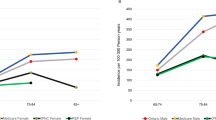
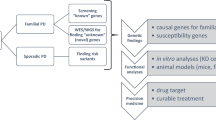
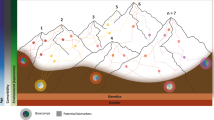
Abstract
The term ‘endemic parkinsonism’ refers to diseases that manifest with a dominant parkinsonian syndrome, which can be typical or atypical, and are present only in a particular geographically defined location or population. Ten phenotypes of endemic parkinsonism are currently known: three in the Western Pacific region; two in the Asian-Oceanic region; one in the Caribbean islands of Guadeloupe and Martinique; and four in Europe. Some of these disease entities seem to be disappearing over time and therefore are probably triggered by unique environmental factors. By contrast, other types persist because they are exclusively genetically determined. Given the geographical clustering and potential overlap in biological and clinical features of these exceptionally interesting diseases, this Review provides a historical reference text and offers current perspectives on each of the 10 phenotypes of endemic parkinsonism. Knowledge obtained from the study of these disease entities supports the hypothesis that both genetic and environmental factors contribute to the development of neurodegenerative diseases, not only in endemic parkinsonism but also in general. At the same time, this understanding suggests useful directions for further research in this area.
Key points
-
Existing definitions and classification schemes for endemic parkinsonism all have limitations.
-
Foci of endemic parkinsonism are clustered by geographic region as well as clinical features.
-
Endemic parkinsonism has a highly heterogeneous pathological background.
-
Some clusters of endemic parkinsonism are associated with exposure to neurotoxic environmental factors.
-
Other clusters of endemic parkinsonism have a primarily genetic cause.
-
Further study of endemic parkinsonism could illuminate future research into neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nuytemans, K. et al. Founder mutation p.R1441C in the leucine-rich repeat kinase 2 gene in Belgian Parkinson’s disease patients. Eur. J. Hum. Genet. 16, 471–479 (2008).
Plato, C. H. C. et al. Amyotrophic lateral sclerosis and parkinsonism–dementia complex of Guam: changing incidence rates during the past 60 years. Am. J. Epidemiol. 157, 149–157 (2003).
Spencer, P. S., Nunn, P. B., Hugon, J., Ludolph, A. & Roy, D. N. Motorneurone disease on Guam: possible role of a food neurotoxin. Lancet 1, 965 (1985).
Spencer, P. et al. Guam amyotrophic lateral sclerosis–parkinsonism–dementia linked to a plant excitant neurotoxin. Science 237, 517–522 (1987).
Spencer, P. S. et al. Discovery and partial characterization of primate motor-system toxins. Ciba Found. Symp. 126, 221–238 (1987).
Cox, P. A., Davis, D. A., Mash, D. C. & Metcalf, J. S. Do vervets and macaques respond differently to BMAA? Neurotoxicology 57, 310–311 (2016).
Spencer, P. S., Garner, C. E., Palmer, V. S. & Kisby, G. E. Vervets and macaques: similarities and differences in their responses to l-BMAA. Neurotoxicology 56, 284–286 (2016).
Cox, P. A. & Sacks, O. W. Cycad neurotoxins, consumption of flying foxes and ALS-PDC disease in Guam. Neurology 58, 976–979 (2002).
Marler, T. E., Lee, V. & Shaw, C. Cycad toxins and neurological disorders on Guam: defining theoretical and experimental standards for correlating human disease with environmental toxins. Hortscience 40, 1598–1606 (2005).
Spencer, P. S., Ohta, M. & Palmer, V. S. Cycad use and motor neurone disease in Kii peninsula of Japan. Lancet 2, 1462–1463 (1987).
Spencer, P. S., Palmer, V. S., Herman, A. & Asmedi, A. Cycad use and motor neurone disease in Irian Jaya. Lancet 2, 1273–1274 (1987).
Caparros-Lefebvre, D., Steele, J., Kotake, Y. & Ohta, S. Geographic isolates of atypical parkinsonism and tauopathy in the tropics: possible synergy of neurotoxins. Mov. Disord. 21, 1769–1770 (2006).
Lannuzel, A., Ruberg, M. & Michel, P. P. Atypical parkinsonism in the Caribbean island of Guadeloupe: etiological role of the mitochondrial complex I inhibitor annonacin. Mov. Disord. 23, 2122–2128 (2008).
Zimmerman, H. M. Progress report of work in the laboratory of pathology during May, 1945. Guam, US Naval Medical Research Unit number 2, June 1 (Unpublished Navy Memorandum, Sealed ‘Secret’) (Department of the Navy, 1945).
Mulder, D. W., Kurland, L. T. & Iriarte, L. L. G. Neurologic diseases on the island of Guam. US Armed Forces Med. J. 5, 39 (1954).
Kurland, L. T. & Mulder, D. W. Epidemiologic investigations of amyotrophic lateral sclerosis. 1. Preliminary report of geographical distribution with special reference to the Mariana Islands including clinical and pathological observations. Neurology 4, 438–448 (1954).
Kurland, L. T. & Mulder, D. W. Epidemiologic investigations of amyotrophic lateral sclerosis. 2. Familial aggregations indicative of dominant inheritance. Neurology 5, 182–196 (1955).
Hirano, A. My academic life in neuropathology. J. Neuropathol. Exp. Neurol. 69, 760–766 (2010).
Hirano, A. Hirano bodies and related neuronal inclusions. Neuropathol. Appl. Neurobiol. 20, 3–11 (1994).
Steele, J. C. Parkinsonism–dementia complex of Guam. Mov. Disord. 20, S99–S107 (2005).
Steele, J. C. et al. Defining neurodegeneration on Guam by targeted genomic sequencing. Ann. Neurol. 77, 458–468 (2015).
Spencer, P. S. Etiology of retinal and cerebellar pathology in Western Pacific amyotrophic lateral sclerosis and parkinsonism–dementia complex. Eye Brain 12, 97–104 (2020).
Spencer, P. S. et al. Kampo medicine and muro disease (amyotrophic lateral sclerosis and parkinsonism–dementia complex): postscript and historical footnote. eNeurologicalsci 22, 100308 (2021).
Hirano, A., Malamud, N. & Kurland, L. T. Parkinsonism–dementia complex, an endemic disease on the island of Guam: II. Pathological features. Brain 84, 662–679 (1961).
Hirano, A., Malamud, N., Elizan, T. S. & Kurland, L. T. Amyotrophic lateral sclerosis and parkinsonism–dementia complex on Guam. Further pathologic studies. Arch. Neurol. 15, 35–51 (1966).
Oyanagi, K. & Wada, M. Neuropathology of parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam: an update. J. Neurol. 246, 19–27 (1999).
Oyanagi, K. et al. Amyotrophic lateral sclerosis of Guam: the nature of the neuropathological findings. Acta Neuropathol. 88, 405–412 (1994).
Yamazaki, M. et al. Alpha-synuclein inclusions in amygdala in the brains of patients with the parkinsonism–dementia complex of Guam. J. Neuropathol. Exp. Neurol. 59, 585–591 (2000).
Forman, M. S. et al. Tau and α-synuclein pathology in amygdala of parkinsonism–dementia complex patients of Guam. Am. J. Pathol. 160, 1725–1731 (2002).
Miklossy, J. et al. Enduring involvement of tau, β-amyloid, α-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism–dementia complex of Guam (ALS/PDC). Acta Neuropathol. 116, 625–637 (2008).
Verheijen, B. M., Oyanagi, K. & Leeuwen, F. W. Dysfunction of protein quality control in parkinsonism–dementia complex of Guam. Front. Neurol. 9, 173 (2018).
Spencer, P., Palmer, V. S. & Kisby, G. K. Western Pacific ALS-PDC: evidence implicating cycad neurotoxins. J. Neurol. Sci. 419, 117185 (2020).
Laqueur, G. L., Mickelsen, O., Whitting, M. G. & Kurland, L. T. Carcinogenic properties of nuts from Cycas circinalis L. indigenous to Guam. J. Natl Cancer Inst. 31, 919–951 (1963).
Kurland, L. T. An appraisal to the neurotoxicity of cycad and the etiology of amyotrophic lateral sclerosis on Guam. Fed. Proc. 31, 1540–1542 (1972).
Gajdusek, D. C. & Salazar, A. M. Amyotrophic lateral sclerosis and parkinsonian syndromes in high incidence among the Auyu and Jakai people of West new Guinea. Neurology 32, 107–126 (1982).
Gajdusek, D. C. Motor neuron disease in natives of New Guinea. N. Engl. J. Med. 268, 473–476 (1963).
Spencer, P. S., Palmer, V., Ohta, M. & Herman, A. in Amyotrophic Lateral Sclerosis: Recent Advances in Research and Treatment (eds Tsubaki, T. & Yase, Y.) 35–40 (Excerpta Medica, 1988).
Cox, P. A., Banack, S. A. & Murch, S. J. Biomagification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamoro people of Guam. Proc. Natl Acad. Sci. USA 100, 13380–13383 (2003).
Ince, P. G. & Codd, G. A. Return of the cycad hypothesis — does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol. Appl. Neurobiol. 31, 345–353 (2005).
Banack, S. A., Murch, S. J. & Cox, P. A. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J. Ethnopharmacol. 106, 97–104 (2006).
Wiles, G. J. The status of fruit bats on Guam. Pac. Sci. 41, 1–4 (1987).
Morton, J. M. & Wiles, G. J. Observations of Mariana fruit bats (Pteropus mariannus) in the upper Talofofo watershed on southern Guam. Micronesica 34, 155–163 (2002).
Wiles, G. J. & Johnson, N. C. Population size and natural history of Mariana fruit bats (Chiroptera: Pteropodidae) on Sarigan, Mariana Islands. Pac. Sci. 58, 585–596 (2004).
Monson, C. S., Banack, S. A. & Cox, P. A. Conservation implications of Chamorro consumption of flying foxes as a possible cause of amyotrophic lateral sclerosis–parkinsonism dementia complex in Guam. Cons. Biol. 17, 678–686 (2003).
Foss, A. J., Chernoff, N. & Aubel, M. T. The analysis of undervaterizated ß-methylamino-l-alanine (BMAA), BAMA, AEG & 2,4-DAB in Pteropus mariannus mariannus specimens using HILIC-LC-MS/MS. Toxicon 152, 150–159 (2018).
Steele, J. C. & McGeer, P. L. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology 70, 1984–1990 (2008).
Cox, T. A., McDarby, J. V., Lavine, L., Steele, J. C. & Calne, D. B. A retinopathy on Guam with high prevalence in Lytico–Bodig. Ophthalmology 96, 1731–1735 (1989).
Kisby, G. E. & Spencer, P. S. Genotoxic damage during brain development presages prototypical neurodegenerative disease. Front. Neurol. 15, 752153 (2021).
Ahlskog, J. E. et al. Guamanian neurodegenerative disease: investigation of the calcium metabolism/heavy metal hypothesis. Neurology 45, 1340–1344 (1995).
Durlach, J. et al. Are age-related neurodegenerative diseases linked with various types of magnesium depletion? Magnes. Res. 10, 339–353 (1997).
Yanagihara, R. et al. Calcium and vitamin D metabolism in Guamanian Chamorros with amyotrophic lateral sclerosis and parkinsonism–dementia. Ann. Neurol. 15, 42–48 (1984).
Poorkaj, P. et al. TAU as susceptibility gene for amyotrophic sclerosis–parkinsonism–dementia complex of Guam. Arch. Neurol. 58, 1871–1878 (2001).
Sieh, W. et al. Identification of novel susceptibility loci for Guam neurodegenerative disease: challenges of genome scans in genetic isolates. Hum. Mol. Genet. 18, 3725–3738 (2009).
Dombroski, B. A. et al. C9orf72 hexanucleotide repeat expansion and Guam amyotrophic lateral sclerosis–parkinsonism–dementia complex. JAMA Neurol. 70, 742–745 (2013).
No author listed. ‘Kishu Koza no shou fuko no hito’ [An unhappy story of a unfilial man from Koza Village of Kii]. Honcho Koji Innen Shu Vol. 4, Edo (Tokyo) (Yorozuya Kinbe, 1689); translated into early modern Japanese in Iseido Sosho 847–908 (Kyoto Shibunkaku, 1970).
Kuzuhara, S. ‘Endemic paraplegia of Koza in Kii’ in Honcho Koji Innen Shu published in 1689 is probably the earliest description of amyotrophic lateral sclerosis of Kii Peninsula: presentation of the original and investigation of factuality. Rinsho Shinkeigaku 61, 815–824 (2021).
Miura, K. Amyotrophische lateral sklerose unter dem bilde von sog. bulbaerparalyse. Seishin Shinkeigaku Zasshi 10, 366–369 (1911).
Kimura, K., Yase, Y. & Higashi, Y. Epidemiological and geomedical studies on ALS and allied diseases in Kii peninsula (Japan). Preliminary report. Proc. Jpn. Acad. Sci. 37, 417–420 (1961).
Kimura, K. et al. Epidemiological and geomedical studies on amyotrophic lateral sclerosis. Dis. Nerv. Syst. 24, 155–159 (1963).
Shiraki, H. & Yase, Y. Amyotrophic lateral sclerosis in Japan. in Handbook of Clinical Neurology Vol. 22, part II (eds Vinken, P. J. & Bruyn, G. W.) 353–419 (Elsevier, 1975).
Yoshida, S. Environmental factors in western Pacific foci of ALS and a possible pathogenetic role of aluminum (Al) in motor neuron degeneration [in Japanese with English abstract]. Rinsho Shinkeigaku 31, 1310–1312 (1991).
Kuzuhara, S. et al. Familial amyotrophic lateral sclerosis and parkinsonism–dementia complex of the Kii peninsula of Japan: clinical and neuropathological study and tau analysis. Ann. Neurol. 49, 501–511 (2001).
Mimuro, M., Yoshida, M., Kuzuhara, S. & Kokubo, Y. Amyotrophic lateral sclerosis and parkinsonism–dementia complex of the Hohara focus of the Kii peninsula: a multiple proteinopathy? Neuropathology 38, 98–107 (2018).
Mimuro, M., Kokubo, Y. & Kuzuhara, S. Similar topographic distribution of neurofibrillary tangles in amyotrophic lateral sclerosis and parkinsonism–dementia complex in people living in the Kii peninsula of Japan suggests a single tauopathy. Acta Neuropathol. 113, 653–658 (2007).
Shiraki, H. & Yase, Y. Amyotrophic lateral sclerosis and parkinsonism–dementia in the Kii peninsula: comparison with the same disorders in Guam and with Alzheimer’s disease. in Handbook of Clinical Neurology: Diseases of the Motor System (eds Vinken, P. J. et al.) 273–300 (Elsevier, 1991).
Oyanagi, K. et al. Distinct pathological features of the Gallyas- and tau-positive glia in the parkinsonism–dementia complex and amyotrophic lateral sclerosis of Guam. J. Neuropathol. Exp. Neurol. 56, 308–316 (1997).
Yamazaki, M. et al. Tau-positive fine granules in the cerebral white matter: a novel finding among tauopathies exclusive to parkinsonism–dementia complex of Guam. J. Neuropathol. Exp. Neurol. 64, 839–846 (2005).
Kokubo, Y. & Kuzuhara, S. Neurofibrillary tangles in ALS and parkinsonism–dementia complex focus in Kii, Japan. Neurology 63, 2399–2401 (2004).
Itoh, N. et al. Biochemical and ultrastructural study of neurofibrillary tangles in amyotrophic lateral sclerosis/parkinsonism–dementia complex in the Kii peninsula of Japan. J. Neuropathol. Exp. Neurol. 62, 791–798 (2003).
Spencer, P. S. et al. Kampo medicine and Muro disease (amyotrophic lateral sclerosis and parkinsonism–dementia complex). eNeurologicalSci 18, 100230 (2020).
Kuzuhara, S. & Kokubo, Y. in Amyotrophic Lateral Sclerosis and the Frontotemporal Dementias (ed. Strong, M. J.) 39–54 (Oxford Univ. Press, 2012).
Tomiyama, H. et al. Mutation analyses in amyotrophic lateral sclerosis/parkinsonism–dementia complex of the Kii peninsula, Japan. Mov. Disord. 23, 2344–2348 (2008).
Hara, K. et al. TRPM7 is not associated with amyotrophic lateral sclerosis–parkinsonism dementia complex in the Kii peninsula of Japan. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 310–313 (2010).
Hara, K. et al. Molecular-genetic analysis of amyotrophic lateral sclerosis/parkinsonism–dementia complex (ALS/PDC) in the Kii peninsula [in Japanese]. Rinsho Shinkeigaku 47, 974–976 (2007).
Ishiura, H. et al. C9ORF72 repeat expansion in amyotrophic lateral sclerosis in the Kii peninsula of Japan. Arch. Neurol. 69, 1154–1158 (2012).
Kokubo, Y. et al. An immigrant family with Kii amyotrophic lateral sclerosis/parkinsonism–dementia complex. Neurol. Sci. 43, 1423–1425 (2022).
Spencer, P. S., Kisby, G. E. & Ludolp, A. C. Long-latency neurodegenerative disease in the western Pacific. Geriatrics 46, 37–42 (1991).
Okumiya, K. et al. Amyotrophic lateral sclerosis and parkinsonism in Papua, Indonesia: 2001–2012 survey results. BMJ Open 4, e004353 (2014).
Spencer, P., Palmer, V. S. & Ludolph, A. C. On the decline and etiology of high-incidence motor system disease in West papua (Southwest New Guinea). Mov. Disord. 20, 119–126 (2005).
Gibbs, C. J. & Gajdusek, D. C. An update on long-term in vivo and in vitro studies designed to identify a virus as the cause of amyotrophic lateral sclerosis, parkinsonism dementia, and Parkinson’s disease. Adv. Neurol. 36, 343–353 (1982).
Gajdusek, D. C. in Amyotrophic Lateral Sclerosis — Concepts in Pathogenesis and Etiology (ed. Hudson, A. J.) 317–325 (Univ. Toronto Press, 1990).
Spencer, P. S. Parkinsonism and motor neuron disorders: lessons from the Western Pacific. J. Neurol. Sci. 433, 120021 (2022).
Spencer, P. S., Palmer, V. S. & Kisby, G. E. Seeking environmental causes of neurodegenerative disease and envisioning primary prevention. Neurotoxicology 56, 269–283 (2016).
Kisby, G. E., Kabel, H., Hugon, J. & Spencer, P. Damage and repair of nerve cell DNA in toxic stress. Drug Metab. Rev. 31, 589–618 (1999).
Esclaire, F. et al. The Guam cycad toxin methylazoxymethanol damages neuronal DNA and modulate tau mRNA expression and excitotoxicity. Exp. Neurol. 155, 11–21 (1999).
Spencer, P. S. Hypothesis: etiologic and molecular mechanistic leads for sporadic neurodegenerative diseases based on experience with Western Pacific ALS/PDC. Front. Neurol. 10, 754 (2019).
Vallely, A. & Tetu, M. A familial cluster of Parkinson’s disease identified in Milne Bay province, Papua New Guinea. PNG Med. J. 42, 27–31 (1999).
Fernandez, H. H. & Rosales, R. L. Uncovering the mystery from the Philippine island of Panay. Int. J. Neurosci. 121, 1–2 (2011).
Laabs, B. H. et al. Identifying genetic modifiers of age-associated penetrance in X-linked dystonia-parkinsonism. Nat. Commun. 12, 3216 (2021).
Rosales, R. L. X-linked dystonia parkinsonism: clinical phenotype, genetics and therapeutics. J. Mov. Disord. 3, 32–38 (2010).
Pauly, M. L. et al. Expanding data collection for the MDSGene database: X-linked dystonia-parkinsonism as use case example. Mov. Disord. 35, 1933–1938 (2020).
Ng, A. R., Jamora, R. D. G. & Rosales, R. L. X‐linked dystonia parkinsonism: crossing a new threshold. J. Neur. Transm. 128, 567–573 (2021).
Sprenger, A. et al. Eye movement deficits in X-linked dystonia–parkinsonism are related to striatal degeneration. Parkinsonism Relat. Disord. 61, 170–178 (2019).
Lee, L. V., Munoz, E. L., Tan, K. T. & Reyes, M. T. Sex linked recessive dystonia parkinsonism of Panay, Philippines (XDP). J. Clin. Mol. Pathol. 54, 362–368 (2001).
Goto, S. et al. Functional anatomy of the basal ganglia in X-linked recessive dystonia–parkinsonism. Ann. Neurol. 58, 7–17 (2005).
Goto, S. et al. Defects in the striatal neuropeptide Y system in X-linked dystonia–parkinsonism. Brain 136, 1555–1567 (2013).
Kawarai, T., Morigaki, R., Kaji, R. & Goto, S. Clinicopathological phenotype and genetics of X-linked dystonia–parkinsonism (XDP; DYT3; Lubag). Brain Sci. 7, 72 (2017).
Hanssen, H. et al. Imaging gradual neurodegeneration in a basal ganglia model disease. Ann. Neurol. 86, 517–526 (2019).
Rosales, R. L., Ng, A. R., Delos Santos, M. M. & Fernandez, H. H. The broadening application of chemodenervation in X-linked dystonia–parkinsonism (part II): an open-label experience with botulinum toxin-A (Dysport®) injections for oromandibular, lingual, and truncal dystonias. Int. J. Neurosci. 121, 44–56 (2011).
Brüggemann, N. et al. Association of pallidal neurostimulation and outcome predictors with X-linked dystonia–parkinsonism. JAMA Neurol. 76, 211–216 (2019).
Aneichyk, T. et al. Dissecting the causal mechanism of X-linked dystonia–parkinsonism by integrating genome and transcriptome assembly. Cell 172, 897–909 (2018).
Lüth, T. et al. Elucidating hexanucleotide repeat number and methylation within the X-linked dystonia–parkinsonism (XDP)-related SVA retrotransposon in TAF1 with nanopore sequencing. Genes 13, 126 (2022).
Di Lazzaro, G. et al. X-linked parkinsonism: phenotypic and genetic heterogeneity. Mov. Disord. 36, 1511–1525 (2021).
Reyes, C. J. et al. Brain regional differences in hexanucleotide repeat length in X-linked dystonia–parkinsonism using nanopore sequencing. Neurol. Genet. 7, e608 (2021).
Westenberger, A. et al. A hexanucleotide repeat modifies expressivity of X-linked dystonia–parkinsonism. Ann. Neurol. 85, 812–822 (2019).
Bragg, D. C. et al. Disease onset in X-linked dystonia–parkinsonism correlates with expansion of a hexameric repeat within an SVA retrotransposon in TAF1. Proc. Natl Acad. Sci. USA 114, E11020–E11028 (2017).
Trinh, J. et al. Mosaic divergent repeat interruption in XDP influence repeat stability and disease onset. Brain 146, 1075–1082 (2023).
Angibaud, G., Gaultier, C. & Rascol, O. Atypical parkinsonism and Annonaceae consumption in New Caledonia. Mov. Disord. 19, 603–605 (2004).
Caparros-Lefebvre, D. Atypical parkinsonism in New Caledonia: comparisons with Guadeloupe and association with Annonaceae consumption. Mov. Disord. 19, 604–606 (2004).
Lannuzel, A. et al. Further evidence for a distinctive atypical degenerative parkinsonism in the Caribbean: a new cluster in the French West Indian Island of Martinique. J. Neurol. Sci. 388, 214–219 (2018).
Caparros-Lefebvre, D. & Elbaz, A. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a case–control study. Lancet 354, 281–286 (1999).
Caparros-Lefebvre, D. et al. Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy. Brain 125, 801–811 (2002).
Lannuzel, A. et al. Clinical varieties and epidemiological aspects of amyotrophic lateral sclerosis in the Caribbean Island of Guadeloupe: a new focus of ALS associated with parkinsonism. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 216–223 (2015).
Moghdamtousi, S. Z. et al. Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 16, 15625–15658 (2015).
Lannuzel, A. et al. Toxicity of Annonaceae for dopaminergic neurons: potential role in atypical parkinsonism in Guadeloupe. Mov. Disord. 17, 84–90 (2002).
De Sousa, O. V., Vieira, G. D. V., de Pinho, J. D. J. R., Yamamoto, C. H. & Alves, M. S. Antinociceptive and anti-inflammatory activities of the ethanol extract of Annona muricata L. leaves in animal models. Int. J. Mol. Sci. 11, 2067–2078 (2010).
Mishra, S., Ahmad, S., Kumar, N. & Sharma, B. K. Annona muricata (the cancer killer): a review. Glob. J. Pharm. Res. 2, 1613–1618 (2013).
Ragasa, C. Y., Soriano, G., Torres, O. B., Don, M. J. & Shen, C. C. Acetogenins from Annona muricata. Pharm. J. 4, 32–37 (2012).
Lannuzel, A. et al. The mitochondrial complex I inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience 121, 287–296 (2003).
Champy, P. et al. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem. 88, 63–69 (2004).
Lannuzel, A. et al. Atypical parkinsonism in Guadeloupe: a common risk factor for two closely related phenotypes? Brain 130, 816–827 (2007).
Steele, J. C., Caparros-Lefebvre, D., Lees, A. J. & Sacks, O. W. Progressive supranuclear palsy and its relation to pacific foci of the parkinsonism–dementia complex and Guadeloupean parkinsonism. Parkinsonism Relat. Disord. 9, 39–54 (2002).
DeCock, V. C. et al. REM sleep behavior disorder in patients with Guadeloupean parkinsonism, a tauopathy. Sleep 30, 1026–1032 (2007).
Camuzat, A. et al. The PSP-associated MAPT H1 subhaplotype in Guadeloupean atypical parkinsonism. Mov. Disord. 16, 2384–2391 (2008).
Kedari, T. S. & Khan, A. A. Guyabano (Annona muricata): a review of its traditional uses, phytochemistry and pharmacology. Am. J. Res. Comm. 2, 247–268 (2014).
Spencer, P. S. & Palmer, V. S. Food plant chemicals linked with neurological and neurodegenerative disease. Adv. Neurotoxicol. 1, 247–267 (2017).
Martí Massó, J. F., Zarranz, J. J., Otaegui, D. & López de Munain, A. Neurogenetic disorders in the Basque population. Ann. Hum. Genet. 79, 57–75 (2015).
Paisán-Ruíz, C. et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600 (2004).
Mata, I. F. et al. LRRK2 R1441G in Spanish patients with Parkinson’s disease. Neurosci. Lett. 382, 309–311 (2005).
Simón-Sánchez, J. et al. Parkinson’s disease due to the R1441G mutations in dardarin: a founder effect in the Basques. Mov. Disord. 21, 1954–1959 (2006).
González-Fernández, M. C. et al. LRRK2-associated parkinsonism is a major cause of disease in Northern Spain. Parkinsonism Relat. Disord. 13, 509–515 (2007).
Deng, H., Wang, P. & Jankovic, J. The genetics of Parkinson’s disease. Ageing Res. Rev. 42, 72–85 (2018).
López de Munain, A., Martí Massó, J. F. & Pérez Tur, J. The discovery of dardarin gene 15 years later: a globalized local history. Mov. Disord. 35, 708 (2020).
Martí-Massó, J. F. et al. Neuropathology of Parkinson’s disease with the R1441G mutation in LRRK2. Mov. Disord. 24, 1998–2001 (2009).
Mata, I. F. et al. LRRK2 mutations are a common cause of Parkinson’s disease in Spain. Eur. J. Neurol. 13, 391–394 (2006).
Ruíz-Martínez, J. et al. Penetrance in Parkinson’s disease related to the LRRK2 R1441G mutation in the Basque-Country (Spain). Mov. Disord. 25, 2340–2345 (2010).
Gorostidi, A., Ruiz-Martinez, J., Lopez de Munain, A., Alzualde, A. & Marti-Masso, J. F. LRRK2 G2019S and R1441G mutations associated with Parkinson’s disease are common in the Basque Country but relative prevalence is determined by ethnicity. Neurogenetics 10, 157–159 (2009).
Hurles, M. E. et al. Recent male-mediated gene flow over linguistic barrier in Iberia, suggested by analysis of a Y-chromosomal DNA polymorphism. Am. J. Hum. Genet. 65, 1437–1448 (1999).
Simpson, C. H. et al. Prevalence of the LRRK2 variants in Parkinson’s disease: a comprehensive review. Parkinsonism Relat. Disord. 98, 103–113 (2022).
Zimprich, A. et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson’s disease. Am. J. Hum. Genet. 89, 168–175 (2011).
Struhal, W. et al. VPS35 Parkinson’s disease phenotype resembles the sporadic disease. J. Neural Transm. 121, 755–759 (2014).
Vilariño-Güell, C. et al. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89, 162–167 (2011).
Sheerin, U. M. et al. Screening for VPS35 mutations in Parkinson’s disease. Neurobiol. Aging 33, 838.e1–5 (2012).
Ando, M. et al. VPS35 mutation in Japanese patients with typical Parkinson’s disease. Mov. Disord. 27, 1413–1417 (2012).
Chen, Y., Chang, Y., Lan, M., Chen, P. & Lin, C. H. Identification of VPS35 p.D620N mutation related Parkinson’s disease in a Taiwanese family with successful bilateral subthalamic nucleus deep brain stimulation: a case report and literature review. BMC Neurol. 17, 191 (2017).
Caparros-Lefebvre, D. et al. A geographical cluster of progressive supranuclear palsy in Northern France. Neurology 85, 1293–1300 (2015).
Caparros-Lefebvre, D. Food toxins and the Caribbean Parkinson plus types. Rev. Neurol. 175, 641–643 (2019).
Batelkova, K., Kolejka, J. & Pokorny, J. Landscape synthesis and geographical information systems as part of natural landscape assessment for regional planning: case study: Horňácko. Geography 101, 296–309 (1996).
Mensikova, K. et al. Prevalence of neurodegenerative parkinsonism in an isolated population in south-eastern Moravia, Czech Republic. Eur. J. Epidemiol. 28, 833–836 (2013).
Mensikova, K. et al. Epidemiological study of neurodegenerative parkinsonism in Hornacko, a specific region of south-eastern Moravia, Czech Republic. Cesk. Slov. Neurol. N. 77/110, 714–720 (2014).
Frolec, V., Holy, D., & Hornácko, J. R. The Life and Culture of the People from Moravian-Slovakian Borderlands (Blok, 1996).
Menšíková, K. et al. Atypical parkinsonism of progressive supranuclear palsy-parkinsonism (PSP-P) phenotype with rare variants in FBXO7 and VPS35 genes associated with Lewy body pathology. Acta Neuropathol. 137, 171–173 (2019).
Kolarikova, K. et al. High throughput sequencing haplotype analysis indicates in the CHMP2B gene a potential risk factor for endemic parkinsonism in Southeastern Moravia, Czech Republic. Life 12, 121 (2022).
Kolarikova, K. et al. Whole exome sequencing study in isolated South-Eastern Moravia (Czech Republic) population indicates heterogenous genetic background for parkinsonism development. Front. Neurosci. 16, 817713 (2022).
Bartoníková, T. et al. New endemic familial parkinsonism in south Moravia, Czech Republic and its genetic background. Medicine 97, e12313 (2018).
Lagrange, E. et al. An amyotrophic lateral sclerosis hot spot in the French Alps associated with genotoxic fungi. J. Neurol. Sci. 427, 117558 (2021).
Schulzova, V. et al. Agaritine content of 53 Agaricus species collected from nature. Food Addit. Contam. 26, 82–93 (2009).
Patocka, J., Pita, R. & Kuca, K. Gyromitrin, mushroom toxin of Gyromitra spp. Mil. Med. Sci. Lett. 81, 61–67 (2012).
Steele, J. C. & Guzman, T. Observations about amyotrophic lateral sclerosis and the parkinsonism–dementia complex of Guam with regard to epidemiology and etiology. Can. J. Neurol. Sci. 14, 358–362 (1987).
Román, G. C. Neuroepidemiology of amyotrophic lateral sclerosis: clues to aetiology and pathogenesis. J. Neurol. Neurosurg. Psychiatr. 61, 131–137 (1996).
de Langavant, L. C. et al. Annonaceae consumption worsens disease severity and cognitive deficits in degenerative parkinsonism. Mov. Disord. 37, 2355–2366 (2022).
Sacks, O. The Island of the Colorblind (Knopf-Doubleday, 1997).
Acknowledgements
The research work of authors is supported by the European Regional Development Fund — Project ENOCH (No. Z.02.1.01/0.0/0.0/16_019/0000868 to L.T., D.H., K.K. and M.S.); a grant from the Ministry of Health of the Czech Republic for the conceptual development of a research organization (FNOL 0098892) RVO FNOL 2022 to K.M., L.T. and D.H.; and Ministry of Health of the Czech Republic grant NV19-14-00090 to K.M., L.T., D.H. and R.M. The authors thank A. Johnson and S.E. Cook for language editing and thank A. Vydrova and Z. Malinska for providing the Supplementary figures. The authors thank the Third World Medical Research Foundation for providing the URL for the video ‘The poison that waits?’. The authors also thank J. Stankus for preparing the original version of Fig. 1, and D. Konickova for preparing the original version of Fig.3.
Author information
Authors and Affiliations
Contributions
K.M., J.C.S., R.R., P.S., A.L., Y.U., R.S., L.T., S.B., G.R. and P.K. researched data for the manuscript and/or contributed to its conceptualization. J.C.S., C.C., P.S., A.L., Y.U., S.G.-R., R.M., R. Vodicka and S.B. substantially contributed to discussions of the article content. P.K., K.M., R.R., D.H., L.T. and K.K. wrote the manuscript. P.H., P.O., R. Vrtel, M.P., M.B., L.B., G.R. and M.S. undertook critical reading of the manuscript. Figures and Supplementary information were provided by P.S., D.H. and L.T. (Fig. 5), S.B. (Fig. 4), L.B., M.S. and K.M. (Fig. 1), R.R. (Fig. 2, part C) and A.L. All authors reviewed and edited the entire manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Kiyomitsu Oyanagi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
MDSGene: https://www.mdsgene.org/
OMIM: https://www.omim.org/
The Poison that Waits?: https://vimeo.com/1621281
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Menšíková, K., Steele, J.C., Rosales, R. et al. Endemic parkinsonism: clusters, biology and clinical features. Nat Rev Neurol 19, 599–616 (2023). https://doi.org/10.1038/s41582-023-00866-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00866-3
This article is cited by
-
Questioning the cycad theory of Kii ALS–PDC causation
Nature Reviews Neurology (2024)
-
Reply to: Questioning the cycad theory of Kii ALS–PDC causation
Nature Reviews Neurology (2024)