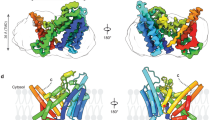
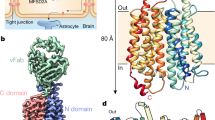
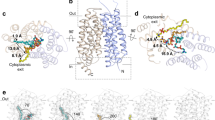
Abstract
Phosphatidylcholine and phosphatidylethanolamine, the two most abundant phospholipids in mammalian cells, are synthesized de novo by the Kennedy pathway from choline and ethanolamine, respectively1,2,3,4,5,6. Despite the essential roles of these lipids, the mechanisms that enable the cellular uptake of choline and ethanolamine remain unknown. Here we show that the protein encoded by FLVCR1, whose mutation leads to the neurodegenerative syndrome posterior column ataxia and retinitis pigmentosa7,8,9, transports extracellular choline and ethanolamine into cells for phosphorylation by downstream kinases to initiate the Kennedy pathway. Structures of FLVCR1 in the presence of choline and ethanolamine reveal that both metabolites bind to a common binding site comprising aromatic and polar residues. Despite binding to a common site, FLVCR1 interacts in different ways with the larger quaternary amine of choline in and with the primary amine of ethanolamine. Structure-guided mutagenesis identified residues that are crucial for the transport of ethanolamine, but dispensable for choline transport, enabling functional separation of the entry points into the two branches of the Kennedy pathway. Altogether, these studies reveal how FLVCR1 is a high-affinity metabolite transporter that serves as the common origin for phospholipid biosynthesis by two branches of the Kennedy pathway.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cryo-EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) under the accession codes EMD-42107 (choline-bound FLVCR1), EMD-42108 (ethanolamine-bound FLVCR1), EMD-42109 (endogenous choline-bound FLVCR1), EMD-42110 (endogenous choline-bound FLVCR1, from images collected in 1 mM ethanolamine) and EMD-42111 (endogenous ligand-bound FLVCR1). Atomic coordinates have been deposited in the Protein Data Bank (PDB) under the accession codes 8UBW (choline-bound FLVCR1), 8UBX (ethanolamine-bound FLVCR1), 8UBY (endogenous choline-bound FLVCR1), 8UBZ (endogenous choline-bound FLVCR1, from images collected in 1 mM ethanolamine) and 8UC0 (endogenous ligand-bound FLVCR1). The atomic coordinates of previously published structures of E. coli SotB (PDB 6KKL) in an inward-facing state and E. coli DgoT (PDB 6E9N) in an inward-facing state were used in this study. Co-dependencies between FLVCR1 and all genes computed from CRISPR DepMap Chronos 2023Q2 used in this study were downloaded from https://depmap.org/portal/all. Source data are provided with this paper.
Code availability
The code written to perform the FLVCR1 DepMap coessentiality analysis is available at https://github.com/artemkhan/Coessentiality_DepMAP_FLVCR1.git.
References
Vance, J. E. Phospholipid synthesis and transport in mammalian cells. Traffic 16, 1–18 (2015).
Patel, D. & Witt, S. N. Ethanolamine and phosphatidylethanolamine: partners in health and disease. Oxid. Med. Cell. Longev. 2017, 4829180 (2017).
Kent, C. Phospholipid metabolism in mammals. Encycl. Biol. Chem. 3, 314–320 (2004).
Kennedy, E. P. Sailing to Byzantium. Annu. Rev. Biochem. 61, 1–28 (1992).
Gibellini, F. & Smith, T. K. The Kennedy pathway–de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62, 414–428 (2010).
Kennedy, E. P. Synthesis of phosphatides in isolated mitochondria: II. Incorporation of choline into lecithin. J. Biol. Chem. 209, 525–535 (1954).
Yanatori, I., Yasui, Y., Miura, K. & Kishi, F. Mutations of FLVCR1 in posterior column ataxia and retinitis pigmentosa result in the loss of heme export activity. Blood Cells Mol. Dis. 49, 60–66 (2012).
Rajadhyaksha, A. M. et al. Mutations in FLVCR1 cause posterior column ataxia and retinitis pigmentosa. Am. J. Hum. Genet. 87, 643–654 (2010).
Khan, A. A. & Quigley, J. G. Heme and FLVCR-related transporter families SLC48 and SLC49. Mol. Aspects Med. 34, 669–682 (2013).
Corbin, K. D. & Zeisel, S. H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 28, 159–165 (2012).
Kenny, T. C. et al. Integrative genetic analysis identifies FLVCR1 as a plasma-membrane choline transporter in mammals. Cell Metab. https://doi.org/10.1016/j.cmet.2023.04.003 (2023).
Tsuchiya, M., Tachibana, N., Nagao, K., Tamura, T. & Hamachi, I. Organelle-selective click labeling coupled with flow cytometry allows pooled CRISPR screening of genes involved in phosphatidylcholine metabolism. Cell Metab. 35, 1072–1083 (2023).
Kvarnung, M. et al. Mutations in FLVCR2 associated with Fowler syndrome and survival beyond infancy. Clin. Genet. 89, 99–103 (2016).
Meyer, E. et al. Mutations in FLVCR2 are associated with proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome (Fowler syndrome). Am. J. Hum. Genet. 86, 471–478 (2010).
Lalonde, E. et al. Unexpected allelic heterogeneity and spectrum of mutations in Fowler syndrome revealed by next-generation exome sequencing. Hum. Mutat. 31, 918–923 (2010).
Drew, D., North, R. A., Nagarathinam, K. & Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 121, 5289–5335 (2021).
Sauve, S., Williamson, J., Polasa, A. & Moradi, M. Ins and outs of rocker switch mechanism in major facilitator superfamily of transporters. Membranes 13, 462 (2023).
Yan, N. Structural biology of the major facilitator superfamily transporters. Annu. Rev. Biophys. 44, 257–283 (2015).
Zhang, X. C., Zhao, Y., Heng, J. & Jiang, D. Energy coupling mechanisms of MFS transporters. Protein Sci. 24, 1560–1579 (2015).
Okuda, T. et al. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 3, 120–125 (2000).
Ferguson, S. M. et al. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc. Natl Acad. Sci. USA 101, 8762–8767 (2004).
Iwamoto, H., Blakely, R. D. & De Felice, L. J. Na+, Cl−, and pH dependence of the human choline transporter (hCHT) in Xenopus oocytes: the proton inactivation hypothesis of hCHT in synaptic vesicles. J. Neurosci. 26, 9851–9859 (2006).
Mödinger, Y., Schön, C., Wilhelm, M. & Hals, P.-A. Plasma kinetics of choline and choline metabolites after a single dose of SuperbaBoostTM krill oil or choline bitartrate in healthy volunteers. Nutrients 11, 2548 (2019).
Garguilo, M. G. & Michael, A. C. Amperometric microsensors for monitoring choline in the extracellular fluid of brain. J. Neurosci. Methods 70, 73–82 (1996).
Brehm, R., Lindmar, R. & Löffelholz, K. Muscarinic mobilization of choline in rat brain in vivo as shown by the cerebral arterio-venous difference of choline. J. Neurochem. 48, 1480–1485 (1987).
Bianchi, L. et al. Extracellular levels of amino acids and choline in human high grade gliomas: an intraoperative microdialysis study. Neurochem. Res. 29, 325–334 (2004).
Plagemann, P. G. Choline metabolism and membrane formation in rat hepatoma cells grown in suspension culture. 3. Choline transport and uptake by simple diffusion and lack of direct exchange with phosphatidylcholine. J. Lipid Res. 12, 715–724 (1971).
Oswald, C. et al. Crystal structures of the choline/acetylcholine substrate-binding protein ChoX from Sinorhizobium meliloti in the liganded and unliganded-closed states. J. Biol. Chem. 283, 32848–32859 (2008).
Bärland, N. et al. Mechanistic basis of choline import involved in teichoic acids and lipopolysaccharide modification. Sci. Adv. 8, eabm1122 (2022).
Holm, L. Dali server: structural unification of protein families. Nucleic Acids Res. 50, W210–W215 (2022).
Xiao, Q. et al. Visualizing the nonlinear changes of a drug-proton antiporter from inward-open to occluded state. Biochem. Biophys. Res. Commun. 534, 272–278 (2021).
Leano, J. B. et al. Structures suggest a mechanism for energy coupling by a family of organic anion transporters. PLoS Biol. 17, e3000260 (2019).
Quigley, J. G. et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell 118, 757–766 (2004).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576 (2017).
Wainberg, M. et al. A genome-wide atlas of co-essential modules assigns function to uncharacterized genes. Nat. Genet. 53, 638–649 (2021).
Jackson, B. T. Identification of metabolic networks by genetic co-essentiality analysis. Nat. Rev. Mol. Cell Biol. 24, 378 (2023).
Lykidis, A., Wang, J., Karim, M. A. & Jackowski, S. Overexpression of a mammalian ethanolamine-specific kinase accelerates the CDP-ethanolamine pathway. J. Biol. Chem. 276, 2174–2179 (2001).
Vermeulen, P. S., Geelen, M. J. H. & van Golde, L. M. G. Substrate specificity of CTP:phosphoethanolamine cytidylyltransferase purified from rat liver. Biochim. Biophys. Acta Lipids Lipid Metab. 1211, 343–349 (1994).
Taylor, A., Grapentine, S., Ichhpuniani, J. & Bakovic, M. Choline transporter-like proteins 1 and 2 are newly identified plasma membrane and mitochondrial ethanolamine transporters. J. Biol. Chem. 296, 100604 (2021).
Navale, A. M. & Paranjape, A. N. Glucose transporters: physiological and pathological roles. Biophys. Rev. 8, 5–9 (2016).
Goehring, A. et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat. Protoc. 9, 2574–2585 (2014).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Suloway, C. et al. Fully automated, sequential tilt-series acquisition with Leginon. J. Struct. Biol. 167, 11–18 (2009).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Scheres, S. H. W. in The Resolution Revolution: Recent Advances in cryoEM Vol. 579 (ed. Crowther, R. A.) 125–157 (Academic, 2016).
Terwilliger, T. C., Ludtke, S. J., Read, R. J., Adams, P. D. & Afonine, P. V. Improvement of cryo-EM maps by density modification. Nat. Methods 17, 923–927 (2020).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature 628, 450–457 (2024).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Pavelka, A. et al. CAVER: algorithms for analyzing dynamics of tunnels in macromolecules. IEEE/ACM Trans. Comput. Biol. Bioinform. 13, 505–517 (2016).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Acknowledgements
We thank M. J. de la Cruz and the Simons Electron Microscopy Center staff for help with data acquisition; the Memorial Sloan Kettering Cancer Center High Performance Computing group for assistance with data processing; the members of the laboratories for comments on the manuscript; and L. Finley for discussions. R.K.H. is supported by the National Institutes of Health (NIH) National Cancer Institute Cancer Center Support Grant P30-CA008748 and is a Searle Scholar. T.C.K. is supported by the NIH National Institute of Diabetes and Digestive and Kidney Diseases (F32 DK127836), the Shapiro-Silverberg Fund for the Advancement of Translational Research and a Merck Postdoctoral Fellowship at The Rockefeller University. K.B. is supported by the NIH National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK123323-01), and a Mark Foundation Emerging Leader Award, and is a Searle Scholar and a Pew-Stewart Scholar. Some of this work was carried out at the Simons Electron Microscopy Center at the New York Structural Biology Center, with major support from the Simons Foundation (SF349247).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.S. and R.K.H.; methodology: Y.S., T.C.K., A.K., K.B. and R.K.H.; formal analysis: Y.S. and R.K.H.; investigation, Y.S., T.C.K., K.B. and R.K.H.; writing (original draft): Y.S. and R.K.H.; funding acquisition, T.C.K., K.B. and R.K.H.
Corresponding author
Ethics declarations
Competing interests
R.K.H. is a consultant for F. Hoffmann-La Roche. K.B. is a scientific adviser to Nanocare Pharmaceuticals and Atavistik Bio. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Camilo Perez and Terry Smith for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Regulation of FLVCR1-mediated choline uptake.
a, Background subtracted uptake of 20 nM [methyl-3H]choline by vesicles containing FLVCR1 after 30 min in buffer at pH 4.5-10.5. Background was measured by uptake of 20 nM [methyl-3H]choline into the protein-free control liposomes. n = 3 technically independent samples. b, Background subtracted uptake of 20 nM [methyl-3H]choline by FLVCR1 proteoliposome after 30 min in buffer in the presence or absence of Na+, K+, Ca2+, or Mg2+. Background was measured by uptake of 20 nM [methyl-3H]choline into the protein-free control liposomes. n = 3 technically independent samples.
Extended Data Fig. 2 Validation of cryo-EM structures of FLVCR1.
a-c Representative cryo-EM images and two-dimensional class averages of human FLVCR1 in 1 mM choline (a), 1 mM ethanolamine (b) or without added substrate (c). Number of collected cryo-EM images are shown. d-h, Plots showing Fourier shell correlations between two independent half-maps (black) and between density-modified map and refined atomic model (red) for choline-bound FLVCR1 (d), ethanolamine-bound FLVCR1 (e), endogenous choline-bound FLVCR1 obtained from FLVCR1 incubated with 1 mM ethanolamine (f), endogenous choline-bound FLVCR1 obtained from FLVCR1 without exogenous ligand incubation (g), and endogenous ligand-bound FLVCR1 obtained from FLVCR1 without exogenous ligand incubation (h). Dashed lines are indicated at FSC = 0.5 and FSC = 0.143. i-m, Local resolution plots of choline-bound FLVCR1 (i), ethanolamine-bound FLVCR1 (j), endogenous-choline bound FLVCR1 obtained from FLVCR1 incubated with 1 mM ethanolamine (k), endogenous choline-bound FLVCR1 obtained from FLVCR1 without exogenous substrate incubation (l), and endogenous ligand-bound FLVCR1 obtained from FLVCR1 without exogenous ligand incubation (m).
Extended Data Fig. 3 Cryo-EM analysis of hFLVCR1.
Flow-chart summarizing Cryo-EM image acquisition and processing of human FLVCR1 without added substrate (a), in 1 mM choline (b), or 1 mM ethanolamine (c). Number of collected cryo-EM images and selected particles are shown.
Extended Data Fig. 4 Choline-bound FLVCR1 adopts an inward-facing conformation.
a, Superposition of TM1-6 and TM7-12. RMSD = 3.5 Å. b, Central cavity viewed from the cytosolic side. Aspartate and glutamate residues in and near the entrance to the central cavity are shown as sticks. c, Central section of choline-bound FLVCR1 is contiguous with choline shown as sticks. d, Blue spheres depict the minimum radius of the central cavity in the choline-bound state as a function of position. e-f, Superposition of choline-bound FLVCR1 (blue) with E. coli SotB (D; PDB: 6KKL; RMSD = 2.3 Å; red) (e) or E. coli DgoT (E; PDB: 6E9N; RMSD = 2.6 Å; cyan) (f).
Extended Data Fig. 5 Sequence alignment of SLC49A family members and identified disease-associated substitutions.
a, Sequence alignment of human FLVCR1 (SLC49A1), human FLVCR2 (SLC49A2), human MFSD7 (SLC49A3), human DIRC2 (SLC49A4) and Drosophila CG1358 performed using Clustal Omega54 and pyBoxshade (https://github.com/mdbaron42/pyBoxshade). Substrate-binding site residues are highlighted by red boxes. Residues whose substitution leads to PCARP or Fowler syndrome are highlighted by blue and green boxes, respectively. b, Structure of choline-bound FLVCR1 (left) and Alphafold2 model of FLVCR2 (right)55 with residues whose mutation leads to PCARP and Fowler syndrome highlighted in blue and green, respectively. FLVCR1 substrate binding site residues and the corresponding residues in FLVCR2 are highlighted in red.
Extended Data Fig. 6 Cryo-EM structures of FLVCR1 determined in a substrate-free condition.
a-b, Cryo-EM density maps and atomic models of FLVCR1 in endogenous choline (a) and endogenous ligand-bound (b) states. c-d, Central slice of the central cavity of choline-bound (c) and endogenous ligand-bound (d) states, with surface colored white. Choline is shown as sticks in c. Endogenous ligand is not modeled or shown in d. e, Superposition of choline-bound (grey) and endogenous choline-bound (blue) states. f, Superposition of substrate-binding sites in choline-bound (grey) and endogenous choline-bound (blue) states.
Extended Data Fig. 7 Cryo-EM structures of FLVCR1 determined in 1mM ethanolamine.
a-b, Cryo-EM density maps of endogenous choline-bound FLVCR1 (a) and ethanolamine-bound FLVCR1 (b) states, determined from particles imaged in the presence of 1 mM ethanolamine. c, Superposition of ethanolamine-bound (gold) and choline-bound (blue) states. d, Substrate-binding site in endogenous choline-bound FLVCR1 state, determined from particles imaged in the presence of 1 mM ethanolamine. Residues and modelled substrates are shown as sticks. Density is shown as a grey isosurface and contoured at 3.0 σ. e-f, Coordination of ethanolamine in the substrate-binding site in ethanolamine-bound state (e) and choline in the substrate-binding site in the choline-bound state (f). Polar interactions are shown as dashed lines and distances as solid lines.
Extended Data Fig. 8 FLVCR1 substrate-binding site mutants.
a, FSEC analysis of wild-type or substrate-binding site mutants fused to mCerulean expressed in FLVCR1-knockout HEK293T cells. b, Western blot analysis of FLVCR1-knockout HEK293T cells expressing a vector control or wild-type or mutant FLVCR1 cDNA. GAPDH was ran on a separate gel as sample processing controls. For western blot source data, see Supplementary Fig. 1. c, Cumulative log2 fold change in cell number of HEK293T cells and FLVCR1-knockout HEK293T cells expressing a vector control or wild-type or mutant FLVCR1 cDNA. n = 3 biologically independent samples. (P-values: HEK293T WT and HEK293T FLVCR1 KO + FLVCR1 WT cDNA; p = 3x10−3, HEK293T FLVCR1 KO + FLVCR1 WT cDNA and HEK293T FLVCR1 KO + FLVCR1 Q214A cDNA; p = 9x10−1, HEK293T FLVCR1 KO + Vector and HEK293T FLVCR1 KO + FLVCR1 W125A cDNA; p = 9x10−1, HEK293T FLVCR1 KO + Vector and HEK293T FLVCR1 KO + FLVCR1 Y153A cDNA; p = 1x10−1, HEK293T FLVCR1 KO + FLVCR1 WT cDNA and HEK293T FLVCR1 KO + Vector; p = 8x10−5).
Extended Data Fig. 9 Incorporation of labeled choline into betaine requires FLVCR1.
Schematic for tracing [1,2-13C2] choline into betaine (left) and the abundance of betaine M + 2 after incubation with 21.5 µM [1,2-13C2] choline for 1 h in FLVCR1-knockout HEK293T cells expressing a vector control or wild-type or mutant FLVCR1 cDNA (right). n = 3 biologically independent samples.
Supplementary information
Supplementary Fig. 1
Raw, uncropped western blot image of Extended Data Fig. 8b. GAPDH was run on a separate gel as a sample processing control.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Son, Y., Kenny, T.C., Khan, A. et al. Structural basis of lipid head group entry to the Kennedy pathway by FLVCR1. Nature 629, 710–716 (2024). https://doi.org/10.1038/s41586-024-07374-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07374-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.