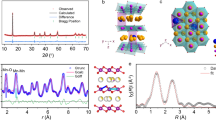
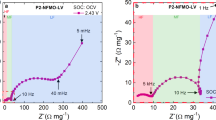
Abstract
In P2-type layered transition metal (TM) oxides, which are typical cathode materials for Na-ion batteries, the presence of Li within the TM layer could lead to the formation of specific Na–O–Li configurations that trigger additional oxygen redox at high charging voltages. However, the prismatic-type (P-type) to octahedral-type (O-type) phase transition and irreversible TM migration could be simultaneously aggravated in high state of charge, resulting in structural distortion. Here we demonstrate that excessive desodiation of P2-Na0.67Li0.1Fe0.37Mn0.53O2 (NLFMO) induces the formation of neighbouring O-type stacking faults with an intergrowth structure (that is, interlacing of O- and P-type layers), which leads to out-of-lattice Li migration and irreversible oxygen loss. We show that, by controlling the depth of charge to tailor the intergrowth structure, a P-type stacking state can be uniformly interspersed between the O-type stacking state, thereby avoiding neighbouring O-type stacking faults. Adjusting the O/P intergrowth structure leads to both reversible migration of Li/TM ions and reversible anionic redox in the NLFMO cathode. We thereby achieve a high-performance pouch cell (with an energy density of 165 W h kg−1 based on the entire weight of the cell) with both cationic and anionic redox activities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings in this study are available within the paper and the Supplementary Information. Source data are provided with this paper.
References
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Li, B. & Xia, D. Anionic redox in rechargeable lithium batteries. Adv. Mater. 29, 1701054 (2017).
Li, B., Yan, H., Zuo, Y. & Xia, D. Tuning the reversibility of oxygen redox in lithium-rich layered oxides. Chem. Mater. 29, 2811–2818 (2017).
Yabuuchi, N. et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 11, 512–517 (2012).
Wang, P.-F., You, Y., Yin, Y.-X. & Guo, Y.-G. Layered oxide cathodes for sodium-ion batteries: phase transition, air stability, and performance. Adv. Energy Mater. 8, 1701912 (2018).
Wang, P.-F. et al. Both cationic and anionic redox chemistry in a P2-type sodium layered oxide. Nano Energy 69, 104474 (2020).
House, R. A. et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 577, 502–508 (2020).
Rong, X. et al. Structure-induced reversible anionic redox activity in Na layered oxide cathode. Joule 2, 125–140 (2018).
Cao, X. et al. Restraining oxygen loss and suppressing structural distortion in a newly Ti-substituted layered oxide P2-Na0.66Li0.22Ti0.15Mn0.63O2. ACS Energy Lett. 4, 2409–2417 (2019).
Cao, X. et al. Stabilizing reversible oxygen redox chemistry in layered oxides for sodium-ion batteries. Adv. Energy Mater. 10, 1903785 (2020).
Xu, J. et al. Identifying the critical role of Li substitution in P2-Nax[LiyNizMn1–y–z]O2 (0 < x, y, z < 1) intercalation cathode materials for high-energy Na-ion batteries. Chem. Mater. 26, 1260–1269 (2014).
Cao, X. et al. Triggering and stabilizing oxygen redox chemistry in layered Li[Na1/3Ru2/3]O2 enabled by stable Li–O–Na configuration. ACS Energy Lett. 7, 2349–2356 (2022).
Lu, Z. & Dahn, J. R. In situ X-ray diffraction study of P2-Na2/3Ni1/3Mn2/3O2. J. Electrochem. Soc. 148, 1225–1229 (2001).
Singh, G. et al. High-voltage Mg-doped Na0.67Ni0.3–xMgxMn0.7O2 (x = 0.05, 0.1) Na-ion cathodes with enhanced stability and rate capability. Chem. Mater. 28, 5087–5094 (2016).
Somerville, J. W. et al. Nature of the 'Z'-phase in layered Na-ion battery cathodes. Energy Environ. Sci. 12, 2223–2232 (2019).
Pang, W. K. et al. Interplay between electrochemistry and phase evolution of the P2-type Nax(Fe1/2Mn1/2)O2 cathode for use in sodium-ion batteries. Chem. Mater. 27, 3150–3158 (2015).
Peng-Fei, W. et al. An abnormal 3.7 volt O3-type sodium-ion battery cathode. Angew. Chem. Int. Ed. 57, 8178–8183 (2018).
Wang, P.-F. et al. Ti-substituted NaNi0.5Mn0.5−xTixO2 cathodes with reversible O3−P3 phase transition for high-performance sodium-ion batteries. Adv. Mater. 29, 1700210 (2017).
Bassey, E. N. et al. Structural origins of voltage hysteresis in the Na-ion cathode P2-Na0.67[Mg0.28Mn0.72]O2: a combined spectroscopic and density functional theory study. Chem. Mater. 33, 4890–4906 (2021).
Cao, X. et al. Reversible anionic redox chemistry in layered Li4/7[□1/7Mn6/7]O2 enabled by stable Li–O-vacancy configuration. Joule 6, 1290–1303 (2022).
Cao, X. et al. Stabilizing anionic redox chemistry in a Mn-based layered oxide cathode constructed by Li-deficient pristine state. Adv. Mater. 33, 2004280 (2020).
Cui, C. et al. Structure and interface design enable stable Li-rich cathode. J. Am. Chem. Soc. 142, 8918–8927 (2020).
Yang, L. et al. Lithium-doping stabilized high-performance P2-Na0.66Li0.18Fe0.12Mn0.7O2 cathode for sodium ion batteries. J. Am. Chem. Soc. 141, 6680–6689 (2019).
Liu, J., Huq, A., Moorhead-Rosenberg, Z., Manthiram, A. & Page, K. Nanoscale Ni/Mn ordering in the high voltage spinel cathode LiNi0.5Mn1.5O4. Chem. Mater. 28, 6817–6821 (2016).
Zhang, B. et al. Reliable impedance analysis of Li-ion battery half-cell by standardization on electrochemical impedance spectroscopy (EIS). J. Chem. Phys. 158, 054202 (2023).
Zhang, H. et al. Titration mass spectroscopy (TMS): a quantitative analytical technology for rechargeable batteries. Nano Lett. 22, 9972–9981 (2022).
Oishi, M. et al. Direct observation of reversible charge compensation by oxygen ion in Li-rich manganese layered oxide positive electrode material, Li1.16Ni0.15Co0.19Mn0.50O2. J. Power Sources 276, 89–94 (2015).
Hu, E. et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 3, 690–698 (2018).
Rong, X. et al. Anionic redox reaction-induced high-capacity and low-strain cathode with suppressed phase tansition. Joule 3, 503–517 (2019).
Zhao, C., Ding, F., Lu, Y., Chen, L. & Hu, Y.-S. High-entropy layered oxide cathodes for sodium-ion batteries. Angew. Chem. Int. Ed. 59, 264–269 (2020).
Ning, F. et al. Inhibition of oxygen dimerization by local symmetry tuning in Li-rich layered oxides for improved stability. Nat. Commun. 11, 4973 (2020).
Li, Q. et al. Tuning both anionic and cationic redox chemistry of Li-rich Li1.2Mn0.6Ni0.2O2 via a 'three-in-one' strategy. Chem. Mater. 32, 9404–9414 (2020).
Casas-Cabanas, M., Reynaud, M., Rikarte, J., Horbach, P. & Rodríguez-Carvajal, J. FAULTS: a program for refinement of structures with extended defects. J. Appl. Crystallogr. 49, 2259–2269 (2016).
Talaie, E., Duffort, V., Smith, H. L., Fultz, B. & Nazar, L. F. Structure of the high voltage phase of layered P2-Na2/3[Mn1/2Fe1/2]O2 and the positive effect of Ni substitution on its stability. Energy Environ. Sci. 8, 2512–2523 (2015).
Lebens-Higgins, Z. W. et al. Revisiting the charge compensation mechanisms in LiNi0.8Co0.2−yAlyO2 systems. Mater. Horiz. 6, 2112–2123 (2019).
Cheng, C. et al. Enhancing the reversibility of lattice oxygen redox through modulated transition metal–oxygen covalency for layered battery electrodes. Adv. Mater. 34, e2201152 (2022).
Cao, X. et al. Achieving stable anionic redox chemistry in Li-excess O2-type layered oxide cathode via chemical ion-exchange strategy. Energy Storage Materials 38, 1–8 (2021).
Zhao, C. et al. Coexistence of (O2)n− and trapped molecular O2 as the oxidized species in P2-type sodium 3d layered oxide and stable interface enabled by highly fluorinated electrolyte. J. Am. Chem. Soc. 143, 18652–18664 (2021).
Yabuuchi, N., Yoshida, H. & Komaba, S. Crystal structures and electrode performance of alpha-NaFeO2 for rechargeable sodium batteries. J. Electrochem. 80, 716–719 (2012).
Dräger, C. et al. Observation of electrochemically active Fe3+/Fe4+ in LiCo0.8Fe0.2MnO4 by in situ Mössbauer spectroscopy and X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 21, 89–95 (2019).
Li, Q. et al. Improving the oxygen redox reversibility of Li-rich battery cathode materials via Coulombic repulsive interactions strategy. Nat. Commun. 13, 1123 (2022).
Sharpe, R. et al. Redox chemistry and the role of trapped molecular O2 in Li-rich disordered rocksalt oxyfluoride cathodes. J. Am. Chem. Soc. 142, 21799–21809 (2020).
Mortemard de Boisse, B. et al. Intermediate honeycomb ordering to trigger oxygen redox chemistry in layered battery electrode. Nat. Commun. 7, 11397 (2016).
Kong, W. et al. Tailoring Co3d and O2p band centers to inhibit oxygen escape for stable 4.6 V LiCoO2 cathodes. Angew. Chem. Int. Ed. 133, 27308–27318 (2021).
CICE energiGUNE. FAULTS software https://cicenergigune.com/en/faults (2016).
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (grant nos. 22021001, 22179111, 22109186, 52250402, 52025025 and 22288102), the Ministry of Science and Technology of China (grant no. 2021YFA1201900), the Basic Research Program of Tan Kah Kee Innovation Laboratory (grant no. RD2021070401), the Principal Fund from Xiamen University (grant no. 20720210015), the Fundamental Research Funds for the Central Universities (grant no. 20720220010) and the Beijing Natural Science Foundation (grant no. Z190010). H.L. and J.C. acknowledge support from the US National Science Foundation under grant no. DMR-1809372. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. H.L. and J.C. were supported by the National Science Foundation under grant no. DMR-1809372. G.-L.X. and K.A. thank the Clean Vehicle Consortium, US–China Clean Energy Research Centre (CERC-CVC2) for support. This research also employed the resources of the Shanghai Synchrotron Radiation Facility BL11B, BL14B1 and BL02B02 beamline stations (SSRF, under contract nos. 2021-SSRF-PT-017208, 2022-SSRF-PT-019758 and 2022-SSRF-PT-021637), the Hefei National Synchrotron Radiation Laboratory (NSRL-USTC, under contract nos. 2021-HLS-PT-004529, 2021-HLS-PT-004156 and 2021-HLS-PT-004241), the Beijing Synchrotron Radiation Laboratory 1W1B, 4B9A, 4B7B, 3W1B and 4B9B beamline stations (under contract nos. 2021-BEPC-PT-005771, 2021-BEPC-PT-005765, 2021-BEPC-PT-005760 and 2022-BEPC-PT-006478) and the China Spallation Neutron Source (CSNS, under contract nos. P1621080200036 and P1621122000008). The authors appreciate the help from D. Wong, C. Schulz and M. Bartkowiak for RIXS characterization (proposal info: 221-11099-ST) at beamline U41-PEAXIS of BESSY II, Helmholtz-Zentrum Berlin für Materialien und Energie, Berlin, Germany. We thank W. He (Chimie du Solide-Energie, Collège de France, France), L. Pan (Hokkaido University, Japan), C. Li (East China Normal University) and J. Serrano Sevillano (CIC energiGUNE, Spain) for help with and discussion on in situ XRD, Mossbäuer characterization, ssNMR and the FAULTS program.
Author information
Authors and Affiliations
Contributions
X.W. and Y. Qiao contributed to the design of the research and performed the experimental data analysis. X.W. conducted the materials synthesis, electrochemistry and cell performance. Q.Z. and L.G. conducted the STEM experiments and related data analysis. H.L. and J.C. conducted the soft XAS experiments. C.Z. conducted the hard XAS experiments with the help of C.-J.S. and I.H. X.W. and Y.T. conducted the analysis of XAS results. B.Z., G.Z. and Y.T. helped to conduct the XRD experiment and FAULTS simulation. H.L., Z.H. and Y.X. conducted the ND/nPDF experiments and related data analysis. H.Z. and S.Z. conducted the TiMS/DEMS and HR-TEM experiment, respectively. Q.W. conducted the RIXS experiments and related data analysis. Y.S. conducted the DFT calculations and MD simulations. Y. Qiao conducted the analysis of Fe-MS and ssNMR characterizations. Y.S., Q.W., G.-L.X., L.G. and Y. Qiao supervised the work. All authors discussed the results and co-wrote and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Seung-Taek Myung, Dorthe B. Ravnsbæk, Chongmin Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31, discussion and Tables 1–5.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhang, Q., Zhao, C. et al. Achieving a high-performance sodium-ion pouch cell by regulating intergrowth structures in a layered oxide cathode with anionic redox. Nat Energy 9, 184–196 (2024). https://doi.org/10.1038/s41560-023-01425-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-023-01425-2