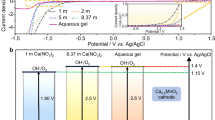
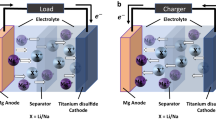
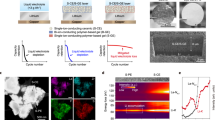
Abstract
High-performance, cost-efficient electrolyte systems are sought after for high-energy-density multivalent metal batteries. However, the expensive precursor and complex synthesis process hinders exploration of cathode electrode/electrolyte interfaces and solvation structures. Here we developed a universal cation replacement method to prepare low-cost, high-reversibility magnesium and calcium electrolytes derived from a zinc organoborate solvation structure. By rationally adjusting the precursor chain length and F-substitution degree, we can fine tune anion participation in the primary solvation shell. A completely dissociated Mg organoborate electrolyte enables high current endurance and enhanced electrochemical kinetics, whereas the Ca organoborate electrolyte with strong coordination/B–H inclusion offers a stable solid–electrolyte interphase with high coulombic efficiency. A rechargeable 53.4 Wh kg−1 Mg metal prototype is achieved with a 30 μm Mg anode, a low electrolyte/sulfur ratio (E/S = 5.58 μl mg−1) and a modified separator/interlayer. This work provides innovative strategies for reversible electrolyte systems and high-energy-density multivalent metal batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data that support the main findings are available in the main text and the Supplementary Information.
Code availability
The Python codes for Mg2+/Ca2+ solvation analysis are available at https://github.com/liuqilei/zhejiang_university.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Obama, B. The irreversible momentum of clean energy. Science 355, aam6284 (2017).
Cao, Y., Li, M., Lu, J., Liu, J. & Amine, K. Bridging the academic and industrial metrics for next-generation practical batteries. Nat. Nanotechnol. 14, 200–207 (2019).
Liang, Y., Dong, H., Aurbach, D. & Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 5, 646–656 (2020).
Liang, Z. & Ban, C. Strategies to enable reversible magnesium electrochemistry: from electrolytes to artificial solid–electrolyte interphases. Angew. Chem. Int. Ed. 60, 11036–11047 (2021).
Wang, D. et al. Plating and stripping calcium in an organic electrolyte. Nat. Mater. 17, 16–20 (2018).
Hou, S. et al. Solvation sheath reorganization enables divalent metal batteries with fast interfacial charge transfer kinetics. Science 374, 172–178 (2021).
Dong, H. et al. High-power Mg batteries enabled by heterogeneous enolization redox chemistry and weakly coordinating electrolytes. Nat. Energy 5, 1043–1050 (2020).
Muldoon, J., Bucur, C. B. & Gregory, T. Fervent hype behind magnesium batteries: an open call to synthetic chemists—electrolytes and cathodes needed. Angew. Chem. Int. Ed. 56, 12064–12084 (2017).
Gummow, R. J., Vamvounis, G., Kannan, M. B. & He, Y. Calcium-ion batteries: current state-of-the-art and future perspectives. Adv. Mater. 30, 1801702 (2018).
Mohtadi, R., Tutusaus, O., Arthur, T. S., Zhao-Karger, Z. & Fichtner, M. The metamorphosis of rechargeable magnesium batteries. Joule 5, 581–617 (2021).
Attias, R., Salama, M., Hirsch, B., Goffer, Y. & Aurbach, D. Anode–electrolyte interfaces in secondary magnesium batteries. Joule 3, 27–52 (2019).
See, K. A. et al. The interplay of Al and Mg speciation in advanced Mg battery electrolyte solutions. J. Am. Chem. Soc. 138, 328–337 (2016).
Li, W. et al. Synthesis, crystal structure, and electrochemical properties of a simple magnesium electrolyte for magnesium/sulfur batteries. Angew. Chem. Int. Ed. 55, 6406–6410 (2016).
Xu, Y., Li, W., Zhou, G., Pan, Z. & Zhang, Y. A non-nucleophilic mono-Mg2+ electrolyte for rechargeable Mg/S battery. Energy Storage Mater. 14, 253–257 (2018).
Wang, H. et al. Reversible electrochemical interface of mg metal and conventional electrolyte enabled by intermediate adsorption. ACS Energy Lett. 5, 200–206 (2019).
Li, Z. et al. Establishing a stable anode–electrolyte interface in Mg batteries by electrolyte additive. ACS Appl. Mater. Interfaces 13, 33123–33132 (2021).
Nguyen, D.-T. et al. A high-performance magnesium triflate-based electrolyte for rechargeable magnesium batteries. Cell Rep. Phys. Sci. 1, 100265 (2020).
Xiao, J. et al. Stable solid electrolyte interphase in situ formed on magnesium-metal anode by using a perfluorinated alkoxide-based all-magnesium salt electrolyte. Adv. Mater. 34, e2203783 (2022).
Muldoon, J. et al. Corrosion of magnesium electrolytes: chlorides—the culprit. Energy Environ. Sci. 6, 482–487 (2013).
Attias, R. et al. The role of surface adsorbed Cl– complexes in rechargeable magnesium batteries. ACS Catal. 10, 7773–7784 (2020).
Zhao-Karger, Z. et al. Toward highly reversible magnesium–sulfur batteries with efficient and practical Mg(B(hfip)4)2 electrolyte. ACS Energy Lett. 3, 2005–2013 (2018).
Shyamsunder, A., Blanc, L. E., Assoud, A. & Nazar, L. F. Reversible calcium plating and stripping at room temperature using a borate salt. ACS Energy Lett. 4, 2271–2276 (2019).
Li, Z., Fuhr, O., Fichtner, M. & Zhao-Karger, Z. Towards stable and efficient electrolytes for room-temperature rechargeable calcium batteries. Energy Environ. Sci. 12, 3496–3501 (2019).
Tang, K. et al. A stable solid electrolyte interphase for magnesium metal anode evolved from a bulky anion lithium salt. Adv. Mater. 32, e1904987 (2020).
Ren, W. et al. An efficient bulky Mg(B(Otfe)4)2 electrolyte and its derivatively general design strategy for rechargeable magnesium batteries. ACS Energy Lett. 6, 3212–3220 (2021).
Tutusaus, O. et al. An efficient halogen-free electrolyte for use in rechargeable magnesium batteries. Angew. Chem. Int. Ed. 54, 7900–7904 (2015).
Luo, J., Bi, Y., Zhang, L., Zhang, X. & Liu, T. L. A stable, non-corrosive perfluorinated pinacolatoborate Mg electrolyte for rechargeable Mg batteries. Angew. Chem. Int. Ed. 58, 6967–6971 (2019).
Zhao-Karger, Z., Gil Bardaji, M. E., Fuhr, O. & Fichtner, M. A new class of non-corrosive, highly efficient electrolytes for rechargeable magnesium batteries. J. Mater. Chem. A 5, 10815–10820 (2017).
Paskevicius, M. et al. Metal borohydrides and derivatives—synthesis, structure and properties. Chem. Soc. Rev. 46, 1565–1634 (2017).
Keyzer, EvanN. et al. A general synthetic methodology to access magnesium aluminate electrolyte systems for Mg batteries. J. Mater. Chem. A 7, 2677–2685 (2019).
Zhu, Y. et al. Effective synthesis of magnesium borohydride via B–O to B–H bond conversion. Chem. Eng. J. 432, 134322 (2022).
Hiyoshizo, K. et al. Facile reduction of benzenethiol ester under mild conditions with zinc borohydride. Chem. Lett. 15, 1003–1004 (1986).
Forero-Saboya, J. et al. Understanding the nature of the passivation layer enabling reversible calcium plating. Energy Environ. Sci. 13, 3423–3431 (2020).
Li, S. et al. Synergistic dual-additive electrolyte enables practical lithium-metal batteries. Angew. Chem. Int. Ed. 59, 14935–14941 (2020).
Li, S. et al. High-efficacy and polymeric solid–electrolyte interphase for closely packed Li electrodeposition. Adv. Sci. 8, 2003240 (2021).
Li, S. et al. Structured solid electrolyte interphase enable reversible Li electrodeposition in flame-retardant phosphate-based electrolyte. Energy Storage Mater. 42, 628–635 (2021).
McClary, S. A. et al. A heterogeneous oxide enables reversible calcium electrodeposition for a calcium battery. ACS Energy Lett. 7, 2792–2800 (2022).
Wang, X. et al. Glassy Li metal anode for high-performance rechargeable Li batteries. Nat. Mater. 19, 1339–1345 (2020).
Son, S. B. et al. An artificial interphase enables reversible magnesium chemistry in carbonate electrolytes. Nat. Chem. 10, 532–539 (2018).
Niu, C. et al. Self-smoothing anode for achieving high-energy lithium metal batteries under realistic conditions. Nat. Nanotechnol. 14, 594–601 (2019).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019).
Fang, C. et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 6, 987–994 (2021).
Lu, T., Sobtop. Version 1.0 (dev3.1); http://sobereva.com/soft/Sobtop
Schauperl, M. et al. Non-bonded force field model with advanced restrained electrostatic potential charges (RESP2). Commun. Chem. 3, 44 (2020).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Zhang, J. & Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 23, 20323–20328 (2021).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Cancès, E., Mennucci, B. & Tomasi, J. A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 107, 3032–3041 (1997).
Miertus, S., Scrocco, E. & Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 55, 117–129 (1981).
Hohenstein, E. G., Chill, S. T. & Sherrill, C. D. Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theory Comput. 4, 1996–2000 (2008).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Performance of SM6, SM8, and SMD on the SAMPL1 test set for the prediction of small-molecule solvation free energies. J. Phys. Chem. B 113, 4538–4543 (2009).
Acknowledgements
We owe our gratitude to Y. Yao and H. Dong for their help on this work. S.L. thanks his colleagues who still helped him complete this article after graduation. We thank L. Wu and S. Chang at the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University, for the technical assistance on Cryo-EM. We thank N. Zheng at State Key Laboratory of Chemical Engineering in Zhejiang University for performing SEM and Raman. We thank Y. Lu at the school of Materials Science and Engineering in Zhejiang University for performing XPS. We thank D. Chen from Shiyanjia Lab (www.shiyanjia.com) for providing invaluable assistance with the X-ray single-crystal analysis. We acknowledge financial support from the Natural Science Foundation of China (22022813, 21878268), the National Key R&D Program of China (2018YFA0209600), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01006), the Key R&D Program of Zhejiang Province (2019C01155) and the Fundamental Research Funds for China Central Universities (DUT22LAB608).
Author information
Authors and Affiliations
Contributions
S.L. conceived the idea and designed the experiments. S.L. and J.Z. synthesized the electrolytes. Q.L. and H.C. performed the DFT/MD calculation. S.Z. provided the PVA separator. H.C. and X.W. synthesized and fabricated sulfur electrodes. L.F., W.Z. and Q.W. prepared GO/Cu membranes. All authors participated in writing the manuscript. Y.L. supervised the project.
Corresponding author
Ethics declarations
Competing interests
S.L., H.C., J.Z., S.Z. and Y.L. declare that this work has been filed as Chinese Patent Application number 2023102361925. All other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Toshihiko Mandai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–62 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Zhang, J., Zhang, S. et al. Cation replacement method enables high-performance electrolytes for multivalent metal batteries. Nat Energy 9, 285–297 (2024). https://doi.org/10.1038/s41560-023-01439-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01439-w
This article is cited by
-
Synthesis via cation replacement reactions
Nature Energy (2024)
-
A rechargeable Ca/Cl2 battery
Science China Chemistry (2024)