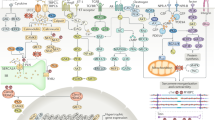
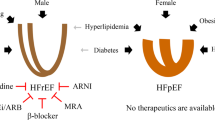
Abstract
Heart failure with preserved ejection fraction (HFpEF) is a major, worldwide health-care problem. Few therapies for HFpEF exist because the pathophysiology of this condition is poorly defined and, increasingly, postulated to be diverse. Although perturbations in other organs contribute to the clinical profile in HFpEF, altered cardiac structure, function or both are the primary causes of this heart failure syndrome. Therefore, studying myocardial tissue is fundamental to improve pathophysiological insights and therapeutic discovery in HFpEF. Most studies of myocardial changes in HFpEF have relied on cardiac tissue from animal models without (or with limited) confirmatory studies in human cardiac tissue. Animal models of HFpEF have evolved based on theoretical HFpEF aetiologies, but these models might not reflect the complex pathophysiology of human HFpEF. The focus of this Review is the pathophysiological insights gained from studies of human HFpEF myocardium. We outline the rationale for these studies, the challenges and opportunities in obtaining myocardial tissue from patients with HFpEF and relevant comparator groups, the analytical approaches, the pathophysiological insights gained to date and the remaining knowledge gaps. Our objective is to provide a roadmap for future studies of cardiac tissue from diverse cohorts of patients with HFpEF, coupling discovery biology with measures to account for pathophysiological diversity.
Key points
-
Few studies of cardiac tissue from patients with heart failure with preserved ejection fraction (HFpEF) and comparator groups have been published.
-
Most of these studies were small and showed variability in tissue source, case–control ascertainment and analytical approaches.
-
Cardiac tissue samples from patients with HFpEF show variable degrees of myocardial fibrosis, hypertrophy, microvascular rarefaction, T-tubule disruption, systolic and diastolic dysfunction and impaired metabolism.
-
Only eight candidate pathophysiological pathways have been examined in hypothesis-driven studies of cardiac tissue from patients with HFpEF, and these studies have not led to consensus on its pathophysiology.
-
Only four studies used discovery transcriptomics or proteomic technologies in cardiac tissue from patients with HFpEF and comparators, and showed intriguing, but highly variable, findings.
-
Studies of heart tissue in large and diverse cohorts of patients with HFpEF are urgently needed, with discovery multiomics, appropriate bioinformatic analyses and rigorous validation to address pathophysiological diversity and gain novel therapeutic insights.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Savarese, G. et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 118, 3272–3287 (2022).
Borlaug, B. A., Sharma, K., Shah, S. J. & Ho, J. E. Heart failure with preserved ejection fraction: JACC scientific statement. J. Am. Coll. Cardiol. 81, 1810–1834 (2023).
Dunlay, S. M., Roger, V. L. & Redfield, M. M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 14, 591–602 (2017).
Redfield, M. M. & Borlaug, B. A. Heart failure with preserved ejection fraction: a review. JAMA 329, 827–838 (2023).
Desai, A. S., Lam, C. S. P., McMurray, J. J. V. & Redfield, M. M. How to manage heart failure with preserved ejection fraction: practical guidance for clinicians. JACC Heart Fail. 11, 619–636 (2023).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43 (2012).
Kumanyika, S. & Dietz, W. H. Solving population-wide obesity – progress and future prospects. N. Engl. J. Med. 383, 2197–2200 (2020).
Mossadeghi, B. et al. Multimorbidity and social determinants of health in the US prior to the COVID-19 pandemic and implications for health outcomes: a cross-sectional analysis based on NHANES 2017-2018. BMC Public. Health 23, 887 (2023).
Chamberlain, A. M. et al. Multimorbidity in heart failure: a community perspective. Am. J. Med. 128, 38–45 (2015).
Teramoto, K. et al. Epidemiology and clinical features of heart failure with preserved ejection fraction. Card. Fail. Rev. 8, e27 (2022).
Borlaug, B. A. et al. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiologic targets. Cardiovasc. Res. 118, 3434–3450 (2022).
Campbell, R. T. et al. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? J. Am. Coll. Cardiol. 60, 2349–2356 (2012).
Mohammed, S. F. et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ. Heart Fail. 5, 710–719 (2012).
Paulus, W. J. & Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Joseph, J. et al. Genetic architecture of heart failure with preserved versus reduced ejection fraction. Nat. Commun. 13, 7753 (2022).
Bozkurt, B. et al. Universal definition and classification of heart failure: a Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 23, 352–380 (2021).
Charles, C. J., Rademaker, M. T., Scott, N. J. A. & Richards, A. M. Large animal models of heart failure: reduced vs. preserved ejection fraction. Animals 10, 1906 (2020).
Smith, A. N. et al. Genomic, proteomic, and metabolic comparisons of small animal models of heart failure with preserved ejection fraction: a tale of mice, rats, and cats. J. Am. Heart Assoc. 11, e026071 (2022).
Shapiro, B. P. et al. Mineralocorticoid signaling in transition to heart failure with normal ejection fraction. Hypertension 51, 289–295 (2008).
Schiattarella, G. G. et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568, 351–356 (2019).
Conceicao, G., Heinonen, I., Lourenco, A. P., Duncker, D. J. & Falcao-Pires, I. Animal models of heart failure with preserved ejection fraction. Neth. Heart J. 24, 275–286 (2016).
Valero-Munoz, M., Backman, W. & Sam, F. Murine models of heart failure with preserved ejection fraction: a “fishing expedition”. JACC Basic. Transl. Sci. 2, 770–789 (2017).
Lerman, L. O. et al. Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 73, e87–e120 (2019).
Tong, D. et al. Female sex is protective in a preclinical model of heart failure with preserved ejection fraction. Circulation 140, 1769–1771 (2019).
Du, X. J. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc. Res. 63, 510–519 (2004).
Selvaraj, S. et al. Systolic blood pressure in heart failure with preserved ejection fraction treated with sacubitril/valsartan. J. Am. Coll. Cardiol. 75, 1644–1656 (2020).
Hackam, D. G. & Redelmeier, D. A. Translation of research evidence from animals to humans. JAMA 296, 1731–1732 (2006).
Vyas, M. V., Gros, R. & Hackam, D. G. Translation of cardiovascular animal models to human randomized trials. Am. J. Cardiol. 137, 141 (2020).
McGonigle, P. & Ruggeri, B. Animal models of human disease: challenges in enabling translation. Biochem. Pharmacol. 87, 162–171 (2014).
Bishu, K. et al. Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation 124, 2882–2891 (2011).
Takimoto, E. et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 11, 214–222 (2005).
Bermejo, J. et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur. Heart J. 39, 1255–1264 (2018).
Desai, A. S. et al. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am. Heart J. 162, 966–972.e10 (2011).
Sangaralingham, S. J., Kuhn, M., Cannone, V., Chen, H. H. & Burnett, J. C. Natriuretic peptide pathways in heart failure: further therapeutic possibilities. Cardiovasc. Res. 118, 3416–3433 (2023).
Lewis, G. A. et al. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat. Med. 27, 1477–1482 (2021).
Hahn, V. S. et al. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail. 8, 712–724 (2020).
Aoki, T. et al. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. Comparison between preserved and reduced ejection fraction heart failure. Circ. J. 75, 2605–2613 (2011).
Kasner, M. et al. Multimodality imaging approach in the diagnosis of chronic myocarditis with preserved left ventricular ejection fraction (MCpEF): the role of 2D speckle-tracking echocardiography. Int. J. Cardiol. 243, 374–378 (2017).
Kasner, M. et al. Functional iron deficiency and diastolic function in heart failure with preserved ejection fraction. Int. J. Cardiol. 168, 4652–4657 (2013).
Kasner, M. et al. Simultaneous estimation of NT-proBNP on top to mitral flow Doppler echocardiography as an accurate strategy to diagnose diastolic dysfunction in HFNEF. Int. J. Cardiol. 149, 23–29 (2011).
Kasner, M. et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 57, 977–985 (2011).
Lee, D. I. et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 519, 472–476 (2015).
Westermann, D. et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 4, 44–52 (2011).
Yang, J. et al. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat. Commun. 7, 13710 (2016).
Aslam, M. I. et al. Reduced right ventricular sarcomere contractility in heart failure with preserved ejection fraction and severe obesity. Circulation 143, 965–967 (2021).
Hahn, V. S. et al. Myocardial gene expression signatures in human heart failure with preserved ejection fraction. Circulation 143, 120–134 (2021).
Hahn, V. S. et al. Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation 147, 1147–1161 (2023).
Besler, C. et al. Evaluation of phosphodiesterase 9A as a novel biomarker in heart failure with preserved ejection fraction. ESC Heart Fail. 8, 1861–1872 (2021).
Borbely, A. et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation 111, 774–781 (2005).
Franssen, C. et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 4, 312–324 (2016).
Friebel, J. et al. Protease-activated receptor 2 deficiency mediates cardiac fibrosis and diastolic dysfunction. Eur. Heart J. 40, 3318–3332 (2019).
Hamdani, N. et al. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. Eur. Heart J. 30, 1863–1872 (2009).
Kolijn, D. et al. Enhanced cardiomyocyte function in hypertensive rats with diastolic dysfunction and human heart failure patients after acute treatment with soluble guanylyl cyclase (sGC) activator. Front. Physiol. 11, 345 (2020).
Kolijn, D. et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 117, 495–507 (2021).
Kühl, U. et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112, 1965–1970 (2005).
Pabel, S. et al. Empagliflozin directly improves diastolic function in human heart failure. Eur. J. Heart Fail. 20, 1690–1700 (2018).
Tong, D. et al. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ. Res. 128, 1629–1641 (2021).
Tschöpe, C. et al. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation 111, 879–886 (2005).
van Heerebeek, L. et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113, 1966–1973 (2006).
van Heerebeek, L. et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126, 830–839 (2012).
van Heerebeek, L. et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117, 43–51 (2008).
Yousefi, K. et al. osteopontin promotes left ventricular diastolic dysfunction through a mitochondrial pathway. J. Am. Coll. Cardiol. 73, 2705–2718 (2019).
Lopez, B., Gonzalez, A., Querejeta, R., Larman, M. & Diez, J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J. Am. Coll. Cardiol. 48, 89–96 (2006).
Querejeta, R. et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation 110, 1263–1268 (2004).
Hull, J. V. et al. Risks of right heart catheterization and right ventricular biopsy: a 12-year, single-center experience. Mayo Clin. Proc. 98, 419–431 (2023).
LeWinter, M. M. et al. Abundance, localization, and functional correlates of the advanced glycation end-product carboxymethyl lysine in human myocardium. Physiol. Rep. 5, e13462 (2017).
Robinson, E. L. et al. Dissecting the transcriptome in cardiovascular disease. Cardiovasc. Res. 118, 1004–1019 (2022).
Muehlschlegel, J. D. et al. Using next-generation RNA sequencing to examine ischemic changes induced by cold blood cardioplegia on the human left ventricular myocardium transcriptome. Anesthesiology 122, 537–550 (2015).
Stone, G. et al. Sex differences in gene expression in response to ischemia in the human left ventricular myocardium. Hum. Mol. Genet. 28, 1682–1693 (2019).
Chaffin, M. et al. Single-nucleus profiling of human dilated and hypertrophic cardiomyopathy. Nature 608, 174–180 (2022).
Liu, C. F. et al. Whole-transcriptome profiling of human heart tissues reveals the potential novel players and regulatory networks in different cardiomyopathy subtypes of heart failure. Circ. Genom. Precis. Med. 14, e003142 (2021).
Margulies, K. B. et al. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ. Res. 96, 592–599 (2005).
Abdellatif, M. et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci. Transl. Med. 13, eabd7064 (2021).
Power, B. M. & Van Heerden, P. V. The physiological changes associated with brain death – current concepts and implications for treatment of the brain dead organ donor. Anaesth. Intensive Care 23, 26–36 (1995).
Khush, K. K. et al. Left ventricular dysfunction associated with brain death: results from the Donor Heart Study. Circulation 148, 822–833 (2023).
Lei, I. et al. “The secret life of human donor hearts”: an examination of transcriptomic events during cold storage. Circ. Heart Fail. 13, e006409 (2020).
Flam, E. et al. Integrated landscape of cardiac metabolism in end-stage human nonischemic dilated cardiomyopathy. Nat. Cardiovasc. Res. 1, 817–829 (2022).
Mohammed, S. F. et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131, 550–559 (2015).
Mohammed, S. F. et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2, 113–122 (2014).
Fayyaz, A. U. et al. Histologic and proteomic remodeling of the pulmonary veins and arteries in a porcine model of chronic pulmonary venous hypertension. Cardiovasc. Res. 119, 268–282 (2023).
Judge, D. P. & Brown, E. E. Bringing autopsies into the molecular genetic era. Circulation 137, 2727–2729 (2018).
Thiene, G. & Saffitz, J. E. Autopsy as a source of discovery in cardiovascular medicine: then and now. Circulation 137, 2683–2685 (2018).
Cocariu, E. A. et al. Correlations between the autolytic changes and postmortem interval in refrigerated cadavers. Rom. J. Intern. Med. 54, 105–112 (2016).
Doll, S. et al. Region and cell-type resolved quantitative proteomic map of the human heart. Nat. Commun. 8, 1469 (2017).
Herrington, D. M. et al. Proteomic architecture of human coronary and aortic atherosclerosis. Circulation 137, 2741–2756 (2018).
Tucker, N. R. et al. Transcriptional and cellular diversity of the human heart. Circulation 142, 466–482 (2020).
Garmany, R. et al. Multi-omic architecture of obstructive hypertrophic cardiomyopathy. Circ. Genom. Precis. Med. 16, e003756 (2023).
Litvinukova, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Liu, C. F. et al. Global analysis of histone modifications and long-range chromatin interactions revealed the differential cistrome changes and novel transcriptional players in human dilated cardiomyopathy. J. Mol. Cell Cardiol. 145, 30–42 (2020).
Liu, J. et al. Transcriptional and immune landscape of cardiac sarcoidosis. Circ. Res. 131, 654–669 (2022).
Reichart, D. et al. Pathogenic variants damage cell composition and single cell transcription in cardiomyopathies. Science 377, eabo1984 (2022).
van Heesch, S. et al. The translational landscape of the human heart. Cell 178, 242–260.e29 (2019).
Nirschl, J. J. et al. A deep-learning classifier identifies patients with clinical heart failure using whole-slide images of H&E tissue. PLoS ONE 13, e0192726 (2018).
Peyster, E. G. et al. An automated computational image analysis pipeline for histological grading of cardiac allograft rejection. Eur. Heart J. 42, 2356–2369 (2021).
Kitata, R. B., Yang, J. C. & Chen, Y. J. Advances in data-independent acquisition mass spectrometry towards comprehensive digital proteome landscape. Mass. Spectrom. Rev. 42, 2324–2348 (2023).
Messner, C. B. et al. Mass spectrometry-based high-throughput proteomics and its role in biomedical studies and systems biology. Proteomics 23, e2200013 (2023).
Cui, Y. et al. Single-cell transcriptome analysis maps the developmental track of the human heart. Cell Rep. 26, 1934–1950.e5 (2019).
Gladka, M. M. et al. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulator of fibroblasts activation. Circulation 138, 166–180 (2018).
He, S. et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 40, 1794–1806 (2022).
Blaser, M. C., Kraler, S., Luscher, T. F. & Aikawa, E. Multi-omics approaches to define calcific aortic valve disease pathogenesis. Circ. Res. 128, 1371–1397 (2021).
Ferrucci, L. et al. Transcriptomic and proteomic of gastrocnemius muscle in peripheral artery disease. Circ. Res. 132, 1428–1443 (2023).
Joshi, A., Rienks, M., Theofilatos, K. & Mayr, M. Systems biology in cardiovascular disease: a multiomics approach. Nat. Rev. Cardiol. 18, 313–330 (2021).
Guo, Y., Zhao, S., Li, C. I., Sheng, Q. & Shyr, Y. RNAseqPS: a web tool for estimating sample size and power for RNAseq experiment. Cancer Inform. 13, 1–5 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Saccenti, E. & Timmerman, M. E. Approaches to sample size determination for multivariate data: applications to PCA and PLS-DA of omics data. J. Proteome Res. 15, 2379–2393 (2016).
Squair, J. W. et al. Confronting false discoveries in single-cell differential expression. Nat. Commun. 12, 5692 (2021).
Ahmad, S. et al. The functional consequences of sodium channel NaV 1.8 in human left ventricular hypertrophy. ESC Heart Fail 6, 154–163 (2019).
Chaanine, A. H. et al. Mitochondrial morphology, dynamics, and function in human pressure overload or ischemic heart disease with preserved or reduced ejection fraction. Circ. Heart Fail. 12, e005131 (2019).
D’Assante, R. et al. Myocardial expression of somatotropic axis, adrenergic signalling, and calcium handling genes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. ESC Heart Fail. 8, 1681–1686 (2021).
Das, S. et al. Transcriptomics of cardiac biopsies reveals differences in patients with or without diagnostic parameters for heart failure with preserved ejection fraction. Sci. Rep. 9, 3179 (2019).
Davila, A. et al. Adenosine kinase inhibition augments conducted vasodilation and prevents left ventricle diastolic dysfunction in heart failure with preserved ejection fraction. Circ. Heart Fail. 12, e005762 (2019).
Donaldson, C. et al. Myosin cross-bridge dynamics in patients with hypertension and concentric left ventricular remodeling. Circ. Heart Fail. 5, 803–811 (2012).
Falcao-Pires, I. et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation 124, 1151–1159 (2011).
Frisk, M. et al. Etiology-dependent impairment of diastolic cardiomyocyte calcium homeostasis in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 77, 405–419 (2021).
Gao, Q., He, S., Peng, Y., Su, P. & Zhao, L. Proteomic profiling of epicardial fat in heart failure with preserved versus reduced and mildly reduced ejection fraction. J. Cell Mol. Med. 27, 727–735 (2023).
He, S. et al. Proteomic analysis of epicardial adipose tissue from heart disease patients with concomitant heart failure with preserved ejection fraction. Int. J. Cardiol. 362, 118–125 (2022).
Heymans, S. et al. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 112, 1136–1144 (2005).
Hulsmans, M. et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 215, 423–440 (2018).
Merino, D. et al. BMP-7 attenuates left ventricular remodelling under pressure overload and facilitates reverse remodelling and functional recovery. Cardiovasc. Res. 110, 331–345 (2016).
Perez-Belmonte, L. M. et al. Expression of epicardial adipose tissue thermogenic genes in patients with reduced and preserved ejection fraction heart failure. Int. J. Med. Sci. 14, 891–895 (2017).
Peterzan, M. A. et al. Cardiac energetics in patients with aortic stenosis and preserved versus reduced ejection fraction. Circulation 141, 1971–1985 (2020).
Runte, K. E. et al. Relaxation and the role of calcium in isolated contracting myocardium from patients with hypertensive heart disease and heart failure with preserved ejection fraction. Circ. Heart Fail. 10, e004311 (2017).
Sato, M. et al. Proteome analysis demonstrates involvement of endoplasmic reticulum stress response in human myocardium with subclinical left ventricular diastolic dysfunction. Geriatr. Gerontol. Int. 21, 577–583 (2021).
Sebastiao, M. J. et al. Unveiling human proteome signatures of heart failure with preserved ejection fraction. Biomedicines 10, 2943 (2022).
Stadiotti, I. et al. Pressure overload activates DNA-damage response in cardiac stromal cells: a novel mechanism behind heart failure with preserved ejection fraction? Front. Cardiovasc. Med. 9, 878268 (2022).
Trum, M. et al. Empagliflozin inhibits Na+/H+ exchanger activity in human atrial cardiomyocytes. ESC Heart Fail. 7, 4429–4437 (2020).
Zhang, Y. et al. Phenotypic characterization of primary cardiac fibroblasts from patients with HFpEF. PLoS ONE 17, e0262479 (2022).
Zile, M. R. et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131, 1247–1259 (2015).
Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 79, e263–e421 (2022).
Mohammed, S. F., Majure, D. T. & Redfield, M. M. Zooming in on the microvasculature in heart failure with preserved ejection fraction. Circ. Heart Fail. 9, e003272 (2016).
Burkhoff, D. et al. Levosimendan improves hemodynamics and exercise tolerance in PH-HFpEF: results of the randomized placebo-controlled HELP trial. JACC Heart Fail. 9, 360–370 (2021).
Capone, F., Vettor, R. & Schiattarella, G. G. Cardiometabolic HFpEF: NASH of the heart. Circulation 147, 451–453 (2023).
Krajcova, A. et al. High resolution respirometry to assess function of mitochondria in native homogenates of human heart muscle. PLoS ONE 15, e0226142 (2020).
Sabbah, M. S. et al. Obese-inflammatory phenotypes in heart failure with preserved ejection fraction. Circ. Heart Fail. 13, e006414 (2020).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Schiattarella, G. G. et al. Xbp1s-FoxO1 axis governs lipid accumulation and contractile performance in heart failure with preserved ejection fraction. Nat. Commun. 12, 1684 (2021).
Wu, L., Sowers, J. R., Zhang, Y. & Ren, J. Targeting DNA damage response in cardiovascular diseases: from pathophysiology to therapeutic implications. Cardiovasc. Res. 119, 691–709 (2023).
Packer, M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J. Am. Coll. Cardiol. 71, 2360–2372 (2018).
Little, W. C. et al. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J. Card. Fail. 11, 191–195 (2005).
Van Tassell, B. W. et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ. Heart Fail. 11, e005036 (2018).
Van Tassell, B. W. et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 113, 321–327 (2014).
Yura, Y., Sano, S. & Walsh, K. Clonal hematopoiesis: a new step linking inflammation to heart failure. JACC Basic. Transl. Sci. 5, 196–207 (2020).
Lindskog, C. et al. The human cardiac and skeletal muscle proteomes defined by transcriptomics and antibody-based profiling. BMC Genomics 16, 475 (2015).
Kanemaru, K. et al. Spatially resolved multiomics of human cardiac niches. Nature 619, 801–810 (2023).
Pieske, B. et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 40, 3297–3317 (2019).
Popovic, D. et al. Ventricular stiffening and chamber contracture in heart failure with higher ejection fraction. Eur. J. Heart Fail. 25, 657–668 (2023).
Pietzner, M. et al. Mapping the proteo-genomic convergence of human diseases. Science 374, eabj1541 (2021).
Rasooly, D. et al. Genome-wide association analysis and Mendelian randomization proteomics identify drug targets for heart failure. Nat. Commun. 14, 3826 (2023).
Shah, S. J., Butler, J., Shah, S. H., Kamphaus, T. N. & Sachdev, V. Accelerating therapeutic discoveries for heart failure: a new public-private partnership. Nat. Rev. Drug. Discov. 21, 781–782 (2022).
Levac, D., Colquhoun, H. & O’Brien, K. K. Scoping studies: advancing the methodology. Implement. Sci. 5, 69 (2010).
Munn, Z. et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 18, 143 (2018).
Peters, M. D. et al. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 13, 141–146 (2015).
Acknowledgements
A.U.F. and K.E.K. are supported by T32 HL007111. B.A.B. is supported by R01 HL128526, R01 HL162828, U01 HL160226 and W81XWH2210245 from the US Department of Defense, and a grant from the Accelerating Medicines Partnership for Heart Failure through the Foundation for the National Institutes of Health (FNIH). S.D. is supported by HL162828 and U01HL160226. K.B.M. is supported by R01 HL149891 and a grant from the Accelerating Medicines Partnership for Heart Failure through the FNIH. Y.W. is supported by HL 148339, DK 117910 and a grant from the Cardiovascular Department, Mayo Clinic, Rochester, MN. M.M.R. is supported by HL162828, U01 HL160226 and a grant from the Accelerating Medicines Partnership for Heart Failure through the FNIH.
Author information
Authors and Affiliations
Contributions
A.U.F., M.E., L.J.P., K.E.K. and M.M.R. researched data for the article. A.U.F. and M.M.R. contributed substantially to discussion of the content and wrote the manuscript. All authors reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Michele Senni, Walter J. Paulus and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
The review strategy to identify all studies of human myocardial tissue in heart failure with preserved ejection fraction (HFpEF) was performed in accordance with the systematic scoping review guidelines150,151,152. Databases, including Ovid MEDLINER, Ovid EMBASE, Scopus and Web of Science, were searched in English from their inception until 17 August 2022. An experienced librarian (L.J.P.), with input from the rest of the authors, designed and executed the search strategy using controlled vocabulary and keywords for human tissue and HFpEF or diastolic heart failure or dysfunction. Inclusion criteria included: (1) original investigation, (2) study of human cardiac tissue solely or for validation of findings observed in animal models, and (3) tissue obtained from patients with clinical diagnosis of HFpEF or rigorously documented diastolic dysfunction. Use of human cardiac tissue as non-HFpEF comparator was recorded but not required for inclusion. Studies that relied solely on imaging or other non-tissue collection procedures to characterize myocardial properties were excluded. Two investigators (A.U.F. and M.E.) reviewed titles, abstracts and figures from the search results and excluded articles clearly not meeting the predefined eligibility criteria. Three investigators (A.U.F., M.E. and M.M.R.) independently reviewed the remaining studies in detail and excluded those that did not meet the selection criteria or that were restricted to specific heart failure aetiologies (infiltrative or hypertrophic cardiomyopathy). Abstracted data included study type (human-only versus animal model plus human tissue), heart failure diagnostic criteria, ejection fraction criteria for HFpEF diagnosis, and comparator groups (heart failure with reduced ejection fraction (HFrEF) or comparators without heart failure (non-failing comparators)). Within HFpEF, other comparators were noted (Supplementary Box 1). Additionally, group sizes, age, whether both sexes were included, type of tissue (myocardium versus adipose), biopsy site and biopsy acquisition method were recorded. When human tissue studies were performed as part of an animal model-based study, only findings pertinent to the human tissue studies were presented. A total of 6,465 articles were identified from the database search and 14 from other sources (such as authors’ previous knowledge or included in the reference list of identified articles) and 4,083 duplicates were removed (Supplementary Fig. 1a). After title, abstract and figure review, 2,254 articles not meeting the inclusion criteria were excluded. After full-text assessment, another 86 studies did not meet inclusion criteria. Ultimately, 56 studies qualified for inclusion20,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,66,73,78,79,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128, which included 11 (refs. 37,38,42,45,52,55,58,61,64,66,114) identified in cited references or by investigator pre-existing knowledge, and three47,115,124 that were published subsequent to the literature search end-date. The included studies were from 2004 to 2023 (Supplementary Fig. 1b), with 28 (50%) studies published since 2018. Most studies were published in high-impact journals (Supplementary Fig. 1c). Some relevant studies might have been missed by our search and review strategies, and new studies might have emerged during the review and publication process. Our summaries were brief and focused on mechanistic insights and did not detail the strengths and weaknesses of each study.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fayyaz, A.U., Eltony, M., Prokop, L.J. et al. Pathophysiological insights into HFpEF from studies of human cardiac tissue. Nat Rev Cardiol (2024). https://doi.org/10.1038/s41569-024-01067-1
Accepted:
Published:
DOI: https://doi.org/10.1038/s41569-024-01067-1