–TM5 helix interface by mutagenesis of Glu1223.41, a critical residue in GPCR structure. J. Mol. Biol. 376, 1305–1319 (2008).
Olsen, R. H. J. et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–84 (2020).
Duan, J. et al. Cryo-EM structure of an activated VIP1 receptor–G protein complex revealed by a NanoBiT tethering strategy. Nat. Commun. 11, 4121 (2020).
Punjani, A. & Fleet, D. J. 3D variability analysis: resolving continuous flexibility and discrete heterogeneity from single particle cryo-EM. J. Struct. Biol. 213, 107702 (2021).
Picard, L. P., Schonegge, A. M. & Bouvier, M. Structural insight into G protein-coupled receptor signaling efficacy and bias between Gs and β-arrestin. ACS Pharmacol. Transl. Sci. 2, 148–154 (2019).
Okude, J. et al. Identification of a conformational equilibrium that determines the efficacy and functional selectivity of the μ-opioid receptor. Angew. Chem. Int. Ed. Engl. 54, 15771–15776 (2015).
Wingler, L. M. et al. Angiotensin analogs with divergent bias stabilize distinct receptor conformations. Cell 176, 468–478 (2019).
Suomivuori, C. M. et al. Molecular mechanism of biased signaling in a prototypical G protein-coupled receptor. Science 367, 881–887 (2020).
Violin, J. D., Crombie, A. L., Soergel, D. G. & Lark, M. W. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol. Sci. 35, 308–316 (2014).
Wingler, L. M., McMahon, C., Staus, D. P., Lefkowitz, R. J. & Kruse, A. C. Distinctive activation mechanism for angiotensin receptor revealed by a synthetic nanobody. Cell 176, 479–490 (2019).
Wingler, L. M. et al. Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science 367, 888–892 (2020).
Lagane, B. et al. Mutation of the DRY motif reveals different structural requirements for the CC chemokine receptor 5-mediated signaling and receptor endocytosis. Mol. Pharmacol. 67, 1966–1976 (2005).
Barak, L. S., Oakley, R. H., Laporte, S. A. & Caron, M. G. Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc. Natl Acad. Sci. USA 98, 93–98 (2001).
Nakajima, K. & Wess, J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol. Pharmacol. 82, 575–582 (2012).
Bouley, R. et al. Functional role of the NPXXY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am. J. Physiol. Cell Physiol. 285, C750–C762 (2003).
Hunyady, L., Bor, M., Baukal, A. J., Balla, T. & Catt, K. J. A conserved NPLFY sequence contributes to agonist binding and signal transduction but is not an internalization signal for the type 1 angiotensin II receptor. J. Biol. Chem. 270, 16602–16609 (1995).
Prioleau, C., Visiers, I., Ebersole, B. J., Weinstein, H. & Sealfon, S. C. Conserved helix 7 tyrosine acts as a multistate conformational switch in the 5HT2C receptor. Identification of a novel “locked-on” phenotype and double revertant mutations. J. Biol. Chem. 277, 36577–36584 (2002).
Yang, D. et al. G protein-coupled receptors: structure- and function-based drug discovery. Signal Transduct. Target. Ther. 6, 7 (2021).
Zhao, C. et al. Structural insights into sphingosine-1-phosphate recognition and ligand selectivity of S1PR3–Gi signaling complexes. Cell Res https://doi.org/10.1038/s41422-021-00567-w (2021)
Yuan, Y. et al. Structures of signaling complexes of lipid receptors S1PR1 and S1PR5 reveal mechanisms of activation and drug recognition. Cell Res https://doi.org/10.1038/s41422-021-00566-x (2021).
Xia, R. et al. Cryo-EM structure of the human histamine H1 receptor/Gq complex. Nat. Commun. 12, 2086 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zivanov, J., Nakane, T. & Scheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Yin, J. et al. Structure of a D2 dopamine receptor–G-protein complex in a lipid membrane. Nature 584, 125–129 (2020).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Wang, R. Y. et al. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. eLife 5, e17219 (2016).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Inoue, A. et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell 177, 1933–1947 (2019).
Hilger, D. et al. Structural insights into differences in G protein activation by family A and family B GPCRs. Science 369, eaba3373 (2020)
Fiser, A., Do, R. K. G. & Sali, A. Modeling of loops in protein structures. Protein Sci. 9, 1753–1773 (2000).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Zhang, H., Pluhackova, K., Jiang, Z. & Bockmann, R. A. Binding characteristics of sphingosine-1-phosphate to ApoM hints to assisted release mechanism via the ApoM calyx-opening. Sci. Rep. 6, 30655 (2016).
Berendsen, H. J. C., Vanderspoel, D. & Vandrunen, R. Gromacs—a message-passing parallel molecular-dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995).
Hoover, W. G. Canonical dynamics—equilibrium phase-space distributions. Phys. Rev. A Gen. Phys. 31, 1695–1697 (1985).
Parrinello, M. & Rahman, A. Crystal structure and pair potentials: a molecular-dynamics study. Phys. Rev. Lett. 45, 4 (1980).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 3 (1993).
McGibbon, R. T. et al. MDTraj: a modern open library for the analysis of molecular dynamics trajectories. Biophys. J. 109, 1528–1532 (2015).
Harris, C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Acknowledgements
We thank the Cryo-EM facility of Harbin Institute of Technology for sample screening and data collection. We thank P. Lu at Westlake University for instruction on ligand docking. This work was supported by the Startup Funds of HIT Center for Life Sciences and the National Natural Science Foundation of China (32070048 to Y.H.). We thank H.E. Xu of Shanghai Institute of Materia Medica, Chinese Academy of Sciences, for the original LgBiT and pFastbac-2×MBP plasmids. We thank K. Sato, S. Nakano and A. Inoue at Tohoku University for their assistance in plasmid preparation and cell-based GPCR assays. This work was supported by KAKENHI 21H04791 (A.I.), 21H051130 (A.I.) and JPJSBP120213501 (A.I.) from the Japan Society for the Promotion of Science (JSPS); Grant-in-Aid for JSPS Fellows 19J11256 (K.K.); the PRIME JP19gm5910013 (A.I.), the LEAP JP20gm0010004 (A.I.) and the BINDS JP20am0101095 (A.I.) from the Japan Agency for Medical Research and Development (AMED); JST Moonshot Research and Development Program JPMJMS2023 (A.I.) from the Japan Science and Technology Agency (JST); Daiichi Sankyo Foundation of Life Science (A.I.); Takeda Science Foundation (A.I.); Ono Medical Research Foundation (A.I.) and The Uehara Memorial Foundation (A.I.).
Author information
Authors and Affiliations
Contributions
Y.H. initiated the project. Y.H. and A.I. designed the experiments, analyzed data and supervised the whole project. Z.X. made the expression constructs, purified the proteins, prepared and screened the grids, made the mutations and performed BRET2 assays. T.I. performed the MD simulation and analyzed data. K.K. and R.K. performed pilot studies for the NanoBiT GPCR signaling assays and edited the manuscript. Y.Q. and R.X. made the mutations and prepared the plasmids. M.-X.S. and X.-H.C. set up the BRET2 experiments. A.Z. and C.G. collected data with Y.H., and Y.H. solved the structures. Z.H. analyzed the data and edited the manuscript. Y.H., A.I. and T.I. wrote the manuscript. All authors contributed to data interpretation and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Jesús Giraldo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sample preparation of S1PR1/Gi complexes.
a, Snake-shape diagram of the S1PR1-LgBiT construct used in the complex assembling. The diagram is adopted and modified from the GPCRdb (https://www.gpcrdb.org/protein/s1pr1_human/). The pink color highlights the position where the F1333.41W mutation is introduced. b, The BRET2 Gi dissociation assay to examine the F133W3.41 activity to S1P. Data are presented as mean values ± SEM, n = 3-4. c, The BRET2 Gi dissociation assay to examine the LgBiT-fused construct activity to S1P. Data are presented as mean values ± SEM, n = 3-4. d, Size exclusion column profile of S1PR1/Gi complex. e, Bias signaling activity of S1P, FTY720-P and BAF312. Gi dissociation, β-arrestin recruitment and β-arrestin endosomal translocation responses in the wild-type S1PR1 were tested by the NanoBiT-based assays. For the individual experiments performed in parallel, pharmacological parameters were normalized to S1P and presented as a logarithm of relative intrinsic activity (RAi; EC50/Emax value relative to that of S1P; Methods). Data are presented as mean values ± SEM, n = 6-7 (dots). Adjusted P values were calculated by the two-way ANOVA with the Dunnett’s post-hoc test.
Extended Data Fig. 2 Representative data process of S1PR1/Gi complexes.
A flow-chart of the cryo-EM data process of the S1PR1/Gi complex.
Extended Data Fig. 3 Local resolution of S1PR1/Gi complexes.
a, Local resolution analysis of the S1PR1/Gi complexes. b, FSC curve of the S1PR1/Gi complexes refined by the Non-uniform refinement in cryoSPARC, the resolution is assessed by model to map, FSC = 0.5.
Extended Data Fig. 4 Representative density map of S1PR1/Gi complexes and 3D variability analysis.
a-c, The cryo-EM density map of the representative regions of the S1PR1/Gi complexes. The density map is drawn by pymol at the contour level of 3.0. d, Representative image of the 3D variability analysis of the S1PR1 cryo-EM maps. The yellow arrow marks the position of ligand and the red arrow marks TM1.
Extended Data Fig. 5 Ligand binding pocket of S1PR1.
a, Cross section of a hydrophobicity analysis in the S1P-bound S1PR1 structure. b, An electrostatic potential surface analysis of the S1P-binding pocket. c, Mutant study to assess ligands response by the NanoBiT-Gi dissociation assay. Dashed lines in the mutant panels represent the wild-type (WT) S1PR1 response. Data are presented as mean values ± SEM, n = 3-5. Note that in many data points, error bars are smaller than the size of symbols and thus are not visible. For details of the ∆pEC50, Emax and error values, see Supplementary Table 2.
Extended Data Fig. 6 Mutant studies assessing ligand recognition.
a, Surface expression of the S1PR1 mutants analyzed by the flow cytometry. b, c, Pharmacological parameters for the Gi-coupling activity analyzed by the NanoBiT-Gi dissociation assay. For the individual experiments performed in parallel, data were normalized to the wild-type (WT) S1PR1 and presented as surface expression level (a), Emax (b) and ∆pEC50 (c). Data are presented as mean values ± SEM, n = 3-5 (dots). NA, parameter not available owing to a lack of ligand response. Mutants with lowered surface expression levels (E1213.29A, W2696.48A and E2947.36×35A) are shown as grey bars.
Extended Data Fig. 7 MD simulation study and mutant study assessing the ligand binding modes.
a, Key residues distance distributions with S1P (cyan), FTY720-P (green), BAF312 (purple), and ML056 (pink). Left panel, K34N-term and oxygens in the negatively-charged head groups (phosphate groups in S1P, FTY720-P, and ML056; carboxyl group in BAF312); middle panel, R1203.28 and the negatively-charged head groups; right panel, R1203.28 and N1012.60 or E1213.29. b, Representative snapshots of the key polar residues (K34N-term, N1012.60, R1203.28, and E1213.29) with the ligands during the simulations. Black dashed lines represent hydrogen bonds. c, The related position of ligands to W2696.48. FTY: FTY720-P, green color. BAF: BAF312, blue color. S1P, cyan color. d, The hydrophobic interaction between the lower part of the ligand binding pocket and the distal end of the benzyloxy oxime moiety of BAF312. e, Functional study for the F1253.33A mutation assessed by the BRET2 Gi dissociation assay. Data are presented as mean values ± SEM, n = 3-4. f, The outward displacement (arrow) of F2736.52 upon ligand binding.
Extended Data Fig. 8 MD simulation study assessing ligand-induced S1PR1 activation.
a, Distance distribution matrix of the key residues with S1P (cyan), FTY720-P (green), BAF312 (purple), and ML056 (pink). b, Interaction traces of three replicas (blue, red, and orange) with each ligand. Distance of the nearest non-hydrogen atom pairs is drawn with thick 2-ns moving average traces and thin unsmoothed traces (2-ps interval). c, Plots of averaged distances in Interactions 1-3 from the three replicas (the same color schemes as in b). Bars and error bars represent mean and SEM, respectively. Dotted lines indicate distances in the ML056-bound S1PR1 structure (PDB ID: 3V2Y). d, Fractions of contacts in Interactions 1-3 from the three replicas. Contact thresholds were set as the distances in the ML056-bound S1PR1. Statistical analyses were performed using the ordinary one-way ANOVA followed by the Dunnett’s post hoc test (c, d).
Extended Data Fig. 9 Key residues and ligand structures of the biased agonism.
a, Flow cytometry-based surface expression analysis for the mutants studied in the β-arrestin assays. The vertical dashed line separates mutants that are included in Fig. 4c (left) and Extended Data Fig. 8b (right). Note that the expression data of the W2696.48A mutant is identical to the one shown in Extended Data Fig. 5c. Data are presented as mean values ± SEM, n = 3-5 (dots). b, Mutant study to assess S1P response of the indicated mutants by the NanoBiT-Gi dissociation assay and the β-arrestin recruitment assay. Dashed lines and the long dashed dotted lines in the mutant panels represent the WT and the mock responses, respectively. Data are presented as mean values ± SEM, n = 3-4. Note that, in many data points, error bars are smaller than the size of symbols and thus are not visible. c, Bias profiles of etrasimod and S1P receptor agonist 1. Gi dissociation, β-arrestin recruitment and β-arrestin endosomal translocation responses in the wild-type S1PR1 were tested by the NanoBiT-based assays. For the individual experiments performed in parallel, data were normalized to S1P and presented as a logarithm of relative intrinsic activity (RAi; EC50/Emax value relative to that of S1P; Methods). Data are presented as mean values ± SEM, n = 3-4.
Extended Data Fig. 10 Comparison with published S1PR structures.
A comparison of S1P-, FTY720-P- and BAF312-bound S1PR1 with published S1P-bound S1PR3, FTY720-P-bound S1PR3 and BAF312-bound S1PR1, respectively. The dashed arrow marks the acyl-chain tail of the S1P molecule pointing the different directions.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and Fig. 1.
Supplementary Video 1
Supplementary Video 1. Molecule simulation of S1P-bound S1PR1.
Supplementary Video 2
Supplementary Video 2. Molecule simulation of FTY720-P-bound S1PR1.
Supplementary Video 3
Supplementary Video 3. 3DVa of S1P-, FTY720-P- and BAF312-bound S1PR1.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
About this article
Cite this article
Xu, Z., Ikuta, T., Kawakami, K. et al. Structural basis of sphingosine-1-phosphate receptor 1 activation and biased agonism. Nat Chem Biol 18, 281–288 (2022). https://doi.org/10.1038/s41589-021-00930-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00930-3
This article is cited by
-
Identification of oleic acid as an endogenous ligand of GPR3
Cell Research (2024)
-
G protein-biased LPAR1 agonism of prototypic antidepressants: Implication in the identification of novel therapeutic target for depression
Neuropsychopharmacology (2024)
-
Structural basis of ligand recognition and design of antihistamines targeting histamine H4 receptor
Nature Communications (2024)
-
β-Arrestin-independent endosomal cAMP signaling by a polypeptide hormone GPCR
Nature Chemical Biology (2024)
-
Applications and prospects of cryo-EM in drug discovery
Military Medical Research (2023)