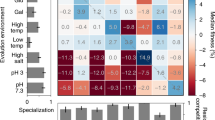
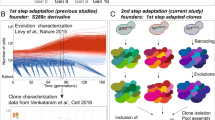
Abstract
Evolution in a static laboratory environment often proceeds via large-effect beneficial mutations that may become maladaptive in other environments. Conversely, natural settings require populations to endure environmental fluctuations. A sensible assumption is that the fitness of a lineage in a fluctuating environment is the time average of its fitness over the sequence of static conditions it encounters. However, transitions between conditions may pose entirely new challenges, which could cause deviations from this time average. To test this, we tracked hundreds of thousands of barcoded yeast lineages evolving in static and fluctuating conditions and subsequently isolated 900 mutants for pooled fitness assays in 15 environments. Here we find that fitness in fluctuating environments indeed often deviates from the time average, leading to fitness non-additivity. Moreover, closer examination reveals that fitness in one component of a fluctuating environment is often strongly influenced by the previous component. We show that this environmental memory is especially common for mutants with high variance in fitness across tested environments. We use a simple mathematical model and whole-genome sequencing to propose mechanisms underlying this effect, including lag time evolution and sensing mutations. Our results show that environmental fluctuations impact fitness and suggest that variance in static environments can explain these impacts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All sequencing data are deposited in Short Read Archive under BioProject identifier PRJNA1103172. The remaining data are available via GitHub at https://github.com/clare-abreu/environmental_memory. Barcoded yeast strains are available from the authors upon request. Source data are provided with this paper.
Code availability
All code used in this paper is available via GitHub at https://github.com/clare-abreu/environmental_memory.
References
Woods, R., Schneider, D., Winkworth, C. L., Riley, M. A. & Lenski, R. E. Tests of parallel molecular evolution in a long-term experiment with Escherichia coli. Proc. Natl Acad. Sci. USA 103, 9107–9112 (2006).
Tenaillon, O. et al. The molecular diversity of adaptive convergence. Science 335, 457–461 (2012).
Venkataram, S. et al. Development of a comprehensive genotype-to-fitness map of adaptation-driving mutations in yeast. Cell 166, 1585–1596.e22 (2016).
Good, B. H., McDonald, M. J., Barrick, J. E., Lenski, R. E. & Desai, M. M. The dynamics of molecular evolution over 60,000 generations. Nature 551, 45–50 (2017).
Rudman, S. M. et al. Direct observation of adaptive tracking on ecological time scales in Drosophila. Science 375, eabj7484 (2022).
Kryazhimskiy, S., Rice, D. P., Jerison, E. R. & Desai, M. M. Global epistasis makes adaptation predictable despite sequence-level stochasticity. Science 344, 1519–1522 (2014).
Couce, A. & Tenaillon, O. A. The rule of declining adaptability in microbial evolution experiments. Front. Genet. 6, 99 (2015).
Kover, P. X. et al. Pleiotropic effects of environment-specific adaptation in Arabidopsis thaliana. New Phytol. 183, 816–825 (2009).
Schick, A., Bailey, S. F. & Kassen, R. Evolution of fitness trade-offs in locally adapted populations of Pseudomonas fluorescens. Am. Nat. 186, S48–S59 (2015).
Li, Y., Petrov, D. A. & Sherlock, G. Single nucleotide mapping of trait space reveals Pareto fronts that constrain adaptation. Nat. Ecol. Evol. 3, 1539–1551 (2019).
Jerison, E. R., Nguyen Ba, A. N., Desai, M. M. & Kryazhimskiy, S. Chance and necessity in the pleiotropic consequences of adaptation for budding yeast. Nat. Ecol. Evol. 4, 601–611 (2020).
Kinsler, G., Geiler-Samerotte, K. & Petrov, D. A. Fitness variation across subtle environmental perturbations reveals local modularity and global pleiotropy of adaptation. eLife 9, e61271 (2020).
Bono, L. M., Smith, L. B., Pfennig, D. W. & Burch, C. L. The emergence of performance trade-offs during local adaptation: insights from experimental evolution. Mol. Ecol. 26, 1720–1733 (2017).
Cooper, T. F. & Lenski, R. E. Experimental evolution with E. coli in diverse resource environments. I. Fluctuating environments promote divergence of replicate populations. BMC Evol. Biol. 10, 11 (2010).
Rodríguez-Verdugo, A. & Ackermann, M. Rapid evolution destabilizes species interactions in a fluctuating environment. ISME J. 15, 450–460 (2021).
Salignon, J., Richard, M., Fulcrand, E., Duplus-Bottin, H. & Yvert, G. Genomics of cellular proliferation in periodic environmental fluctuations. Mol. Syst. Biol. 14, e7823 (2018).
Boyer, S., Hérissant, L. & Sherlock, G. Adaptation is influenced by the complexity of environmental change during evolution in a dynamic environment. PLoS Genet. 17, e1009314 (2021).
Alto, B. W., Wasik, B. R., Morales, N. M. & Turner, P. E. Stochastic temperatures impede RNA virus adaptation. Evolution 67, 969–979 (2013).
Fasanello, V. J., Liu, P., Fay, J. C. & Botero, C. A. Fluctuating selection facilitates the discovery of broadly effective but difficult to reach adaptive outcomes in yeast. Evol. Lett. 8, 243–252 (2024).
Cvijović, I., Good, B. H., Jerison, E. R. & Desai, M. M. Fate of a mutation in a fluctuating environment. Proc. Natl Acad. Sci. USA 112, E5021–E5028 (2015).
Sæther, B.-E. & Engen, S. The concept of fitness in fluctuating environments. Trends Ecol. Evol. 30, 273–281 (2015).
Ketola, T. & Kristensen, T. N. Experimental approaches for testing if tolerance curves are useful for predicting fitness in fluctuating environments. Front. Ecol. Evol. 5, 129 (2017).
Li, Y. et al. Hidden complexity of yeast adaptation under simple evolutionary conditions. Curr. Biol. 28, 515–525.e6 (2018).
Monod, J. The growth of bacterial cultures. Annu. Rev. Microbiol. 3, 371–394 (1949).
Siegal, M. L. Shifting sugars and shifting paradigms. PLoS Biol. 13, e1002068 (2015).
Escalante-Chong, R. et al. Galactose metabolic genes in yeast respond to a ratio of galactose and glucose. Proc. Natl Acad. Sci. USA 112, 1636–1641 (2015).
Basan, M. et al. A universal trade-off between growth and lag in fluctuating environments. Nature 584, 470–474 (2020).
Balakrishnan, R., de Silva, R. T., Hwa, T. & Cremer, J. Suboptimal resource allocation in changing environments constrains response and growth in bacteria. Mol. Syst. Biol. 17, e10597 (2021).
Acar, M., Mettetal, J. T. & van Oudenaarden, A. Stochastic switching as a survival strategy in fluctuating environments. Nat. Genet. 40, 471–475 (2008).
Lambert, G. & Kussell, E. Memory and fitness optimization of bacteria under fluctuating environments. PLoS Genet. 10, e1004556 (2014).
Kronholm, I. & Ketola, T. Effects of acclimation time and epigenetic mechanisms on growth of Neurospora in fluctuating environments. Heredity 121, 327–341 (2018).
Stajic, D., Bank, C. & Gordo, I. Adaptive potential of epigenetic switching during adaptation to fluctuating environments. Genome Biol. Evol. 14, evac065 (2022).
Torres-Garcia, S. et al. Epigenetic gene silencing by heterochromatin primes fungal resistance. Nature 585, 453–458 (2020).
Leung, C., Grulois, D., Quadrana, L. & Chevin, L.-M. Phenotypic plasticity evolves at multiple biological levels in response to environmental predictability in a long-term experiment with a halotolerant microalga. PLoS Biol. 21, e3001895 (2023).
Levy, S. F. et al. Quantitative evolutionary dynamics using high-resolution lineage tracking. Nature 519, 181–186 (2015).
New, A. M. et al. Different levels of catabolite repression optimize growth in stable and variable environments. PLoS Biol. 12, e1001764 (2014).
Zakrzewska, A. et al. Genome-wide analysis of yeast stress survival and tolerance acquisition to analyze the central trade-off between growth rate and cellular robustness. Mol. Biol. Cell 22, 4435–4446 (2011).
Dhar, R., Sägesser, R., Weikert, C. & Wagner, A. Yeast adapts to a changing stressful environment by evolving cross-protection and anticipatory gene regulation. Mol. Biol. Evol. 30, 573–588 (2013).
Ketola, T. & Saarinen, K. Experimental evolution in fluctuating environments: tolerance measurements at constant temperatures incorrectly predict the ability to tolerate fluctuating temperatures. J. Evol. Biol. 28, 800–806 (2015).
Vermeersch, L. et al. On the duration of the microbial lag phase. Curr. Genet. 65, 721–727 (2019).
McDaniel, E. A., Stuecker, T. N., Veluvolu, M., Gasch, A. P. & Lewis, J. A. Independent mechanisms for acquired salt tolerance versus growth resumption induced by mild ethanol pretreatment in Saccharomyces cerevisiae. mSphere 3, e00574-18 (2018).
Rivers, W. Characterizing Aft1/2-Grx3/4 Interaction and the Role of Bol2 During Iron Regulation in Saccharomyces cerevisiae. Senior thesis, Honors College (2019).
Martínez-Pastor, M. T., Perea-García, A. & Puig, S. Mechanisms of iron sensing and regulation in the yeast Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 33, 75 (2017).
IPCC. in Climate Change 2013—The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Ch. 14, 1217–1308 (Cambridge University Press, 2014).
Khan, A. I., Dinh, D. M., Schneider, D., Lenski, R. E. & Cooper, T. F. Negative epistasis between beneficial mutations in an evolving bacterial population. Science 332, 1193–1196 (2011).
Chou, H.-H., Chiu, H.-C., Delaney, N. F., Segrè, D. & Marx, C. J. Diminishing returns epistasis among beneficial mutations decelerates adaptation. Science 332, 1190–1192 (2011).
Reddy, G. & Desai, M. M. Global epistasis emerges from a generic model of a complex trait. eLife 10, e64740 (2021).
Diaz-Colunga, J., Skwara, A., Vila, J. C. C., Bajic, D. & Sanchez, A. Global epistasis and the emergence of function in microbial consortia. Cell 187, 3108–3119.e30 (2024).
Violle, C., Pu, Z. & Jiang, L. Experimental demonstration of the importance of competition under disturbance. Proc. Natl Acad. Sci. USA 107, 12925–12929 (2010).
Abreu, C. I., Woltz, V. L. A., Friedman, J. & Gore, J. Microbial communities display alternative stable states in a fluctuating environment. PLoS Comput. Biol. 16, e1007934 (2020).
Yi, X. & Dean, A. M. Bounded population sizes, fluctuating selection and the tempo and mode of coexistence. Proc. Natl Acad. Sci. USA 110, 16945–16950 (2013).
Letten, A. D., Dhami, M. K., Ke, P.-J. & Fukami, T. Species coexistence through simultaneous fluctuation-dependent mechanisms. Proc. Natl Acad. Sci. USA 115, 6745–6750 (2018).
Mukherjee, A. et al. Coexisting ecotypes in long-term evolution emerged from interacting trade-offs. Nat. Commun. 14, 3805 (2023).
Chesson, P. & Huntly, N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. Am. Nat. 150, 519–553 (1997).
Gilchrist, C. & Stelkens, R. Aneuploidy in yeast: segregation error or adaptation mechanism? Yeast 36, 525–539 (2019).
Beaumont, H. J. E., Gallie, J., Kost, C., Ferguson, G. C. & Rainey, P. B. Experimental evolution of bet hedging. Nature 462, 90–93 (2009).
Bruijning, M., Metcalf, C. J. E., Jongejans, E. & Ayroles, J. F. The evolution of variance control. Trends Ecol. Evol. 35, 22–33 (2020).
Kinsler, G. et al. Extreme sensitivity of fitness to environmental conditions: lessons from #1BigBatch. J. Mol. Evol. 91, 293–310 (2023).
Venkataram, S. BarcodeCounter2. GitHub https://github.com/sandeepvenkataram/BarcodeCounter2 (2021).
Zhao, L., Liu, Z., Levy, S. F. & Wu, S. Bartender: a fast and accurate clustering algorithm to count barcode reads. Bioinformatics 34, 739–747 (2018).
Li, F., Mahadevan, A. & Sherlock, G. An improved algorithm for inferring mutational parameters from bar-seq evolution experiments. BMC Genom. 24, 246 (2023).
Kinsler, G., Li, Y., Sherlock, G. J. & Petrov, D. A shift from pleiotropic to modular adaptation revealed by a high-resolution two-step adaptive walk. Preprint at bioRxiv https://doi.org/10.1101/2024.04.17.589938 (2024).
Acknowledgements
We are grateful to G. Kinsler for patiently training us in the experimental system, as well as for valuable advice about PCR and sequencing, data analysis and whole-genome sequencing analysis. We thank members of the Petrov Lab for helpful comments and suggestions. We thank A. Lyulina for assistance with preliminary experiments; S. Khristich for guidance on molecular biology troubleshooting; K. Xue, K. Solari, K. Schwartz and J. Tarkington for advice on library preparation protocols; and A. Mahadevan for advice on whole-genome sequencing analysis. For additional helpful discussions, we thank Y. Li, B. Good, D. Wong, J. Cremer, J. Gore, M. Dal Bello, M. Dunham and R. Stelkens. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number F32GM145148 (C.I.A.) and Award Number R35GM118165 (D.A.P.).
Author information
Authors and Affiliations
Contributions
C.I.A., S.M. and D.A.P. conceived of the study. C.I.A. and S.M. conducted experiments, collected data and performed analyses, and C.I.A., S.M. and D.A.P. interpreted results. C.I.A. analysed the mathematical model. C.I.A. and D.A.P. acquired funding. C.I.A. wrote the original draft paper, and C.I.A., S.M. and D.A.P. reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Yitzhak Pilpel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Source Data Figs. 2–5
Fitness statistics for all mutants (also available via GitHub).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abreu, C.I., Mathur, S. & Petrov, D.A. Environmental memory alters the fitness effects of adaptive mutations in fluctuating environments. Nat Ecol Evol (2024). https://doi.org/10.1038/s41559-024-02475-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-024-02475-9