Abstract
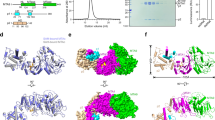
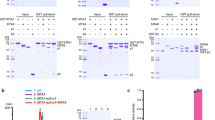
Among RNA 5′-cap structures, γ-phosphate monomethylation is unique to a small subset of noncoding RNAs, 7SK and U6 in humans. 7SK is capped by methylphosphate capping enzyme (MePCE), which has a second nonenzymatic role as a core component of the 7SK ribonuclear protein (RNP), an essential regulator of RNA transcription. We report 2.0- and 2.1-Å X-ray crystal structures of the human MePCE methyltransferase domain bound to S-adenosylhomocysteine (SAH) and uncapped or capped 7SK substrates, respectively. 7SK recognition is achieved by protein contacts to a 5′-hairpin-single-stranded RNA region, thus explaining MePCE’s specificity for 7SK and U6. The structures reveal SAH and product RNA in a near-transition-state geometry. Unexpectedly, binding experiments showed that MePCE has higher affinity for capped versus uncapped 7SK, and kinetic data support a model of slow product release. This work reveals the molecular mechanism of methyl transfer and 7SK retention by MePCE for subsequent assembly of 7SK RNP.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Atomic coordinates and structure factors have been deposited in the Protein Data Bank under the following accession codes: PDB 6DCB (MePCE–SAH–7SK) and PDB 6DCC (MePCE–SAH–me7SK). All other data generated or analyzed in this study are included in the published article (and its supplementary information files) or are available from the corresponding author upon reasonable request.
References
Byszewska, M., Śmietański, M., Purta, E. & Bujnicki, J. M. RNA methyltransferases involved in 5′ cap biosynthesis. RNA Biol. 11, 1597–1607 (2014).
Schapira, M. Structural chemistry of human RNA methyltransferases. ACS Chem. Biol. 11, 575–582 (2016).
Singh, R. & Reddy, R. Gamma-monomethyl phosphate: a cap structure in spliceosomal U6 small nuclear RNA. Proc. Natl Acad. Sci. USA 86, 8280–8283 (1989).
Shumyatsky, G. P., Tillib, S. V. & Kramerov, D. A. B2 RNA and 7SK RNA, RNA polymerase III transcripts, have a cap-like structure at their 5′ end. Nucleic Acids Res. 18, 6347–6351 (1990).
Shimba, S., Buckley, B., Reddy, R., Kiss, T. & Filipowicz, W. Cap structure of U3 small nucleolar RNA in animal and plant cells is different. gamma-monomethyl phosphate cap structure in plant RNA. J. Biol. Chem. 267, 13772–13777 (1992).
Jeronimo, C. et al. Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol. Cell 27, 262–274 (2007).
Muniz, L., Egloff, S. & Kiss, T. RNA elements directing in vivo assembly of the 7SK/MePCE/Larp7 transcriptional regulatory snRNP. Nucleic Acids Res. 41, 4686–4698 (2013).
Brogie, J. E. & Price, D. H. Reconstitution of a functional 7SK snRNP. Nucleic Acids Res. 45, 6864–6880 (2017).
Xue, Y., Yang, Z., Chen, R. & Zhou, Q. A capping-independent function of MePCE in stabilizing 7SK snRNA and facilitating the assembly of 7SK snRNP. Nucleic Acids Res. 38, 360–369 (2010).
Yang, Z., Zhu, Q., Luo, K. & Zhou, Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414, 317–322 (2001).
Nguyen, V. T., Kiss, T., Michels, A. A. & Bensaude, O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414, 322–325 (2001).
Kohoutek, J. P-TEFb: the final frontier. Cell. Div. 4, 19 (2009).
Krueger, B. J. et al. LARP7 is a stable component of the 7SK snRNP while P-TEFb, HEXIM1 and hnRNP A1 are reversibly associated. Nucleic Acids Res. 36, 2219–2229 (2008).
Markert, A. et al. The La-related protein LARP7 is a component of the 7SK ribonucleoprotein and affects transcription of cellular and viral polymerase II genes. EMBO Rep. 9, 569–575 (2008).
He, N. et al. A La-related protein modulates 7SK snRNP integrity to suppress P-TEFb-dependent transcriptional elongation and tumorigenesis. Mol. Cell 29, 588–599 (2008).
Michels, A. A. et al. Binding of the 7SK snRNA turns the HEXIM1 protein into a P-TEFb (CDK9/cyclin T) inhibitor. EMBO J. 23, 2608–2619 (2004).
Muniz, L., Egloff, S., Ughy, B., Jády, B. E. & Kiss, T. Controlling cellular P-TEFb activity by the HIV-1 transcriptional transactivator Tat. PLoS Pathog. 6, e1001152 (2010).
Wassarman, D. A. & Steitz, J. A. Structural analyses of the 7SK ribonucleoprotein (RNP), the most abundant human small RNP of unknown function. Mol. Cell. Biol. 11, 3432–3445 (1991).
Marz, M. et al. Evolution of 7SK RNA and its protein partners in metazoa. Mol. Biol. Evol. 26, 2821–2830 (2009).
Yazbeck, A. M., Tout, K. R. & Stadler, P. F. Detailed secondary structure models of invertebrate 7SK RNAs. RNA Biol. 15, 158–164 (2018).
Uchikawa, E. et al. Structural insight into the mechanism of stabilization of the 7SK small nuclear RNA by LARP7. Nucleic Acids Res. 43, 3373–3388 (2015).
Eichhorn, C. D., Chug, R. & Feigon, J. hLARP7 C-terminal domain contains an xRRM that binds the 3′ hairpin of 7SK RNA. Nucleic Acids Res. 44, 9977–9989 (2016).
Eichhorn, C. D., Yang, Y., Repeta, L. & Feigon, J. Structural basis for recognition of human 7SK long noncoding RNA by the La-related protein Larp7. Proc. Natl Acad. Sci. USA 115, E6457–E6466 (2018).
Singh, R., Gupta, S. & Reddy, R. Capping of mammalian U6 small nuclear RNA in vitro is directed by a conserved stem-loop and AUAUAC sequence: conversion of a noncapped RNA into a capped RNA. Mol. Cell. Biol. 10, 939–946 (1990).
Cosgrove, M. S., Ding, Y., Rennie, W. A., Lane, M. J. & Hanes, S. D. The Bin3 RNA methyltransferase targets 7SK RNA to control transcription and translation. Wiley Interdiscip. Rev. RNA 3, 633–647 (2012).
Cheng, H., Sukal, S., Callender, R. & Leyh, T. S. γ-phosphate protonation and pH-dependent unfolding of the Ras.GTP.Mg2+ complex: a vibrational spectroscopy study. J. Biol. Chem. 276, 9931–9935 (2001).
Copeland, R. A Enzymes: a Practical Introduction to Structure, Mechanism, and Data Analysis. 188–265 (John Wiley & Sons: New York, 2000).
Husain, N. et al. Structural basis for the methylation of A1408 in 16S rRNA by a panaminoglycoside resistance methyltransferase NpmA from a clinical isolate and analysis of the NpmA interactions with the 30S ribosomal subunit. Nucleic Acids Res. 39, 1903–1918 (2011).
Shi, Y. Q. & Rando, R. R. Kinetic mechanism of isoprenylated protein methyltransferase. J. Biol. Chem. 267, 9547–9551 (1992).
Johnson, B. A. & Aswad, D. W. Kinetic properties of bovine brain protein L-isoaspartyl methyltransferase determined using a synthetic isoaspartyl peptide substrate. Neurochem. Res. 18, 87–94 (1993).
Swiercz, R., Person, M. D. & Bedford, M. T. Ribosomal protein S2 is a substrate for mammalian PRMT3 (protein arginine methyltransferase 3). Biochem. J. 386, 85–91 (2005).
Clarke, S. & Banfield, K. in Homocysteine in Health and Disease (eds. Carmel, R. & Jacobsen, D. W.) 63–78 (Cambridge University Press, Cambridge, 2001).
Turner, D. H. & Mathews, D. H. NNDB: the nearest neighbor parameter database for predicting stability of nucleic acid secondary structure. Nucleic Acids Res. 38, D280–D282 (2010).
Martinez, A. et al. Human BCDIN3D monomethylates cytoplasmic histidine transfer RNA. Nucleic Acids Res. 45, 5423–5436 (2017).
Xhemalce, B., Robson, S. C. & Kouzarides, T. Human RNA methyltransferase BCDIN3D regulates microRNA processing. Cell 151, 278–288 (2012).
Warda, A. S. et al. Human METTL16 is a N 6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 18, 2004–2014 (2017).
Pendleton, K. E. et al. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169, 824–835.e14 (2017).
Choi, S., Jung, C.-R., Kim, J.-Y. & Im, D.-S. PRMT3 inhibits ubiquitination of ribosomal protein S2 and together forms an active enzyme complex. Biochim. Biophys. Acta 1780, 1062–1069 (2008).
Didychuk, A. L., Butcher, S. E. & Brow, D. A. The life of U6 small nuclear RNA, from cradle to grave. RNA 24, 437–460 (2018).
Hussain, R. H., Zawawi, M. & Bayfield, M. A. Conservation of RNA chaperone activity of the human La-related proteins 4, 6 and 7. Nucleic Acids Res. 41, 8715–8725 (2013).
Bayfield, M. A., Yang, R. & Maraia, R. J. Conserved and divergent features of the structure and function of La and La-related proteins (LARPs). Biochim. Biophys. Acta 1799, 365–378 (2010).
Maraia, R. J., Mattijssen, S., Cruz-Gallardo, I. & Conte, M. R. The La and related RNA-binding proteins (LARPs): structures, functions, and evolving perspectives. Wiley Interdiscip. Rev. RNA 8, e1430 (2017).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr. 66, 213–221 (2010).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D. Biol. Crystallogr. 65, 1074–1080 (2009).
Kabsch, W. Xds. Acta Crystallogr. D. Biol. Crystallogr. 66, 125–132 (2010).
Painter, J. & Merritt, E. A. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D. Biol. Crystallogr. 62, 439–450 (2006).
Hodel, A., Kim, S.-H. & Brünger, A. T. Model bias in macromolecular crystal structures. Acta Crystallogr. A. 48, 851–858 (1992).
Dolinsky, T. J. et al. PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 35, W522–W525 (2007).
Baker, N. A., Sept, D., Joseph, S., Holst, M. J. & McCammon, J. A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041 (2001).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Lee, W., Tonelli, M. & Markley, J. L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31, 1325–1327 (2015).
Grzesiek, S. & Bax, A. The importance of not saturating water in protein NMR. Application to sensitivity enhancement and NOE measurements. J. Am. Chem. Soc. 115, 12593–12594 (1993).
Vinci, C. R. & Clarke, S. G. Recognition of age-damaged (R,S)-adenosyl-L-methionine by two methyltransferases in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 282, 8604–8612 (2007).
Shah, S. & Friedman, S. H. An ESI-MS method for characterization of native and modified oligonucleotides used for RNA interference and other biological applications. Nat. Protoc. 3, 351–356 (2008).
Jain, K., Jin, C. Y. & Clarke, S. G. Epigenetic control via allosteric regulation of mammalian protein arginine methyltransferases. Proc. Natl Acad. Sci. USA 114, 10101–10106 (2017).
Smietanski, M. et al. Structural analysis of human 2′-O-ribose methyltransferases involved in mRNA cap structure formation. Nat. Commun. 5, 3004 (2014).
Papadopoulos, J. S. & Agarwala, R. COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics 23, 1073–1079 (2007).
Robert, X. & Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 (2014).
Acknowledgements
This work was supported by NIH grant GM107567 to J.F. and American Cancer Society Postdoctoral Fellowship 126777-PF-14-179-01-DMC to C.D.E. We acknowledge NMR equipment grant NIH S10OD016336 and DOE grant DE-FC0302ER63421 for partial support of NMR and X-ray core facilities. The authors thank M. Capel, K. Rajashankar, N. Sukumar, F. Murphy, I. Kourinov, and J. Schuermann of Northeastern Collaborative Access Team (NE-CAT) beamline ID-24 at the Advanced Photon Source (APS) of Argonne National Laboratory, which are supported by NIH grants P41 RR015301 and P41 GM103403. Use of the APS is supported by DOE under contract DE-AC02-06CH11357. We thank Y. Chen for the help with mass spectrometry data collection and analysis in the UCLA MIC mass spectrometry facility, supported in part by NIH instrumentation grant 1S10OD016387; M. Collazo for help with crystal optimization; and K. Jain, J. Lowenson, and S. Clarke for helpful discussions.
Author information
Authors and Affiliations
Contributions
Y.Y. and C.D.E. designed and performed experiments, analyzed data, and wrote the paper; Y.W. prepared RNA samples; D.C. helped with X-ray data collection and processing; and J.F. supervised all aspects of the work, analyzed data, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3 and Supplementary Figures 1–14
Rights and permissions
About this article
Cite this article
Yang, Y., Eichhorn, C.D., Wang, Y. et al. Structural basis of 7SK RNA 5′-γ-phosphate methylation and retention by MePCE. Nat Chem Biol 15, 132–140 (2019). https://doi.org/10.1038/s41589-018-0188-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0188-z
This article is cited by
-
The cell biology of HIV-1 latency and rebound
Retrovirology (2024)
-
The methyl phosphate capping enzyme Bmc1/Bin3 is a stable component of the fission yeast telomerase holoenzyme
Nature Communications (2022)
-
Chemical reversible crosslinking enables measurement of RNA 3D distances and alternative conformations in cells
Nature Communications (2022)
-
A putative cap binding protein and the methyl phosphate capping enzyme Bin3/MePCE function in telomerase biogenesis
Nature Communications (2022)
-
An alternative D. melanogaster 7SK snRNP
BMC Molecular and Cell Biology (2021)