Abstract
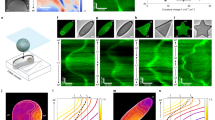
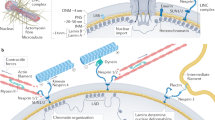
The cell nucleus is enveloped by a complex membrane, whose wrinkling has been implicated in disease and cellular aging. The biophysical dynamics and spectral evolution of nuclear wrinkling during multicellular development remain poorly understood due to a lack of direct quantitative measurements. Here we characterize the onset and dynamics of nuclear wrinkling during egg development in the fruit fly when nurse cell nuclei increase in size and display stereotypical wrinkling behaviour. A spectral analysis of three-dimensional high-resolution live-imaging data from several hundred nuclei reveals a robust asymptotic power-law scaling of angular fluctuations consistent with renormalization and scaling predictions from a nonlinear elastic shell model. We further demonstrate that nuclear wrinkling can be reversed through osmotic shock and suppressed by microtubule disruption, providing tunable physical and biological control parameters for probing the mechanical properties of the nuclear envelope. Our findings advance the biophysical understanding of nuclear membrane fluctuations during early multicellular development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data supporting the findings of this work are available within the paper and its Supplementary Information. Higher-resolution images, point cloud data, spherical harmonic processing codes and spreadsheets containing the experimental data points shown in the plots are available via Figshare at https://doi.org/10.6084/m9.figshare.23800287. Due to file-size limitations, the raw microscopy data are available from the corresponding authors upon request.
Code availability
The code used for numerical simulations is publicly available via GitHub at https://github.com/NicoRomeo/d3shell.
References
Blees, M. K. et al. Graphene kirigami. Nature 524, 204–207 (2015).
Los, J. H., Fasolino, A. & Katsnelson, M. I. Mechanics of thermally fluctuating membranes. npj 2D Mater. Appl. 1, 9 (2017).
Yoo, J. & Aksimentiev, A. In situ structure and dynamics of DNA origami determined through molecular dynamics simulations. Proc. Natl Acad. Sci. USA 110, 20099–20104 (2013).
Kalukula, Y., Stephens, A. D., Lammerding, J. & Gabriele, S. Mechanics and functional consequences of nuclear deformations. Nat. Rev. Mol. Cell Biol. 23, 583–602 (2022).
Lomakin, A. J. et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894 (2020).
Almonacid, M. et al. Active fluctuations of the nuclear envelope shape the transcriptional dynamics in oocytes. Dev. Cell 51, 145–157.e10 (2019).
Biedzinski, S. et al. Microtubules control nuclear shape and gene expression during early stages of hematopoietic differentiation. EMBO J. 39, e103957 (2020).
Brochard, F. & Lennon, J. F. Frequency spectrum of the flicker phenomenon in erythrocytes. J. Phys. 36, 1035–1047 (1975).
Betz, T., Lenz, M., Joanny, J.-F. & Sykes, C. ATP-dependent mechanics of red blood cells. Proc. Natl Acad. Sci. USA 106, 15320–15325 (2009).
Bowick, M. J., Košmrlj, A., Nelson, D. R. & Sknepnek, R. Non-Hookean statistical mechanics of clamped graphene ribbons. Phys. Rev. B 95, 104109 (2017).
Kantsler, V., Segre, E. & Steinberg, V. Vesicle dynamics in time-dependent elongation flow: wrinkling instability. Phys. Rev. Lett. 99, 178102 (2007).
Kokot, G., Faizi, H. A., Pradillo, G. E., Snezhko, A. & Vlahovska, P. M. Spontaneous self-propulsion and nonequilibrium shape fluctuations of a droplet enclosing active particles. Commun. Phys. 5, 91 (2022).
Honerkamp-Smith, A. R., Woodhouse, F. G., Kantsler, V. & Goldstein, R. E. Membrane viscosity determined from shear-driven flow in giant vesicles. Phys. Rev. Lett. 111, 038103 (2013).
Ben-Isaac, E. et al. Effective temperature of red-blood-cell membrane fluctuations. Phys. Rev. Lett. 106, 238103 (2011).
Turlier, H. et al. Equilibrium physics breakdown reveals the active nature of red blood cell flickering. Nat. Phys. 12, 513–519 (2016).
Chu, F.-Y., Haley, S. C. & Zidovska, A. On the origin of shape fluctuations of the cell nucleus. Proc. Natl Acad. Sci. USA 114, 10338–10343 (2017).
Venturini, V. et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370, eaba2644 (2020).
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006).
Mounkes, L. C., Kozlov, S., Hernandez, L., Sullivan, T. & Stewart, C. L. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423, 298–301 (2003).
Nelson, D., Piran, T. & Weinberg, S. Statistical Mechanics of Membranes and Surfaces—Proceedings of the 5th Jerusalem Winter School for Theoretical Physics (World Scientific, 1989).
Košmrlj, A. & Nelson, D. R. Statistical mechanics of thin spherical shells. Phys. Rev. X 7, 011002 (2017).
Aebi, U., Cohn, J., Buhle, L. & Gerace, L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323, 560–564 (1986).
Lammerding, J. et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Investig. 113, 370–378 (2004).
Strambio-De-Castillia, C., Niepel, M. & Rout, M. P. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat. Rev. Mol. Cell Biol. 11, 490–501 (2010).
Knockenhauer, K. E. & Schwartz, T. U. The nuclear pore complex as a flexible and dynamic gate. Cell 164, 1162–1171 (2016).
Hudson, A. M. & Cooley, L. Methods for studying oogenesis. Methods 68, 207–217 (2014).
King, R. C., Rubinson, A. C. & Smith, R. F. Oogenesis in adult Drosophila melanogaster. Growth 20, 121–157 (1956).
McLaughlin, J. M. & Bratu, D. P. Drosophila melanogaster oogenesis: an overview. in Drosophila Oogenesis, Methods in Molecular Biology Vol. 1328 (eds Bratu, D. P. & McNeil, G. P.) 1–20 (Springer, 2015).
Bastock, R. & St Johnston, D. Drosophila oogenesis. Curr. Biol. 18, R1082–R1087 (2008).
Imran Alsous, J. et al. Dynamics of hydraulic and contractile wave-mediated fluid transport during Drosophila oogenesis. Proc. Natl Acad. Sci. USA 118, e2019749118 (2021).
Mahajan-Miklos, S. & Cooley, L. Intercellular cytoplasm transport during Drosophila oogenesis. Dev. Biol. 165, 336–351 (1994).
Lin, H. & Spradling, A. C. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 159, 140–152 (1993).
Tzur, A., Kafri, R., LeBleu, V. S., Lahav, G. & Kirschner, M. W. Cell growth and size homeostasis in proliferating animal cells. Science 325, 167–171 (2009).
Malhas, A. N. & Vaux, D. J. Nuclear Envelope Invaginations and Cancer 523–535 (Springer, 2014).
Fricker, M., Hollinshead, M., White, N. & Vaux, D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 136, 531–544 (1997).
Zilman, A. G. & Granek, R. Undulations and dynamic structure factor of membranes. Phys. Rev. Lett. 77, 4788–4791 (1996).
Andrejevic, J., Lee, L. M., Rubinstein, S. M. & Rycroft, C. H. A model for the fragmentation kinetics of crumpled thin sheets. Nat. Commun. 12, 1470 (2021).
Witten, T. A. Stress focusing in elastic sheets. Rev. Mod. Phys. 79, 643–675 (2007).
Paulose, J., Vliegenthart, G. A., Gompper, G. & Nelson, D. R. Fluctuating shells under pressure. Proc. Natl Acad. Sci. USA 109, 19551–19556 (2012).
Guilak, F., Tedrow, J. R. & Burgkart, R. Viscoelastic properties of the cell nucleus. Biochem. Biophys. Res. Commun. 269, 781–786 (2000).
Funkhouser, C. M. et al. Mechanical model of blebbing in nuclear lamin meshworks. Proc. Natl Acad. Sci. USA 110, 3248–3253 (2013).
Kim, D.-H. et al. Volume regulation and shape bifurcation in the cell nucleus. J. Cell Sci. 128, 3375–3385 (2015).
Dahl, K. N., Kahn, S. M., Wilson, K. L. & Discher, D. E. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 117, 4779–4786 (2004).
Enyedi, B. & Niethammer, P. Nuclear membrane stretch and its role in mechanotransduction. Nucleus 8, 156–161 (2017).
Pécréaux, J., Döbereiner, H.-G., Prost, J., Joanny, J.-F. & Bassereau, P. Refined contour analysis of giant unilamellar vesicles. Eur. Phys. J. E 13, 277–290 (2004).
Landau, L. D. & Lifshitz, E. M. Theory of Elasticity. Number 7 in Course of Theoretical Physics (Elsevier, 2009).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Baumgarten, L. & Kierfeld, J. Buckling of thermally fluctuating spherical shells: parameter renormalization and thermally activated barrier crossing. Phys. Rev. E 97, 052801 (2018).
Schmidt, C. F. et al. Existence of a flat phase in red cell membrane skeletons. Science 259, 952–955 (1993).
Yalonetskaya, A., Mondragon, A. A., Hintze, Z. J., Holmes, S. & McCall, K. Nuclear degradation dynamics in a nonapoptotic programmed cell death. Cell Death Differ. 27, 711–724 (2020).
Düring, G., Josserand, C., Krstulovic, G. & Rica, S. Strong turbulence for vibrating plates: emergence of a Kolmogorov spectrum. Phys. Rev. Fluids 4, 064804 (2019).
Swift, J. et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013).
Schulze, S. R. et al. Molecular genetic analysis of the nested Drosophila melanogaster lamin C gene. Genetics 171, 185–196 (2005).
Riemer, D. et al. Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. J. Cell Sci. 108, 3189–3198 (1995).
Agrawal, V., Pandey, V. & Mitra, D. Active buckling of pressurized spherical shells: Monte Carlo simulation. Preprint at https://arxiv.org/abs/2206.14172 (2022).
Chakrabarti, B. et al. Flexible filaments buckle into helicoidal shapes in strong compressional flows. Nat. Phys. 16, 689–694 (2020).
Loubet, B., Seifert, U. & Lomholt, M. A. Effective tension and fluctuations in active membranes. Phys. Rev. E 85, 031913 (2012).
Vutukuri, H. R. et al. Active particles induce large shape deformations in giant lipid vesicles. Nature 586, 52–56 (2020).
Bausch, A. R. & Kroy, K. A bottom-up approach to cell mechanics. Nat. Phys. 2, 231–238 (2006).
Hampoelz, B. et al. Microtubule-induced nuclear envelope fluctuations control chromatin dynamics in Drosophila embryos. Development 138, 3377–3386 (2011).
Deviri, D. & Safran, S. A. Balance of osmotic pressures determines the nuclear-to-cytoplasmic volume ratio of the cell. Proc. Natl Acad. Sci. USA 119, e2118301119 (2022).
Lemiére, J., Real-Calderon, P., Holt, L. J., Fai, T. G. & Chang, F. Control of nuclear size by osmotic forces in Schizosaccharomyces pombe. eLife 11, e76075 (2022).
Cosgrove, B. D. et al. Nuclear envelope wrinkling predicts mesenchymal progenitor cell mechano-response in 2D and 3D microenvironments. Biomaterials 270, 120662 (2021).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017).
Makhija, E., Jokhun, D. S. & Shivashankar, G. V. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc. Natl Acad. Sci. USA 113, E32–E40 (2016).
Kelpsch, D. J., Groen, C. M., Fagan, T. N., Sudhir, S. & Tootle, T. L. Fascin regulates nuclear actin during Drosophila oogenesis. Mol. Biol. Cell 27, 2965–2979 (2016).
Dialynas, G., Speese, S., Budnik, V., Geyer, P. K. & Wallrath, L. L. The role of Drosophila lamin C in muscle function and gene expression. Development 137, 3067–3077 (2010).
Prasad, M. & Montell, D. J. Cellular and molecular mechanisms of border cell migration analyzed using time-lapse live-cell imaging. Dev. Cell 12, 997–1005 (2007).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Legland, D., Arganda-Carreras, I. & Andrey, P. MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 32, 3532–3534 (2016).
Mietke, A. Dynamics of Active Surfaces. PhD thesis, Technische Univ. Dresden (2018).
Burns, K. J., Vasil, G. M., Oishi, J. S., Lecoanet, D. & Brown, B. P. Dedalus: a flexible framework for numerical simulations with spectral methods. Phys. Rev. Research 2, 023068 (2020).
Lin, L. C.-L. & Brown, F. L. H. Brownian dynamics in Fourier space: membrane simulations over long length and time scales. Phys. Rev. Lett. 93, 256001 (2004).
Reuther, A. et al. Interactive supercomputing on 40,000 cores for machine learning and data analysis. In 2018 IEEE High Performance Extreme Computing Conference (HPEC) 1–6 (IEEE, 2018).
Wieczorek, M. A. & Meschede, M. SHTools: tools for working with spherical harmonics. Geochem. Geophys. Geosyst. 19, 2574–2592 (2018).
Acknowledgements
We thank the MIT SuperCloud and Lincoln Laboratory Supercomputing Center for providing high-performance computing resources that have contributed to the research results reported in this paper. We thank M. Kardar, R. D. Kamm, E. Folker, M. A. Collins and D. P. Holmes for helpful discussions. This work was supported by a MathWorks Science Fellowship (N.R.), NSF Award DMS-1952706 (J.D. and N.R.), Sloan Foundation Grant G-2021-16758 (J.D.), MIT Mathematics Robert E. Collins Distinguished Scholar Fund (J.D.), Feodor Lynen Research Fellowship from the Humboldt Foundation (J.F.T.), Jarve Fund MIT grant (A.C.M. and J.D.) and the National Institute of General Medical Sciences of the National Institutes of Health under award no. R01GM144115 (A.C.M). N.R. and J.F.T. acknowledge participation in the KITP online workshop ‘The Physics of Elastic Films: from Biological Membranes to Extreme Mechanics’ supported in part by the National Science Foundation under grant no. NSF PHY-1748958.
Author information
Authors and Affiliations
Contributions
J.I.A., J.A.J., N.R., J.F.T., J.D. and A.C.M. conceived the project. J.I.A. and J.A.J. designed and conducted the experiments. N.R., K.J.B. and J.F.T. designed and implemented the numerical simulations. N.R. and A.M. performed the analytical calculations. J.A.J. and N.R. performed the image and data analyses. N.R., J.A.J., J.I.A. and J.D. wrote the original paper, with input from all authors. All authors revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Stephanie Höhn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12 and Table 1.
Supplementary Video 1
Three z stacks of the Nup107 signal through individual nurse cell nuclei from egg chambers of time proxies 118, 157 and 216 (from left to right), showing the progression of NE shapes from unwrinkled (left) to heavily wrinkled (right) during development. Scale bar, 5 μm; 10 fps.
Supplementary Video 2
Two time-lapse images of the MIPs of two nurse cell nuclei each from egg chambers expressing Nup107::GFP, showing fluctuations in NE wrinkling over time. The first video shows a lower-resolution video acquired at 1 frame per 40 s (time proxy 160). The second video shows a higher-resolution video acquired at 1 frame every 5 min (time proxy 200). Scale bar, 10 μm; 10 fps; the second video has each frame duplicated four times for an effective rate of 2 fps.
Supplementary Video 3
The z stack through an egg chamber (time proxy 166) expressing Nup107::GFP (green) and stained with SPY555-tubulin to visualize the microtubules (magenta). The white box highlights a region corresponding to the zoomed-in z stack in the latter part of the video. Microtubules are present around the nurse cell nuclei, but their locations do not seem to correlate with the locations of NE wrinkles. The orange arrows point to surface fluctuations without a nearby tubulin signal; the white arrow, to surface fluctuations with a nearby tubulin signal; and the cyan arrow, to a tubulin signal near the undeformed surface. Scale bar, 20 μm; 15 fps.
Supplementary Video 4
Egg chamber (time proxy 172) expressing Nup107::GFP before and after the addition of 9 mg ml–1 colchicine to disrupt the microtubules. After drug addition, nurse cell’s NE wrinkles markedly decrease in amplitude over the next 30 min. This effect is not simply due to the addition of a fresh medium (Supplementary Video 5). The white box in the second and third frames indicates the rough border of the zoomed-in image shown for the rest of the video. Scale bar, 20 μm; 5 fps.
Supplementary Video 5
Egg chamber (time proxy 163) expressing Nup107::GFP before and after the addition of a fresh, identical culture medium, used as a control for colchicine addition. After medium addition, the NE wrinkles show no major change. The white box in the second and third frames indicates the rough border of the zoomed-in image shown for the rest of the video. Scale bar, 20 μm; 5 fps.
Supplementary Video 6
Two time-lapse images showing single optical sections from NEs (Nup107, green), cell membranes (magenta) and the motion of cytoplasmic components (reflected light, grey) in the egg chambers under normal conditions (no inhibitors added). The first time lapse is from an egg chamber of time proxy 190; the second is of time proxy 173. Scale bar, 20 μm; 30 fps.
Supplementary Video 7
Three reflection microscopy time-lapse images showing the effects of colchicine addition on intracellular motion. First time lapse: MIP through 4 μm near the top of a nucleus in one nurse cell; the NE appears as a large, bright object deforming and rotating alongside fluctuations of small objects in the cytoplasm (white dots). Alternating dark and light regions on the right side of the NE are fluctuating wrinkles. Second time lapse: projection through 8 μm of the same egg chamber after the addition of 9 mg ml–1 colchicine. The boxed nucleus corresponds to the nucleus shown in the first portion of the video. A reduction in NE roughness can be seen by the reduction in the linear patterns of the dark and light regions. Reduction in cytoplasmic motion becomes more easily apparent at around 10 min. Third time lapse: projection through 6.5 μm of a nucleus from a different egg chamber, more than 30 min after colchicine was added, to ensure that the slowing in the second time lapse did not result from photodamage. Here, too, the NE is smoother than expected given the egg chamber age, and small cytoplasmic objects are substantially less mobile. Scale bar, 10 μm; 20 fps.
Supplementary Video 8
Egg chamber (time proxy 178) expressing Nup107::GFP before and after the addition of 10 μg ml–1 cytochalasin D to disrupt F-actin. After drug addition, nurse cell’s NE wrinkles show no substantial change. The addition of DMSO alone also causes no major change (Supplementary Video 9). The white box in the second and third frames indicates the rough border of the zoomed-in image shown for the rest of the video. Scale bar, 20 μm; 5 fps.
Supplementary Video 9
Egg chamber (time proxy 170) expressing Nup107::GFP before and after the addition of DMSO, used as a control for cytochalasin D addition. After DMSO addition, the NE wrinkles show no major change. The white box in the second and third frames indicates the rough border of the zoomed-in image shown for the rest of the video. Scale bar, 20 μm; 5 fps.
Supplementary Video 10
Three z stacks through individual nurse cell nuclei (Supplementary Video 1) from egg chambers of time proxy 118, 157 and 216 (from left to right). The Nup107 signal is shown in grey following the background subtraction and Gaussian blur used to preprocess before segmentation; the segmentation output is shown in red. The rightmost nucleus is near the oldest of the nuclei segmented and hence effectively represents a lower bound on the accuracy achieved for segmentations used in reconstructions. Scale bar, 5 μm; 10 fps.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jackson, J.A., Romeo, N., Mietke, A. et al. Scaling behaviour and control of nuclear wrinkling. Nat. Phys. 19, 1927–1935 (2023). https://doi.org/10.1038/s41567-023-02216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-023-02216-y