Abstract
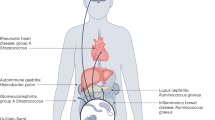
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disorder that primarily affects the joints. One hypothesis for the pathogenesis of RA is that disease begins at mucosal sites as a consequence of interactions between the mucosal immune system and an aberrant local microbiota, and then transitions to involve the synovial joints. Alterations in the composition of the microbial flora in the lungs, mouth and gut in individuals with preclinical and established RA suggest a role for mucosal dysbiosis in the development and perpetuation of RA, although establishing whether these alterations are the specific consequence of intestinal involvement in the setting of a systemic inflammatory process, or whether they represent a specific localization of disease, is an ongoing challenge. Data from mouse models of RA and investigations into the preclinical stages of disease also support the hypothesis that these alterations to the microbiota predate the onset of disease. In addition, several therapeutic options widely used for the treatment of RA are associated with alterations in intestinal microbiota, suggesting that modulation of intestinal microbiota and/or intestinal barrier function might be useful in preventing or treating RA.
Key points
-
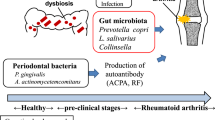
Alterations in the composition of the microbial flora occurs in individuals in the preclinical stages of rheumatoid arthritis (RA) and in those with established RA.
-
DMARDs modify the intestinal microbial composition in patients with RA.
-
Subclinical gut inflammation occurs in some patients with RA and is associated with altered intestinal permeability.
-
Zonulin family peptides are mediators of altered intestinal permeability in RA and their inhibition ameliorates the severity of arthritis in mouse models of disease.
-
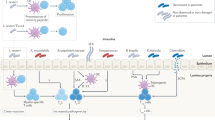
Dysbiosis and altered intestinal permeability could induce chronic activation of innate immune cells.
-
Recirculation of innate immune cells from the gut to the peripheral joints has the potential to support the chronic inflammatory process in at least some patients with RA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aletaha, D. & Smolen, J. S. Diagnosis and management of rheumatoid arthritis. JAMA 320, 1360–1372 (2018).
Catrina, A. I., Deane, K. D. & Scher, J. U. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology 55, 391–402 (2016).
Scher, J. U. et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome 4, 60 (2016).
Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 (2013).
Holers, V. M. et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat. Rev. Rheumatol. 14, 542–557 (2018).
Wells, P. M. et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: a cross-sectional study. Lancet Rheumatol. 2, e418–e427 (2020).
Zhang, X. et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21, 895–905 (2015).
Chen, J. et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 8, 43 (2016).
Marietta, E. V. et al. Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheumatol. 68, 2878–2888 (2016).
Maeda, Y. et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol. 68, 2646–2661 (2016).
Alpizar-Rodriguez, D. et al. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 78, 590–593 (2019).
Inamo, J. Non-causal association of gut microbiome on the risk of rheumatoid arthritis: A Mendelian randomisation study. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2019-216565 (2019).
Alpizar Rodriguez, D., Lesker, T. R., Gilbert, B., Strowig, T. & Finckh, A. Intestinal dysbiosis in RA development: difficulty of establishing causality. Response to: ‘Non-causal association of gut microbiome on the risk of rheumatoid arthritis: a Mendelian randomisation study’ by Inamo. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2019-216637 (2019).
Jeong, Y. et al. Gut microbial composition and function are altered in patients with early rheumatoid arthritis. J. Clin. Med. 8, 693 (2019).
[No authors listed]. News & highlights. Mucosal Immunol. 1, 246–247 (2008).
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. & Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232 (2017).
Quirke, A. M. et al. Bronchiectasis is a model for chronic bacterial infection inducing autoimmunity in rheumatoid arthritis. Arthritis Rheumatol. 67, 2335–2342 (2015).
Bergot, A.-S., Giri, R. & Thomas, R. The microbiome and rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 33, 101497 (2019).
Clarke, A. et al. Heightened autoantibody immune response to citrullinated calreticulin in bronchiectasis: implications for rheumatoid arthritis. Int. J. Biochem. Cell Biol. 89, 199–206 (2017).
Potempa, J., Mydel, P. & Koziel, J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 13, 606–620 (2017).
Mariette, X. et al. Role of good oral hygiene on clinical evolution of rheumatoid arthritis: a randomized study nested in the ESPOIR cohort. Rheumatology 59, 988–996 (2020).
Horta-Baas, G. et al. Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. J. Immunol. Res. 2017, 4835189 (2017).
Salem, F. et al. Gut microbiome in chronic rheumatic and inflammatory bowel diseases: similarities and differences. United European Gastroenterol. J. 7, 1008–1032 (2019).
Rogier, R. et al. Alteration of the intestinal microbiome characterizes preclinical inflammatory arthritis in mice and its modulation attenuates established arthritis. Sci. Rep. 7, 15613 (2017).
Jubair, W. K. et al. Modulation of inflammatory arthritis in mice by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheumatol. 70, 1220–1233 (2018).
Aa, L.-X. et al. Rebalancing of the gut flora and microbial metabolism is responsible for the anti-arthritis effect of kaempferol. Acta Pharmacol. Sin. 41, 73–81 (2020).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Eason, R. J. et al. The helminth product, ES-62 modulates dendritic cell responses by inducing the selective autophagolysosomal degradation of TLR-transducers, as exemplified by PKCδ. Sci. Rep. 6, 37276 (2016).
Doonan, J. et al. The parasitic worm product ES-62 normalises the gut microbiota bone marrow axis in inflammatory arthritis. Nat. Commun. 10, 1554 (2019).
Wu, H.-J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Atarashi, K. et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 (2008).
Horai, R. et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 191, 313–320 (2000).
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Rogier, R. et al. Aberrant intestinal microbiota due to IL-1 receptor antagonist deficiency promotes IL-17- and TLR4-dependent arthritis. Microbiome 5, 63 (2017).
Zhang, Y., Li, Y., Lv, T.-T., Yin, Z.-J. & Wang, X.-B. Elevated circulating Th17 and follicular helper CD4+ T cells in patients with rheumatoid arthritis. APMIS 123, 659–666 (2015).
Alunno, A. et al. Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediators Inflamm. 2015, 751793 (2015).
Pianta, A. et al. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 69, 964–975 (2017).
Chiang, H.-I. et al. An association of gut microbiota with different phenotypes in Chinese patients with rheumatoid arthritis. J. Clin. Med. 8, 1770 (2019).
Van Delft, M. A. M., Van Der Woude, D., Toes, R. E. M. & Trouw, L. A. Secretory form of rheumatoid arthritis-associated autoantibodies in serum are mainly of the IgM isotype, suggesting a continuous reactivation of autoantibody responses at mucosal surfaces. Ann. Rheum. Dis. 78, 146–148 (2019).
Rios, D. et al. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 9, 907–916 (2016).
Jubair, W. et al. Intestinal inflammation and netosis associate with the presence of stool IgA ACPA in subjects at-risk for RA [abstract]. Arthritis Rheumatol. 70 (Suppl. 10), 67 (2018).
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412 (2013).
Johnson, B. M. et al. Gut microbiota differently contributes to intestinal immune phenotype and systemic autoimmune progression in female and male lupus-prone mice. J. Autoimmun. 108, 102420 (2020).
Gomez, A., Luckey, D. & Taneja, V. The gut microbiome in autoimmunity: sex matters. Clin. Immunol. 159, 154–162 (2015).
Gomez, A. et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS ONE 7, e36095 (2012).
Gonçalves dos Santos, G. et al. The neuropathic phenotype of the K/BxN transgenic mouse with spontaneous arthritis: pain, nerve sprouting and joint remodeling. Sci. Rep. 10, 15596 (2020).
Taneja, V. et al. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum. 56, 69–78 (2007).
Behrens, M. et al. Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice. J. Autoimmun. 35, 1–9 (2010).
Sun, Y. et al. Characteristics of gut microbiota in patients with rheumatoid arthritis in Shanghai, China. Front. Cell. Infect. Microbiol. 9, 369 (2019).
Lee, Y. K. & Mazmanian, S. K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 330, 1768–1773 (2010).
Sommer, F. & Bäckhed, F. The gut microbiota — masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013).
Burrello, C. et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat. Commun. 9, 5184 (2018).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Lleal, M. et al. A single faecal microbiota transplantation modulates the microbiome and improves clinical manifestations in a rat model of colitis. EBioMedicine 48, 630–641 (2019).
Tajik, N. et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 11, 1995 (2020).
Liu, X. et al. Role of the gut microbiome in modulating arthritis progression in mice. Sci. Rep. 6, 30594 (2016).
Cypers, H. et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann. Rheum. Dis. 75, 1357–1362 (2016).
Hindryckx, P. et al. Subclinical gut inflammation in spondyloarthritis is associated with a pro-angiogenic intestinal mucosal phenotype. Ann. Rheum. Dis. 70, 2044–2048 (2011).
De Vos, M., Mielants, H., Cuvelier, C., Elewaut, A. & Veys, E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology 110, 1696–1703 (1996).
Mielants, H., Veys, E. M., Cuvelier, C., De Vos, M. & Botelberghe, L. HLA-B27 related arthritis and bowel inflammation. Part 2. Ileocolonoscopy and bowel histology in patients with HLA-B27 related arthritis. J. Rheumatol. 12, 294–298 (1985).
Mielants, H. et al. The evolution of spondyloarthropathies in relation to gut histology. I. Clinical aspects. J. Rheumatol. 22, 2266–2272 (1995).
Schatteman, L. et al. Gut inflammation in psoriatic arthritis: a prospective ileocolonoscopic study. J. Rheumatol. 22, 680–683 (1995).
Ciccia, F. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 74, 1739–1747 (2015).
Ciccia, F. et al. Proinflammatory CX3CR1+CD59+ tumor necrosis factor-like molecule 1A+interleukin-23+ monocytes are expanded in patients with ankylosing spondylitis and modulate innate lymphoid cell 3 immune functions. Arthritis Rheumatol. 70, 2003–2013 (2018).
Viladomiu, M. et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci. Transl Med. 9, eaaf9655 (2017).
Nissinen, R. et al. Immune activation in the small intestine in patients with rheumatoid arthritis. Ann. Rheum. Dis. 63, 1327–1330 (2004).
Marcolongo, R., Bayeli, P. F. & Montagnani, M. Gastrointestinal involvement in rheumatoid arthritis: a biopsy study. J. Rheumatol. 6, 163–173 (1979).
Porzio, V. et al. Intestinal histological and ultrastructural inflammatory changes in spondyloarthropathy and rheumatoid arthritis. Scand. J. Rheumatol. 26, 92–98 (1997).
Bae, J. M., Choo, J. Y., Kim, K. J. & Park, K. S. Association of inflammatory bowel disease with ankylosing spondylitis and rheumatoid arthritis: a nationwide population-based study. Mod. Rheumatol. 27, 435–440 (2017).
Nguyen, Y. et al. Chronic diarrhoea and risk of rheumatoid arthritis: findings from the French E3N-EPIC Cohort Study. Rheumatology 59, 3767–3775 (2020).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Neurath, M. F. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 17, 76–77 (2019).
Vrakas, S. et al. Intestinal bacteria composition and translocation of bacteria in inflammatory bowel disease. PLoS ONE 12, e0170034 (2017).
Smith, M. D., Gibson, R. A. & Brooks, P. M. Abnormal bowel permeability in ankylosing spondylitis and rheumatoid arthritis. J. Rheumatol. 12, 299–305 (1985).
Fasano, A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 9, 69 (2020).
Fasano, A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 10, 1096–1100 (2012).
Thomas, K. E., Sapone, A., Fasano, A. & Vogel, S. N. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in celiac disease. J. Immunol. 176, 2512–2521 (2006).
Clemente, M. G. et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 52, 218–223 (2003).
Sturgeon, C. & Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 4, e1251384 (2016).
Drago, S. et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 41, 408–419 (2006).
Ciccia, F. et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann. Rheum. Dis. 76, 1123–1132 (2017).
El Asmar, R. et al. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123, 1607–1615 (2002).
Evans-Marin, H. et al. Microbiota-dependent involvement of Th17 cells in murine models of inflammatory arthritis. Arthritis Rheumatol. 70, 1971–1983 (2018).
Sato, K. et al. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci. Rep. 7, 6955 (2017).
Flak, M. B. et al. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI insight 4, e125191 (2019).
Arimatsu, K. et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 4, 4828 (2014).
Nakajima, M. et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of Enterobacteria to the liver. PLoS ONE 10, e0134234 (2015).
Elkan, A.-C. et al. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res. Ther. 10, R34 (2008).
Hafstrom, I. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: the effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 40, 1175–1179 (2001).
Lammers, K. M. et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135, 194–204.e3 (2008).
Shimada, S. et al. Involvement of gliadin, a component of wheat gluten, in increased intestinal permeability leading to non-steroidal anti-inflammatory drug-induced small-intestinal damage. PLoS ONE 14, e0211436 (2019).
Tagesson, C. & Bengtsson, A. Intestinal permeability to different-sized polyethyleneglycols in patients with rheumatoid arthritis. Scand. J. Rheumatol. 12, 124–128 (1983).
Jenkins, R. T., Rooney, P. J., Jones, D. B., Bienenstock, J. & Goodacre, R. L. Increased intestinal permeability in patients with rheumatoid arthritis: a side-effect of oral nonsteroidal anti-inflammatory drug therapy? Rheumatology 26, 103–107 (1987).
Mielants, H. et al. Intestinal mucosal permeability in inflammatory rheumatic diseases. I. Role of antiinflammatory drugs. J. Rheumatol. 18, 389–393 (1991).
Bjarnason, I. et al. Intestinal permeability and inflammation in rheumatoid arthritis: effects of non-steroidal anti-inflammatory drugs. Lancet 324, 1171–1174 (1984).
Sigthorsson, G. et al. Intestinal permeability and inflammation in patients on NSAIDs. Gut 43, 506–511 (1998).
Mielants, H. et al. Intestinal mucosal permeability in inflammatory rheumatic diseases. II. Role of disease. J. Rheumatol. 18, 394–400 (1991).
Rodríguez-Lagunas, M. J., Martín-Venegas, R., Moreno, J. J. & Ferrer, R. PGE2 promotes Ca2+-mediated epithelial barrier disruption through EP1 and EP4 receptors in Caco-2 cell monolayers. Am. J. Physiol. Physiol. 299, C324–C334 (2010).
Teng, F. et al. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity 44, 875–888 (2016).
Nielen, M. M. J. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 50, 380–386 (2004).
Rantapää-Dahlqvist, S. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48, 2741–2749 (2003).
Shi, J. et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann. Rheum. Dis. 73, 780–783 (2013).
Demoruelle, M. K. et al. Brief report: Airways abnormalities and rheumatoid arthritis–related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 64, 1756–1761 (2011).
Gizinski, A. M. et al. Rheumatoid arthritis (RA)-specific autoantibodies in patients with interstitial lung disease and absence of clinically apparent articular RA. Clin. Rheumatol. 28, 611–613 (2009).
Klareskog, L. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA–DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 54, 38–46 (2006).
Botton, E., Saraux, A., Laselve, H., Jousse, S. & Le Goff, P. Musculoskeletal manifestations in cystic fibrosis. Joint Bone Spine 70, 327–335 (2003).
Elkayam, O., Segal, R., Lidgi, M. & Caspi, D. Positive anti-cyclic citrullinated proteins and rheumatoid factor during active lung tuberculosis. Ann. Rheum. Dis. 65, 1110–1112 (2006).
Thé, J. & Ebersole, J. L. Rheumatoid factor (RF) distribution in periodontal disease. J. Clin. Immunol. 11, 132–142 (1991).
Kelmenson, L. B. et al. Timing of elevations of autoantibody isotypes prior to diagnosis of rheumatoid arthritis. Arthritis Rheumatol. 72, 251–261 (2020).
Hvatum, M., Kanerud, L., Hällgren, R. & Brandtzaeg, P. The gut-joint axis: cross reactive food antibodies in rheumatoid arthritis. Gut 55, 1240–1247 (2006).
Salmi, M., Andrew, D. P., Butcher, E. C. & Jalkanen, S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J. Exp. Med. 181, 137–149 (1995).
May, E. et al. Identical T-cell expansions in the colon mucosa and the synovium of a patient with enterogenic spondyloarthropathy. Gastroenterology 119, 1745–1755 (2000).
Trollmo, C., Verdrengh, M. & Tarkowski, A. Fasting enhances mucosal antigen specific B cell responses in rheumatoid arthritis. Ann. Rheum. Dis. 56, 130–134 (1997).
Mauro, D., Macaluso, F., Fasano, S., Alessandro, R. & Ciccia, F. ILC3 in axial spondyloarthritis: the gut angle. Curr. Rheumatol. Rep. 21, 37 (2019).
Simoni, Y. et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity 46, 148–161 (2017).
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
Longman, R. S. et al. CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 211, 1571–1583 (2014).
Mauro, D. & Ciccia, F. Gut dysbiosis in spondyloarthritis: cause or effect? Best Pract. Res. Clin. Rheumatol. 33, 101493 (2020).
Li, S., Bostick, J. W. & Zhou, L. Regulation of innate lymphoid cells by aryl hydrocarbon receptor. Front. Immunol. 8, 1909 (2017).
Kim, S.-H., Cho, B.-H., Kiyono, H. & Jang, Y.-S. Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal Peyer’s patches. Sci. Rep. 7, 3980 (2017).
Chun, E. et al. Metabolite-sensing receptor Ffar2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity 51, 871–884.e6 (2019).
Soare, A. et al. Cutting edge: Homeostasis of innate lymphoid cells is imbalanced in psoriatic arthritis. J. Immunol. 200, 1249–1254 (2018).
Cuthbert, R. J. et al. Brief report: Group 3 innate lymphoid cells in human enthesis. Arthritis Rheumatol. 69, 1816–1822 (2017).
Rodríguez-Carrio, J. et al. Brief report: Altered innate lymphoid cell subsets in human lymph node biopsy specimens obtained during the at-risk and earliest phases of rheumatoid arthritis. Arthritis Rheumatol. 69, 70–76 (2017).
Fang, W., Zhang, Y. & Chen, Z. Innate lymphoid cells in inflammatory arthritis. Arthritis Res. Ther. 22, 25 (2020).
Takaki-Kuwahara, A. et al. CCR6+ group 3 innate lymphoid cells accumulate in inflamed joints in rheumatoid arthritis and produce Th17 cytokines. Arthritis Res. Ther. 21, 198 (2019).
Ren, J., Feng, Z., Lv, Z., Chen, X. & Li, J. Natural killer-22 cells in the synovial fluid of patients with rheumatoid arthritis are an innate source of interleukin 22 and tumor necrosis factor-α. J. Rheumatol. 38, 2112–2118 (2011).
Toubal, A., Nel, I., Lotersztajn, S. & Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 19, 643–657 (2019).
Toussirot, E. & Saas, P. MAIT cells: potent major cellular players in the IL-17 pathway of spondyloarthritis? RMD Open 4, e000821 (2018).
Gracey, E. et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann. Rheum. Dis. 75, 2124–2132 (2016).
Treiner, E. Mucosal-associated invariant T cells in inflammatory bowel diseases: bystanders, defenders, or offenders? Front. Immunol. 6, 27 (2015).
Koppejan, H. et al. Altered composition and phenotype of mucosal-associated invariant T cells in early untreated rheumatoid arthritis. Arthritis Res. Ther. 21, 3 (2019).
Leeansyah, E. et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 121, 1124–1135 (2013).
Cho, Y.-N. et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J. Immunol. 193, 3891–3901 (2014).
Kim, M. et al. TNFα and IL-1β in the synovial fluid facilitate mucosal-associated invariant T (MAIT) cell migration. Cytokine 99, 91–98 (2017).
Kurioka, A., Walker, L. J., Klenerman, P. & Willberg, C. B. MAIT cells: new guardians of the liver. Clin. Transl Immunol. 5, e98 (2016).
Nowotschin, S. & Hadjantonakis, A.-K. Use of KikGR a photoconvertible green-to-red fluorescent protein for cell labeling and lineage analysis in ES cells and mouse embryos. BMC Dev. Biol. 9, 49 (2009).
Tsutsui, H., Karasawa, S., Shimizu, H., Nukina, N. & Miyawaki, A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 6, 233–238 (2005).
Proietti, M. et al. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity 41, 789–801 (2014).
Felix, K. M. et al. P2RX7 deletion in T cells promotes autoimmune arthritis by unleashing the Tfh cell response. Front. Immunol. 10, 411 (2019).
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L. & Falzoni, S. The P2X7 receptor in infection and inflammation. Immunity 47, 15–31 (2017).
Gattorno, M. et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 58, 1505–1515 (2008).
Lucas, C., Perdriger, A. & Amé, P. Definition of B cell helper T cells in rheumatoid arthritis and their behavior during treatment. Semin. Arthritis Rheum. 50, 867–872 (2020).
Cao, G. et al. An imbalance between blood CD4+CXCR5+Foxp3+ Tfr cells and CD4+CXCR5+ Tfh cells may contribute to the immunopathogenesis of rheumatoid arthritis. Mol. Immunol. 125, 1–8 (2020).
Bates, N. A. et al. Gut commensal segmented filamentous bacteria fine-tune T follicular regulatory cells to modify the severity of systemic autoimmune arthritis. J. Immunol. https://doi.org/10.4049/jimmunol.2000663 (2021).
Stone, M., Fortin, P. R., Pacheco-Tena, C. & Inman, R. D. Should tetracycline treatment be used more extensively for rheumatoid arthritis? Metaanalysis demonstrates clinical benefit with reduction in disease activity. J. Rheumatol. 30, 2112–2122 (2003).
Amin, A. R. et al. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc. Natl Acad. Sci. USA 93, 14014–14019 (1996).
Zaura, E. et al. Same exposure but two radically different responses to antibiotics: Resilience of the salivary microbiome versus long-term microbial shifts in feces. mBio 6, e01693-15 (2015).
Toivanen, P. et al. Intestinal anaerobic bacteria in early rheumatoid arthritis (RA) [abstract]. Arthritis Res. 4 (Suppl. 1), 5 (2002).
Picchianti-Diamanti, A. et al. Analysis of gut microbiota in rheumatoid arthritis patients: disease-related dysbiosis and modifications induced by etanercept. Int. J. Mol. Sci. 19, 2938 (2018).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Bolin, J. T., Filman, D. J., Matthews, D. A., Hamlin, R. C. & Kraut, J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 Aθ resolution. I. General features and binding of methotrexate. J. Biol. Chem. 257, 13650–13662 (1982).
Scher, J. U., Nayak, R. R., Ubeda, C., Turnbaugh, P. J. & Abramson, S. B. Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat. Rev. Rheumatol. 16, 282–292 (2020).
Krook, A. Effect of metronidazole and sulfasalazine on the normal human faecal flora. Scand. J. Gastroenterol. 16, 587–592 (1981).
Abdollahi-Roodsaz, S., Abramson, S. B. & Scher, J. U. The metabolic role of the gut microbiota in health and rheumatic disease: mechanisms and interventions. Nat. Rev. Rheumatol. 12, 446–455 (2016).
Neumann, V. C., Shinebaum, R., Cooke, E. M. & Wright, V. Effects of sulphasalazine on faecal flora in patients with rheumatoid arthritis: a comparison with penicillamine. Rheumatology 26, 334–337 (1987).
Kanerud, L., Scheynius, A., Nord, C. E. & Hafström, I. Effect of sulphasalazine on gastrointestinal microflora and on mucosal heat shock protein expression in patients with rheumatoid arthritis. Rheumatology 33, 1039–1048 (1994).
Wang, B., He, Y., Tang, J., Ou, Q. & Lin, J. Alteration of the gut microbiota in tumor necrosis factor-α antagonist-treated collagen-induced arthritis mice. Int. J. Rheum. Dis. 23, 472–479 (2020).
Forbes, J. D. et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases – does a common dysbiosis exist? Microbiome 6, 221 (2018).
Aqaeinezhad Rudbane, S. M. et al. The efficacy of probiotic supplementation in rheumatoid arthritis: a meta-analysis of randomized, controlled trials. Inflammopharmacology 26, 67–76 (2018).
Sköldstam, L., Hagfors, L. & Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 62, 208–214 (2003).
Kjeldsen-Kragh, J. et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 338, 899–902 (1991).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Häger, J. et al. The role of dietary fiber in rheumatoid arthritis patients: a feasibility study. Nutrients 11, 2392 (2019).
Zhang, J. et al. Autophagy and mitochondrial dysfunction in adjuvant-arthritis rats treatment with resveratrol. Sci. Rep. 6, 32928 (2016).
Khojah, H. M., Ahmed, S., Abdel-Rahman, M. S. & Elhakeim, E. H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: a clinical study. Clin. Rheumatol. 37, 2035–2042 (2018).
Alrafas, H. R., Busbee, P. B., Nagarkatti, M. & Nagarkatti, P. S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 106, 467–480 (2019).
Naskar, D., Teng, F., Felix, K. M., Bradley, C. P. & Wu, H.-J. J. Synthetic retinoid AM80 ameliorates lung and arthritic autoimmune responses by inhibiting T follicular helper and Th17 cell responses. J. Immunol. 198, 1855–1864 (2017).
Ruane, D. T. & Lavelle, E. C. The role of CD103+ dendritic cells in the intestinal mucosal immune system. Front. Immunol. 2, 25 (2011).
Collins, J., Auchtung, J. M., Schaefer, L., Eaton, K. A. & Britton, R. A. Humanized microbiota mice as a model of recurrent Clostridium difficile disease. Microbiome 3, 35 (2015).
Tan, T. G. et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc. Natl Acad. Sci. USA 113, E8141–E8150 (2016).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Vaahtovuo, J., Munukka, E., Korkeamäki, M., Luukkainen, R. & Toivanen, P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 35, 1500–1505 (2008).
Breban, M. et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann. Rheum. Dis. 76, 1614–1622 (2017).
Kishikawa, T. et al. Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population. Ann. Rheum. Dis. 79, 103–111 (2020).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks V. Taneja, A. Finckh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaiss, M.M., Joyce Wu, HJ., Mauro, D. et al. The gut–joint axis in rheumatoid arthritis. Nat Rev Rheumatol 17, 224–237 (2021). https://doi.org/10.1038/s41584-021-00585-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00585-3
This article is cited by
-
Comprehensive analysis revealed the immunoinflammatory targets of rheumatoid arthritis based on intestinal flora, miRNA, transcription factors, and RNA-binding proteins databases, GSEA and GSVA pathway observations, and immunoinfiltration typing
Hereditas (2024)
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
BMC Medicine (2024)
-
Impaired immune tolerance mediated by reduced Tfr cells in rheumatoid arthritis linked to gut microbiota dysbiosis and altered metabolites
Arthritis Research & Therapy (2024)
-
Associations of cereal fiber intake with rheumatoid arthritis mediated by dietary inflammatory index: insights from NHANES 2011–2020
Scientific Reports (2024)
-
Exploring the mechanism of Celastrol in the treatment of rheumatoid arthritis based on systems pharmacology and multi-omics
Scientific Reports (2024)