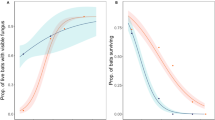
Abstract
The recent introduction of Pseudogymnoascus destructans (the fungal pathogen that causes white-nose syndrome in bats) from Eurasia to North America has resulted in the collapse of North American bat populations and restructured species communities. The long evolutionary history between P. destructans and bats in Eurasia makes understanding host life history essential to uncovering the ecology of P. destructans. In this Review, we combine information on pathogen and host biology to understand the patterns of P. destructans spread, seasonal transmission ecology, the pathogenesis of white-nose syndrome and the cross-scale impact from individual hosts to ecosystems. Collectively, this research highlights how early pathogen detection and quantification of host impacts has accelerated the understanding of this newly emerging infectious disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Langwig, K. E. et al. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol. Lett. 15, 1050–1057 (2012).
Frick, W. F. et al. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682 (2010).
Frick, W. F. et al. Disease alters macroecological patterns of North American bats. Glob. Ecol. Biogeogr. 24, 741–749 (2015).
Turner, G. & Reeder, D. Update of white-nose syndrome in bats, September 2009. Bat Res. News 50, 47–53 (2009).
Reichard, J. D. et al. Interannual survival of Myotis lucifugus (Chiroptera: Vespertilionidae) near the epicenter of white-nose syndrome. Northeast. Nat. 21, N56–N59 (2014).
Dzal, Y., McGuire, L. P., Veselka, N. & Fenton, M. B. Going, going, gone: the impact of white-nose syndrome on the summer activity of the little brown bat (Myotis lucifugus). Biol. Lett. 7, 392–394 (2011).
Francl, K. E., Ford, W. M., Sparks, D. W. & Brack, V. Capture and reproductive trends in summer bat communities in West Virginia: assessing the impact of white-nose syndrome. J. Fish. Wildl. Manag. 3, 33–42 (2012).
Ford, W. M., Britzke, E. R., Dobony, C. A., Rodrigue, J. L. & Johnson, J. B. Patterns of acoustical activity of bats prior to and following white-nose syndrome occurrence. J. Fish. Wildl. Manag. 2, 125–134 (2011).
Powers, K. E., Reynolds, R. J., Orndorff, W., Ford, W. M. & Hobson, C. S. Post-white-nose syndrome trends in Virginias cave bats, 2008–2013. J. Ecol. Nat. Environ. 7, 113–123 (2015).
Powers, K. E. et al. Monitoring the status of gray bats (Myotis grisescens) in Virginia, 2009–2014, and potential impacts of white-nose syndrome. Southeast. Nat. 15, 127–137 (2016).
Reynolds, R. J., Powers, K. E., Orndorff, W., Ford, W. M. & Hobson, C. S. Changes in rates of capture and demographics of Myotis septentrionalis (northern long-eared bat) in western Virginia before and after onset of white-nose syndrome. Northeast. Nat. 23, 195–204 (2016).
Coleman, J. T. H. & Reichard, J. D. Bat white-nose syndrome in 2014: a brief assessment seven years after the discovery of a virulent fungal pathogen in North America. Outlooks Pest Manag. 25, 374–377 (2014).
Turner, G. G., Reeder, D. M. & Coleman, J. T. H. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats and look to the future. Bat Res. News 52, 13–27 (2011).
Nocera, T., Ford, W. M., Silvis, A. & Dobony, C. A. Patterns of acoustical activity of bats prior to and 10 years after WNS on Fort Drum Army Installation, New York. Glob. Ecol. Conserv. 18, e00633 (2019).
Brooks, R. T. Declines in summer bat activity in central New England 4 years following the initial detection of white-nose syndrome. Biodivers. Conserv. 20, 2537–2541 (2011).
Meteyer, C. U. et al. Histopathologic criteria to confirm white-nose syndrome in bats. J. Vet. Diagn. Invest. 21, 411–414 (2009).
Blehert, D. S. et al. Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 227–227 (2009).
Chaturvedi, V. et al. Morphological and molecular characterizations of psychrophilic fungus Geomyces destructans from New York bats with white nose syndrome (WNS). PLoS ONE 5, e10783 (2010).
Gargas, A., Trest, M. T., Christensen, M., Volk, T. J. & Bleher, D. S. Geomyces desctructans sp. nov. associated with bat white-nose syndrome. Mycotaxon 108, 147–154 (2009).
Minnis, A. M. & Lindner, D. L. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol. 117, 638–649 (2013).
Drees, K. P. et al. Phylogenetics of a fungal invasion: origins and widespread dispersal of white-nose syndrome. mBio 8, e01941-17 (2017).
Palmer, J. M., Drees, K. P., Foster, J. T. & Lindner, D. L. Extreme sensitivity to ultraviolet light in the fungal pathogen causing white-nose syndrome of bats. Nat. Commun. 9, 35 (2018).
Reynolds, H. T. & Barton, H. A. Comparison of the white-nose syndrome agent Pseudogymnoascus destructans to cave-dwelling relatives suggests reduced saprotrophic enzyme activity. PLoS ONE 9, e86437 (2014).
Wilson, M. B., Held, B. W., Freiborg, A. H., Blanchette, R. A. & Salomon, C. E. Resource capture and competitive ability of non-pathogenic Pseudogymnoascus spp. and P. destructans, the cause of white-nose syndrome in bats. PLoS ONE 12, e0178968 (2017).
O’Donoghue, A. J. et al. Destructin-1 is a collagen-degrading endopeptidase secreted by Pseudogymnoascus destructans, the causative agent of white-nose syndrome. Proc. Natl Acad. Sci. USA 112, 7478–7483 (2015).
Lorch, J. M. et al. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480, 376–378 (2011).
Warnecke, L. et al. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl Acad. Sci. USA 109, 6999–7003 (2012).
Hoyt, J. R. et al. Environmental reservoir dynamics predict global infection patterns and population impacts for the fungal disease white-nose syndrome. Proc. Natl Acad. Sci. USA 117, 7255–7262 (2020).
Fritze, M. & Puechmaille, S. J. Identifying unusual mortality events in bats: a baseline for bat hibernation monitoring and white-nose syndrome research. Mammal. Rev. 48, 224–228 (2018).
Langwig, K. E. et al. Context dependent conservation responses to wildlife disease. Front. Ecol. Environ. 13, 195–202 (2015).
Lorch, J. M. et al. Snake fungal disease: an emerging threat to wild snakes. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150457 (2016).
Hoyt, J. R. et al. Host persistence or extinction from emerging infectious disease: insights from white-nose syndrome in endemic and invading regions. Proc. R. Soc. B Biol. Sci. https://doi.org/10.1098/rspb.2015.2861 (2016).
Leopardi, S., Blake, D. & Puechmaille, S. J. White-Nose Syndrome fungus introduced from Europe to North America. Curr. Biol. 25, R217–R219 (2015).
Rajkumar, S. S. et al. Clonal genotype of Geomyces destructans among bats with white nose syndrome, New York, USA. Emerg. Infect. Dis. 17, 1273–1276 (2011).
Ren, P. et al. Clonal spread of Geomyces destructans among bats, midwestern and southern United States. Emerg. Infect. Dis. 18, 883–885 (2012).
Puechmaille, S. J. et al. White-nose syndrome: is this emerging disease a threat to European bats? Trends Ecol. Evol. 26, 570–576 (2011).
Campana, M. G. et al. White-nose syndrome fungus in a 1918 bat specimen from France. Emerg. Infect. Dis. https://doi.org/10.3201/eid2309.170875 (2017).
Martínková, N. et al. Increasing incidence of Geomyces destructans fungus in bats from the Czech Republic and Slovakia. PLoS ONE 5, e13853 (2010).
Puechmaille, S. J. et al. Pan-European distribution of white-nose syndrome fungus (Geomyces destructans) not associated with mass mortality. PLoS ONE 6, e19167 (2011).
Zahradníková, A. Jr. et al. Historic and geographic surveillance of Pseudogymnoascus destructans possible from collections of bat parasites. Transbound. Emerg. Dis. 65, 303–308 (2018).
Ruedi, M. et al. Molecular phylogenetic reconstructions identify East Asia as the cradle for the evolution of the cosmopolitan genus Myotis (Mammalia, Chiroptera). Mol. Phylogenet. Evol. 69, 437–449 (2013).
Hosseini, P. R., Dhondt, A. A. & Dobson, A. P. Spatial spread of an emerging infectious disease: conjunctivitis in house finches. Ecology 87, 3037–3046 (2006).
Kilpatrick, A. M. et al. Predicting the global spread of H5N1 avian influenza. Proc. Natl Acad. Sci. USA 103, 19368–19373 (2006).
Lorch, J. M. et al. First detection of bat white-nose syndrome in Western North America. mSphere 1, e00148-16 (2016).
Wilder, A. P., Frick, W. F., Langwig, K. E. & Kunz, T. H. Risk factors associated with mortality from white-nose syndrome among hibernating bat colonies. Biol. Lett. 7, 950–953 (2011).
Lilley, T. M., Anttila, J. & Ruokolainen, L. Landscape structure and ecology influence the spread of a bat fungal disease. Funct. Ecol. 32, 2483–2496 (2018).
Maher, S. P. et al. Spread of white-nose syndrome on a network regulated by geography and climate. Nat. Commun. 3, 1306 (2012).
Davis, W. H. & Hitchcock, H. B. Biology and migration of the bat, Myotis lucifugus, in New England. J. Mammal. 46, 296–313 (1965).
Norquay, K. J. O., Martinez-Nunez, F., Dubois, J. E., Monson, K. M. & Willis, C. K. R. Long-distance movements of little brown bats (Myotis lucifugus). J. Mammal. 94, 506–515 (2013).
Langwig, K. E. et al. Tradeoffs between mobility and infectiousness in the spatial spread of an emerging pathogen. Preprint at bioRxiv https://doi.org/10.1101/2020.05.07.082651 (2020).
Wilder, A. P., Kunz, T. H. & Sorenson, M. D. Population genetic structure of a common host predicts the spread of white-nose syndrome, an emerging infectious disease in bats. Mol. Ecol. 24, 5495–5506 (2015).
Thapa, V. et al. Using a novel partitivirus in Pseudogymnoascus destructans to understand the epidemiology of white-nose syndrome. PLoS Pathog. 12, e1006076 (2016).
Kovacova, V. et al. White-nose syndrome detected in bats over an extensive area of Russia. BMC Vet. Res. 14, 192 (2018).
Holz, P. H., Lumsden, L. F., Marenda, M. S., Browning, G. F. & Hufschmid, J. Two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in southern Australia have diverse fungal skin flora but not Pseudogymnoascus destructans. PLoS ONE 13, e0204282 (2018).
Lilley, T. M. et al. Population connectivity predicts vulnerability to white-nose syndrome in the Chilean myotis (Myotis chiloensis) — a genomics approach. G3 10, 2117–2126 (2020).
Holz, P. et al. Does the fungus causing white-nose syndrome pose a significant risk to Australian bats? Wildl. Res. 46, 657–668 (2019).
Hoyt, J. R. et al. Long-term persistence of Pseudogymnoascus destructans, the causative agent of white-nose syndrome, in the absence of bats. EcoHealth 12, 330–333 (2015).
Lorch, J. M. et al. Distribution and environmental persistence of the causative agent of white-nose syndrome, Geomyces destructans, in bat hibernacula of the eastern United States. Appl. Environ. Microbiol. 79, 1293–1301 (2013).
Campbell, L. J., Walsh, D. P., Blehert, D. S. & Lorch, J. M. Long-term survival of Pseudogymnoascus destructans at elevated temperatures. J. Wildl. Dis. 56, 278–287 (2020).
Verant, M. L., Boyles, J. G., Waldrep, W. Jr. Wibbelt, G. & Blehert, D. S. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE 7, e46280 (2012).
Langwig, K. E. et al. Host and pathogen ecology drive the seasonal dynamics of a fungal disease, white-nose syndrome. Proc. R. Soc. B Biol. Sci. 282, 20142335 (2015).
O’shea, T. J. et al. Bat flight and zoonotic viruses. Emerg. Infect. Dis. 20, 741 (2014).
Perry, R. W. A review of factors affecting cave climates for hibernating bats in temperate North America. Environ. Rev. 21, 28–39 (2013).
Webb, P. I., Speakman, J. R. & Racey, P. A. How hot is a hibernaculum? A review of the temperatures at which bats hibernate. Can. J. Zool. 74, 761–765 (1996).
Langwig, K. E. et al. Drivers of variation in species impacts for a multi-host fungal disease of bats. Phil. Trans. R. Soc. B Biol. Sci. https://doi.org/10.1098/rstb.2015.0456 (2016).
Langwig, K. E. et al. Invasion dynamics of white-nose syndrome fungus, midwestern United States, 2012–2014. Emerg. Infect. Dis. 21, 1023–1026 (2015).
Frick, W. F. et al. Pathogen dynamics during invasion and establishment of white-nose syndrome explain mechanisms of host persistence. Ecology 98, 624–631 (2017).
Zukal, J. et al. White-nose syndrome without borders: Pseudogymnoascus destructans infection tolerated in Europe and Palearctic Asia but not in North America. Sci. Rep. 6, 19829 (2016).
Czenze, Z. J., Jonasson, K. A. & Willis, C. K. Thrifty females, frisky males: winter energetics of hibernating bats from a cold climate. Physiol. Biochem. Zool. 90, 502–511 (2017).
Wilcox, A. et al. Behaviour of hibernating little brown bats experimentally inoculated with the pathogen that causes white-nose syndrome. Anim. Behav. 88, 157–164 (2014).
Meteyer, C. U., Barber, D. & Mandl, J. N. Pathology in euthermic bats with white nose syndrome suggests a natural manifestation of immune reconstitution inflammatory syndrome. Virulence 3, 583–588 (2012).
Meteyer, C. U. et al. Recovery of little brown bats (Myotis lucifugus) from natural infection with Geomyces destructans, white-nose syndrome. J. Wildl. Dis. 47, 618–626 (2011).
Fuller, N. W. et al. Disease recovery in bats affected by white-nose syndrome. J. Exp. Biol. 223, jeb211912 (2020).
Field, K. A. et al. The white-nose syndrome transcriptome: activation of anti-fungal host responses in wing tissue of hibernating bats. PLOS Pathog. 11, e1005168 (2015).
Shelburne, S. A. et al. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine 81, 213–227 (2002).
Pikula, J. et al. White-nose syndrome pathology grading in Nearctic and Palearctic bats. PLoS ONE 12, e0180435 (2017).
Hoyt, J. R. et al. Widespread occurrence of Pseudogymnoascus destructans in northeast China. Emerg. Infect. Dis. 22, 140–142 (2016).
Ballmann, A. E., Torkelson, M. R., Bohuski, E. A., Russell, R. E. & Blehert, D. S. Dispersal hazards of Pseudogymnoascus destructans by bats and human activity at hibernacula in summer. J. Wildl. Dis. 53, 725–735 (2017).
Dobony, C. A. et al. Little brown myotis persist despite exposure to white-nose syndrome. J. Fish Wildl. Manag. 2, 190–195 (2011).
Thomas, D. W., Fenton, M. B. & Barclay, R. M. R. Social behavior of the little brown bat, Myotis lucifugus. I. Mating behavior. Behav. Ecol. Sociobiol. 6, 129–136 (1979).
Parsons, K. N., Jones, G., Davidson-Watts, I. & Greenaway, F. Swarming of bats at underground sites in Britain — implications for conservation. Biol. Conserv. 111, 63–70 (2003).
Jiang, T. et al. Autumn flight activity of the greater horseshoe bat at hibernacula. Anim. Biol. 66, 119–131 (2016).
van Schaik, J. et al. Bats swarm where they hibernate: compositional similarity between autumn swarming and winter hibernation assemblages at five underground sites. PLoS ONE 10, 1 (2015).
Fenton, M. B. Summer activity of Myotis lucifugus (Chiroptera: Vespertilionidae) at hibernacula in Ontario and Quebec. Can. J. Zool. 47, 597–602 (1969).
Kunz, T. H., Wrazen, J. A. & Burnett, C. D. Changes in body mass and fat reserves in pre-hibernating little brown bats (Myotis lucifugus). Ecoscience 5, 8–17 (1998).
Hoyt, J. R. et al. Cryptic connections illuminate pathogen transmission within community networks. Nature 563, 710–713 (2018).
Reeder, D. M. et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE 7, e38920 (2012).
Jonasson, K. A. & Willis, C. K. R. Hibernation energetics of free-ranging little brown bats. J. Exp. Biol. 215, 2141–2149 (2012).
Lučan, R. K. et al. Ectoparasites may serve as vectors for the white-nose syndrome fungus. Parasit. Vectors 9, 16 (2016).
Hudson, H. J. Fungal Biology (CUP Archive, 1992).
Ainsworth, G. Fungal parasites of vertebrates. in Fungal Population: An Advanced Treatise Vol. 211 (Elsevier, 2013).
Zhao, Z., Liu, H., Wang, C. & Xu, J. R. Erratum to: comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics 15, 6 (2014).
Zukal, J. et al. White-nose syndrome fungus: a generalist pathogen of hibernating bats. PLoS ONE 9, e97224 (2014).
Bernard, R. F., Foster, J. T., Willcox, E. V., Parise, K. L. & McCracken, G. F. Molecular detection of the causative agent of white-nose syndrome on Rafinesque’s big-eared bats (Corynorhinus rafinesquii) and two species of migratory bats in the southeastern USA. J. Wildl. Dis. 51, 519–522 (2015).
Turner, G. G. et al. Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white-nose syndrome. J. Wildl. Dis. 50, 566–573 (2014).
USFWS. White-nose syndrome occurrence map - by year. https://www.whitenosesyndrome.org/where-is-wns (2020).
Wibbelt, G. et al. Skin lesions in European hibernating bats associated with Geomyces destructans, the etiologic agent of white-nose syndrome. PLoS ONE 8, e74105 (2013).
Bandouchova, H. et al. Alterations in the health of hibernating bats under pathogen pressure. Sci. Rep. 8, 6067 (2018).
Warnecke, L. et al. Pathophysiology of white-nose syndrome in bats: a mechanistic model linking wing damage to mortality. Biol. Lett. https://doi.org/10.1098/rsbl.2013.0177 (2013).
Herreid, C. F. 2nd, Bretz, W. L. & Schmidt-Nielsen, K. Cutaneous gas exchange in bats. Am. J. Physiol. 215, 506–508 (1968).
Verant, M. L. et al. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiol. 14, 10 (2014).
Cryan, P. M., Uphoff Meteyer, C., Boyles, J. G. & Blehert, D. S. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 8, 135 (2010).
Mayberry, H. W., McGuire, L. P. & Willis, C. K. Body temperatures of hibernating little brown bats reveal pronounced behavioural activity during deep torpor and suggest a fever response during white-nose syndrome. J. Comp. Physiol. B 188, 333–343 (2018).
McGuire, L. P., Mayberry, H. W. & Willis, C. K. White-nose syndrome increases torpid metabolic rate and evaporative water loss in hibernating bats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 313, R680–R686 (2017).
Brownlee-Bouboulis, S. A. & Reeder, D. M. White-nose syndrome-affected little brown myotis (Myotis lucifugus) increase grooming and other active behaviors during arousals from hibernation. J. Wildl. Dis. https://doi.org/10.7589/2012-10-242 (2013).
Lilley, T. M. et al. White-nose syndrome survivors do not exhibit frequent arousals associated with Pseudogymnoascus destructans infection. Front. Zool. 13, 12 (2016).
Carey, C. S. & Boyles, J. G. Interruption to cutaneous gas exchange is not a likely mechanism of WNS-associated death in bats. J. Exp. Biol. 218, 1986–1989 (2015).
Lilley, T. M. et al. Immune responses in hibernating little brown myotis (Myotis lucifugus) with white-nose syndrome. Proc. R. Soc. B Biol. Sci. 284, 20162232 (2017).
Field, K. et al. Anti-fungal immune responses to Pseudogymnoasces destructans in bats affected by white-nose syndrome. J. Immunol. 192 (Suppl. 1), 207.13 (2014).
Reeder, S. M. et al. Pseudogymnoascus destructans transcriptome changes during white-nose syndrome infections. Virulence 8, 1695–1707 (2017).
Harazim, M. et al. Natural selection in bats with historical exposure to white-nose syndrome. BMC Zool. 3, 8 (2018).
Davy, C. M. et al. Transcriptional host–pathogen responses of Pseudogymnoascus destructans and three species of bats with white-nose syndrome. Virulence 11, 781–794 (2020).
Moore, M. S. et al. Energy conserving thermoregulatory patterns and lower disease severity in a bat resistant to the impacts of white-nose syndrome. J. Comp. Physiol. B 188, 163–176 (2018).
Field, K. A. et al. Effect of torpor on host transcriptomic responses to a fungal pathogen in hibernating bats. Mol. Ecol. 27, 3727–3743 (2018).
Johnson, J. S. et al. Antibodies to Pseudogymnoascus destructans are not sufficient for protection against white-nose syndrome. Ecol. Evol. 5, 2203–2014 (2015).
Lilley, T. M. et al. Resistance is futile: RNA-sequencing reveals differing responses to bat fungal pathogen in Nearctic Myotis lucifugus and Palearctic Myotis myotis. Oecologia 191, 295–309 (2019).
Thogmartin, W. E., King, R. A., McKann, P. C., Szymanski, J. A. & Pruitt, L. Population-level impact of white-nose syndrome on the endangered Indiana bat. J. Mammal. 93, 1086–1098 (2012).
Verant, M. L., Meteyer, C. U., Stading, B. & Blehert, D. S. Experimental infection of Tadarida brasiliensis with Pseudogymnoascus destructans, the fungus that causes white-nose syndrome. mSphere 3, e00250-18 (2018).
Rodhouse, T. J. et al. Evidence of region-wide bat population decline from long-term monitoring and Bayesian occupancy models with empirically informed priors. Ecol. Evol. 9, 11078–11088 (2019).
Marroquin, C. M., Lavine, J. O. & Windstam, S. T. Effect of humidity on development of Pseudogymnoascus destructans, the causal agent of bat white-nose syndrome. Northeast. Nat. 24, 54–64 (2017).
Grieneisen, L. E., Brownlee-Bouboulis, S. A., Johnson, J. S. & Reeder, D. M. Sex and hibernaculum temperature predict survivorship in white-nose syndrome affected little brown myotis (Myotis lucifugus). R. Soc. Open Sci. 2, 140470 (2015).
Johnson, J. S. et al. Host, pathogen, and environmental characteristics predict white-nose syndrome mortality in captive little brown myotis (Myotis lucifugus). PLoS ONE https://doi.org/10.1371/journal.pone.0112502 (2014).
Hayman, D. T., Pulliam, J. R., Marshall, J. C., Cryan, P. M. & Webb, C. T. Environment, host, and fungal traits predict continental-scale white-nose syndrome in bats. Sci. Adv. 2, e1500831 (2016).
Verant, M. L. et al. Determinants of Pseudogymnoascus destructans within bat hibernacula: Implications for surveillance and management of white-nose syndrome. J. Appl. Ecol. 55, 820–829 (2018).
McGuire, L. P. et al. White-nose syndrome disease severity and a comparison of diagnostic methods. EcoHealth 13, 60–71 (2016).
Willis, C. K. R., Menzies, A. K., Boyles, J. G. & Wojciechowski, M. S. Evaporative water loss is a plausible explanation for mortality of bats from white-nose syndrome. Integr. Comp. Biol. 51, 364–373 (2011).
Haase, C. G. et al. Incorporating evaporative water loss into bioenergetic models of hibernation to test for relative influence of host and pathogen traits on white-nose syndrome. PLoS ONE 14, e0222311 (2019).
Pannkuk, E. L., Gilmore, D. F., Savary, B. J. & Risch, T. S. Triacylglyceride (TAG) profiles of integumentary lipids isolated from three bat species determined by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS). Can. J. Zool. 90, 1117–1127 (2012).
Pannkuk, E. L. et al. Glycerophospholipid profiles of bats with white-nose syndrome. Physiol. Biochem. Zool. 88, 425–432 (2015).
Avena, C. V. et al. Deconstructing the bat skin microbiome: influences of the host and the environment. Front. Microbiol. 7, 1753 (2016).
Lemieux-Labonté, V., Simard, A., Willis, C. K. & Lapointe, F.-J. Enrichment of beneficial bacteria in the skin microbiota of bats persisting with white-nose syndrome. Microbiome 5, 115 (2017).
Ange-Stark, M. A. et al. White-nose syndrome restructures bat skin microbiomes. Preprint at bioRxiv https://doi.org/10.1101/614842 (2019).
Hoyt, J. R. et al. Bacteria isolated from bats inhibit the growth of Pseudogymnoascus destructans, the causative agent of white-nose syndrome. PLoS ONE 10, e0121329 (2015).
Moore, M. S. et al. Hibernating little brown myotis (Myotis lucifugus) show variable immunological responses to white-nose syndrome. PLoS ONE 8, e58976 (2013).
Rocke, T. E. et al. Virally-vectored vaccine candidates against white-nose syndrome induce anti-fungal immune response in little brown bats (Myotis lucifugus). Sci. Rep. 9, 6788 (2019).
Donaldson, M. E. et al. Profiling the immunome of little brown myotis provides a yardstick for measuring the genetic response to white-nose syndrome. Evolut. Appl. 10, 1076–1090 (2017).
Davy, C. M. et al. White-nose syndrome is associated with increased replication of a naturally persisting coronaviruses in bats. Sci. Rep. 8, 15508 (2018).
Martínková, N. et al. Hibernation temperature-dependent Pseudogymnoascus destructans infection intensity in Palearctic bats. Virulence 9, 1734–1750 (2018).
Langwig, K. E. et al. Resistance in persisting bat populations after white-nose syndrome invasion. Philos. Trans. R. Soc. B: Biol. Sci. 372, 20160044 (2017).
Maslo, B., Valent, M., Gumbs, J. F. & Frick, W. F. Conservation implications of ameliorating survival of little brown bats with white-nose syndrome. Ecol. Appl. 25, 1832–1840 (2015).
Maslo, B. et al. High annual survival in infected wildlife populations may veil a persistent extinction risk from disease. Ecosphere 8, e02001 (2017).
Dowling, Z. R. & O’Dell, D. I. Bat use of an island off the coast of Massachusetts. Northeast. Nat. 25, 362–382 (2018).
Cheng, T. L. et al. Higher fat stores contribute to persistence of little brown bat populations with white-nose syndrome. J. Anim. Ecol. 88, 591–600 (2019).
Bohn, S. et al. Evidence of ‘sickness behaviour’ in bats with white-nose syndrome. Behaviour 153, 981–1003 (2016).
Raberg, L., Sim, D. & Read, A. F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318, 812–814 (2007).
Auteri, G. G. & Knowles, L. L. Decimated little brown bats show potential for adaptive change. Sci. Rep. 10, 3023 (2020).
Gignoux-Wolfsohn, S. A. et al. Genomic signatures of evolutionary rescue in bats surviving white-nose syndrome. Preprint at bioRxiv https://doi.org/10.1101/470294 (2019).
Lilley, T. M. et al. Genome-wide changes in genetic diversity in a population of Myotis lucifugus affected by white-nose syndrome. G3 10, 2007–2020 (2020).
Shuey, M. M., Drees, K. P., Lindner, D. L., Keim, P. & Foster, J. T. Highly sensitive quantitative PCR for the detection and differentiation of Pseudogymnoascus destructans and other Pseudogymnoascus species. Appl. Environ. Microbiol. 80, 1726–1731 (2014).
Muller, L. K. et al. Bat white-nose syndrome: a real-time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans. Mycologia 105, 253–259 (2013).
Davy, C. M. et al. The other white-nose syndrome transcriptome: Tolerant and susceptible hosts respond differently to the pathogen Pseudogymnoascus destructans. Ecol. Evol. 7, 7161–7170 (2017).
Russell, R. E., Thogmartin, W. E., Erickson, R. A., Szymanski, J. & Tinsley, K. Estimating the short-term recovery potential of little brown bats in the eastern United States in the face of white-nose syndrome. Ecol. Model. 314, 111–117 (2015).
Fletcher, Q. E., Webber, Q. M. & Willis, C. K. Modelling the potential efficacy of treatments for white-nose syndrome in bats. J. Appl. Ecol. 57, 1283–1291 (2020).
Cheng, T. L. et al. Efficacy of a probiotic bacterium to treat bats affected by the disease white-nose syndrome. J. Appl. Ecol. 54, 701–708 (2017).
Hoyt, J. R. et al. Field trial of a probiotic bacteria to protect bats from white-nose syndrome. Sci. Rep. 9, 9158 (2019).
Cornelison, C., Gabriel, K., Barlament, C. & Crow, S. Jr. Inhibition of Pseudogymnoascus destructans growth from conidia and mycelial extension by bacterially produced volatile organic compounds. Mycopathologia 177, 1–10 (2014).
Chaturvedi, S. et al. Antifungal testing and high-throughput screening of compound library against Geomyces destructans, the etiologic agent of geomycosis (WNS) in bats. PLoS ONE 6, e17032 (2011).
Court, M. H. et al. Pharmacokinetics of terbinafine in little brown myotis (Myotis lucifugus) infected with Pseudogymnoascus destructans. Am. J. Vet. Res. 78, 90–99 (2017).
Kilpatrick, A. M. et al. Impact of censusing and research on wildlife populations. Conserv. Sci. Pract. 2, e264 (2020).
Weller, T. J. et al. A review of bat hibernacula across the western United States: Implications for white-nose syndrome surveillance and management. PLoS ONE 13, e0205647 (2018).
Garzoli, L. et al. First isolation of Pseudogymnoascus destructans, the fungal causative agent of white-nose disease, in bats from Italy. Mycopathologia 184, 637–644 (2019).
Pavlinić, I., Đaković, M. & Lojkić, I. Pseudogymnoascus destructans in Croatia confirmed. Eur. J. Wildl. Res. 61, 325–328 (2015).
das Neves Paiva-Cardoso, M. et al. First isolation of Pseudogymnoascus destructans in bats from Portugal. Eur. J. Wildl. Res. 60, 645–649 (2014).
Barlow, A. et al. First confirmation of Pseudogymnoascus destructans in British bats and hibernacula. Vet. Rec. 177, 73–73 (2015).
Simonovicova, A., Pangallo, D., Chovanova, K. & Lehotska, B. Geomyces destructans associated with bat disease WNS detected in Slovakia. Biologia 66, 562–564 (2011).
Sachanowicz, K., Stępień, A. & Ciechanowski, M. Prevalence and phenology of white-nose syndrome fungus Pseudogymnoascus destructans in bats from Poland. Cent. Eur. J. Biol. 9, 437–443 (2014).
Hayes, M. A. The geomyces fungi: ecology and distribution. Bioscience 62, 819–823 (2012).
Kendrick, B. The Fifth Kingdom (Hackett Publishing, 2017).
Palmer, J. M. et al. Molecular characterization of a heterothallic mating system in Pseudogymnoascus destructans, the fungus causing white-nose syndrome of bats. G3 4, 1755–1763 (2014).
Tennessee Wildlife Resources Agency. Tennessee Winter Bat Population and White-nose Syndrome Monitoring Reports (Tennessee Wildlife Resources Agency, 2020).
Colatskie, S. Missouri Bat Hibernacula Survey Results of 2011–2017, Following White-Nose Syndrome Arrival (Missouri Department of Conservation, 2017).
Graeter, G. Annual Program Report 2008–2009. 110–115 (Wildlife Diversity Program, Division of Wildlife Management, NC Wildlife Resources Commission, 2009).
Graeter, G. Annual Program Report 2009–2010. 127–137 (Wildlife Diversity Program, Division of Wildlife Management, NC Wildlife Resources Commission, 2010).
Kindel, J. Final Report: White-nose Syndrome Grants to States, F15AC00694 (South Carolina Department of Natural Resources, 2016).
Kindel, J. Final Report: White-nose Syndrome Grants to States SC-E-F16AP00833 (South Carolina Department of Natural Resources, 2017).
Morris, T. & Ferrall, E. 2019 White-nose Syndrome Season Summary (Wildlife Resources Division, Georgia Department of Natural Resources, 2020).
Cressler, C. E., McLeod, D. V., Rozins, C., Van Den Hoogen, J. & Day, T. The adaptive evolution of virulence: a review of theoretical predictions and empirical tests. Parasitology 143, 915–930 (2016).
Lacki, M. J., Hayes, J. P., Kurta, A. & Tuttle, M. D. Bats in Forests: Conservation and Management (JHU Press, 2007).
Miller, K. E. Trophic, habitat, distubance and conservation linkages between bat and aquatic communities in two Connecticut rivers. Doctoral dissertation, Wesleyan University. https://doi.org/10.14418/wes01.3.20 (2013).
Morningstar, D. E., Robinson, C. V., Shokralla, S. & Hajibabaei, M. Interspecific competition in bats and diet shifts in response to white-nose syndrome. Ecosphere 10, e02916 (2019).
Jachowski, D. S. et al. Disease and community structure: white-nose syndrome alters spatial and temporal niche partitioning in sympatric bat species. Divers. Distrib. 20, 1002–1015 (2014).
Kunz, T. H., de Torrez, E. B., Bauer, D., Lobova, T. & Fleming, T. H. in Year in Ecology and Conservation Biology Vol. 1223 Annals of the New York Academy of Sciences (eds Ostfeld, R. S. & Schlesinger, W. H.) 1–38 (Blackwell Science Publishing, 2011).
Maine, J. J. & Boyles, J. G. Bats initiate vital agroecological interactions in corn. Proc. Natl Acad. Sci. https://doi.org/10.1073/pnas.1505413112 (2015).
Frank, E. G. The Effects of Bat Population Losses on Infant Mortality through Pesticide Use in the US. (PhD thesis, Columbia University, 2016).
Wray, A. K. et al. Incidence and taxonomic richness of mosquitoes in the diets of little brown and big brown bats. J. Mammal. 99, 668–674 (2018).
Reiskind, M. H. & Wund, M. A. Experimental assessment of the impacts of northern long-eared bats on ovipositing Culex (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 46, 1037–1044 (2009).
Clare, E. L. et al. The diet of Myotis lucifugus across Canada: assessing foraging quality and diet variability. Mol. Ecol. 23, 3618–3632 (2014).
Acknowledgements
The authors thank S. Yamada for assistance with data curation, N. Fuller, N. Laggan, A. Grimaudo and J. Reichard for helpful comments on the manuscript, and the US National Science Foundation for funding (DEB-1911853).
Author information
Authors and Affiliations
Contributions
J.R.H. and K.E.L. drafted the original figures. All authors contributed to writing the original draft and to the editing of the revised work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Microbiology thanks M. Fisher, N. Martinkova and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hoyt, J.R., Kilpatrick, A.M. & Langwig, K.E. Ecology and impacts of white-nose syndrome on bats. Nat Rev Microbiol 19, 196–210 (2021). https://doi.org/10.1038/s41579-020-00493-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-020-00493-5
This article is cited by
-
Effects of ophidiomycosis on movement, survival, and reproduction of eastern foxsnakes (Pantherophis vulpinus)
Scientific Reports (2024)
-
The effects of spatially-constrained treatment regions upon a model of wombat mange
Journal of Mathematical Biology (2024)
-
Multiresidue analysis of bat guano using GC-MS/MS
Analytical and Bioanalytical Chemistry (2024)
-
Higher white-nose syndrome fungal isolate yields from UV-guided wing biopsies compared with skin swabs and optimal culture media
BMC Veterinary Research (2023)
-
When the host’s away, the pathogen will play: the protective role of the skin microbiome during hibernation
Animal Microbiome (2023)