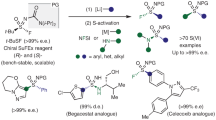
Abstract
Chiral sulfur pharmacophores are crucial for drug discovery in bioscience and medicinal chemistry. While the catalytic asymmetric synthesis of sulfoxides and sulfinate esters with stereogenic-at-sulfur(IV) centres is well developed, the synthesis of chiral sulfinamides remains challenging, which has primarily been attributed to the high nucleophilicity and competing reactions of amines. In this study, we have developed an efficient methodology for the catalytic asymmetric synthesis of chiral sulfinamides and sulfinate esters by the sulfinylation of diverse nucleophiles, including aromatic amines and alcohols, using our bifunctional chiral 4-arylpyridine N-oxides as catalysts. The remarkable results are a testament to the efficiency, versatility and broad applicability of the developed synthetic approach, serving as a valuable tool for the synthesis of sulfur pharmacophores. Mechanistic experiments and density functional theory calculations revealed that the initiation and stereocontrol of this reaction are induced by an acyl transfer catalyst. Our research provides an efficient approach for the construction of optically pure sulfur(IV) centres.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the main text and the Supplementary Information. The X-ray crystallographic coordinates for the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers 2285509 (4o), 2285510 (6y) and 2311965 (7d). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 34, 609–624 (2005).
Chinthakindi, P. K. et al. Sulfonimidamides in medicinal and agricultural chemistry. Angew. Chem. Int. Ed. 56, 4100–4109 (2017).
Mäder, P. & Kattner, L. Sulfoximines as rising stars in modern drug discovery? Current status and perspective on an emerging functional group in medicinal chemistry. J. Med. Chem. 63, 14243–14275 (2020).
Yang, G.-F. et al. Synthesis of chiral sulfonimidoyl chloride via desymmetrizing enantioselective hydrolysis. J. Am. Chem. Soc. 145, 5439–5446 (2023).
Otocka, S., Kwiatkowska, M., Madalińska, L. & Kiełbasiński, P. Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: applications in asymmetric synthesis. Chem. Rev. 117, 4147–4181 (2017).
Lücking, U. Neglected sulfur(VI) pharmacophores in drug discovery: exploration of novel chemical space by the interplay of drug design and method development. Org. Chem. Front. 6, 1319–1324 (2019).
Han, Y. et al. Application of sulfoximines in medicinal chemistry from 2013 to 2020. Eur. J. Med. Chem. 209, 112885 (2021).
Trost, B. M. & Rao, M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 54, 5026–5043 (2015).
Trost, B. M., Ryan, M. C., Rao, M. & Markovic, T. Z. Construction of enantioenriched [3.1.0] bicycles via a ruthenium-catalyzed asymmetric redox bicycloisomerization reaction. J. Am. Chem. Soc. 136, 17422–17425 (2014).
Sedelmeier, J., Hammerer, T. & Bolm, C. C1-Symmetric oxazolinyl sulfoximines as ligands in copper-catalyzed asymmetric Mukaiyama aldol reactions. Org. Lett. 10, 917–920 (2008).
Xu, H., Zuend, S. J., Woll, M. G., Tao, Y. & Jacobsen, E. N. Asymmetric cooperative catalysis of strong Brønsted acid-promoted reactions using chiral ureas. Science 327, 986–990 (2010).
Han, Z., Krishnamurthy, D., Grover, P., Fang, Q. K. & Senanayake, C. H. Properly designed modular asymmetric synthesis for enantiopure sulfinamide auxiliaries from N-sulfonyl-1,2,3-oxathiazolidine-2-oxide agents. J. Am. Chem. Soc. 124, 7880–7881 (2002).
Robak, M. T., Herbage, M. A. & Ellman, J. A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 110, 3600–3740 (2010).
Cogan, D. A., Liu, G., Kim, K., Backes, B. J. & Ellman, J. A. Catalytic asymmetric oxidation of tert-butyl disulfide. Synthesis of tert-butanesulfinamides, tert-butyl sulfoxides, and tert-butanesulfinimines. J. Am. Chem. Soc. 120, 8011–8019 (1998).
Han, Z. S. et al. Design and synthesis of chiral oxathiozinone scaffolds: efficient synthesis of hindered enantiopure sulfinamides and sulfinyl ketimines. Angew. Chem. Int. Ed. 52, 6713–6717 (2013).
Fernández, I., Khiar, N., Llera, J. M. & Alcudia, F. Asymmetric synthesis of alkane- and arenesulfinates of diacetone-d-glucose (DAG): an improved and general route to both enantiomerically pure sulfoxides. J. Org. Chem. 57, 6789–6796 (1992).
Peltier, H. M., Evans, J. W. & Ellman, J. A. Catalytic enantioselective sulfinyl transfer using cinchona alkaloid catalysts. Org. Lett. 7, 1733–1736 (2005).
Liu, G., Cogan, D. A. & Ellman, J. A. Catalytic asymmetric synthesis of tert-butanesulfinamide. Application to the asymmetric synthesis of amines. J. Am. Chem. Soc. 119, 9913–9914 (1997).
Liao, S., Čorić, I., Wang, Q. & List, B. Activation of H2O2 by chiral confined Brønsted acids: a highly enantioselective catalytic sulfoxidation. J. Am. Chem. Soc. 134, 10765–10768 (2012).
Zong, L. et al. Bisguanidinium dinuclear oxodiperoxomolybdosulfate ion pair-catalyzed enantioselective sulfoxidation. Nat. Commun. 7, 13455 (2016).
Ye, X. et al. Enantioselective sulfoxidation catalyzed by a bisguanidinium diphosphatobisperoxotungstate ion pair. Angew. Chem. Int. Ed. 55, 7101–7105 (2016).
Wang, L., Chen, M., Zhang, P., Li, W. & Zhang, J. Palladium/PC-Phos-catalyzed enantioselective arylation of general sulfenate anions: scope and synthetic applications. J. Am. Chem. Soc. 140, 3467–3473 (2018).
Newhouse, T. R., Li, X., Blewett, M. M., Whitehead, C. M. C. & Corey, E. J. A tetradentate ligand for the enantioselective Ti(IV)-promoted oxidation of sulfides to sulfoxides: origin of enantioselectivity. J. Am. Chem. Soc. 134, 17354–17357 (2012).
Aota, Y., Maeda, Y., Kano, T. & Maruoka, K. Efficient synthesis of cyclic sulfoximines from N-propargylsulfinamides through sulfur–carbon bond formation. Chem. Eur. J. 25, 15755–15758 (2019).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines through the S-alkylation of sulfinamides. Angew. Chem. Int. Ed. 58, 17661–17665 (2019).
Aota, Y., Kano, T. & Maruoka, K. Asymmetric synthesis of chiral sulfoximines via the S-arylation of sulfinamides. J. Am. Chem. Soc. 141, 19263–19268 (2019).
Maeda, Y. et al. Practical asymmetric synthesis of chiral sulfoximines via sulfur-selective alkylation. J. Org. Chem. 87, 3652–3660 (2022).
Zou, X. et al. Strain-enabled S-arylation and S-alkenylation of sulfinamides using arynes and cyclic alkynes. Sci. China Chem. 67, 928–935 (2024).
Zou, X., Wang, H. & Gao, B. Synthesis of sulfoximines by copper-catalyzed oxidative coupling of sulfinamides and aryl boronic acids. Org. Lett. 25, 7656–7660 (2023).
Bizet, V., Hendriks, C. M. M. & Bolm, C. Sulfur imidations: access to sulfimides and sulfoximines. Chem. Soc. Rev. 44, 3378–3390 (2015).
Davies, T. Q. et al. Harnessing sulfinyl nitrenes: a unified one-pot synthesis of sulfoximines and sulfonimidamides. J. Am. Chem. Soc. 142, 15445–15453 (2020).
Pickford, H. D. et al. Rapid and scalable halosulfonylation of strain-release reagents. Angew. Chem. Int. Ed. 62, e202213508 (2023).
Ning, Y., Ji, Q., Liao, P., Anderson, E. A. & Bi, X. Silver-catalyzed stereoselective aminosulfonylation of alkynes. Angew. Chem. Int. Ed. 56, 13805–13808 (2017).
Zenzola, M., Doran, R., Degennaro, L., Luisi, R. & Bull, J. A. Transfer of electrophilic NH using convenient sources of ammonia: direct synthesis of NH sulfoximines from sulfoxides. Angew. Chem. Int. Ed. 55, 7203–7207 (2016).
Fang, S. et al. Access to S-stereogenic free sulfoximines via bifunctional phosphonium salt-catalyzed desymmetrization of bisphenols. ACS Catal. 11, 13902–13912 (2021).
Ma, L.-J. et al. Chiral Brønsted-acid-catalyzed asymmetric oxidation of sulfenamide by using H2O2: a versatile access to sulfinamide and sulfoxide with high enantioselectivity. ACS Catal. 9, 1525–1530 (2019).
Chen, Y., Wu, X., Yang, S. & Zhu, C. Asymmetric radical cyclization of alkenes by stereospecific homolytic substitution of sulfinamides. Angew. Chem. Int. Ed. 61, e202201027 (2022).
Evans, D. A. et al. Asymmetric synthesis of chiral organosulfur compounds using N-sulfinyloxazolidinones. J. Am. Chem. Soc. 114, 5977–5985 (1992).
Evans, J. W., Fierman, M. B., Miller, S. J. & Ellman, J. A. Catalytic enantioselective synthesis of sulfinate esters through the dynamic resolution of tert-butanesulfinyl chloride. J. Am. Chem. Soc. 126, 8134–8135 (2004).
Shibata, N. et al. Cinchona alkaloid/sulfinyl chloride combinations: enantioselective sulfinylating agents of alcohols. J. Am. Chem. Soc. 127, 1374–1375 (2005).
Zhang, X., Ang, E. C. X., Yang, Z., Kee, C. W. & Tan, C.-H. Synthesis of chiral sulfinate esters by asymmetric condensation. Nature 604, 298–303 (2022).
Huang, S. et al. Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds. Nat. Chem. 15, 185–193 (2023).
Wojaczyńska, E. & Wojaczyński, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 120, 4578–4611 (2020).
Wang, M. & Jiang, X. Prospects and challenges in organosulfur chemistry. ACS Sustain. Chem. Eng. 10, 671–677 (2022).
Senanayake, C. H., Han, Z. & Krishnamurthy, D. in Organosulfur Chemistry in Asymmetric Synthesis (eds Toru, T. & Bolm, C.) 233–264 (Wiley-VCH, 2008).
Malkov, A. V. & Kočovský, P. Chiral N-oxides in asymmetric catalysis. Eur. J. Org. Chem. 29–36 (2007).
Liu, X. H., Lin, L. L. & Feng, X. M. Chiral N,N′-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 44, 574–587 (2011).
Murray, J. I. et al. Kinetic resolution of 2-substituted indolines by N-sulfonylation using an atropisomeric 4-DMAP-N-oxide organocatalyst. Angew. Chem. Int. Ed. 56, 5760–5764 (2017).
Xie, M.-S. et al. Chiral DMAP-N-oxides as acyl transfer catalysts: design, synthesis, and application in asymmetric Steglich rearrangement. Angew. Chem. Int. Ed. 58, 2839–2843 (2019).
Xie, M.-S. et al. Rational design of 2-substituted DMAP-N-oxides as acyl transfer catalysts: dynamic kinetic resolution of azlactones. J. Am. Chem. Soc. 142, 19226–19238 (2020).
Xie, M.-S. et al. Chiral 4‑aryl-pyridine-N-oxide nucleophilic catalysts: design, synthesis, and application in acylative dynamic kinetic resolution. ACS Catal. 12, 877–891 (2022).
Wang, M., Zhang, Z. & Zhang, W. Design, synthesis, and application of chiral bicyclic imidazole catalysts. Acc. Chem. Res. 55, 2708–2727 (2022).
Liu, S. et al. First catalytic enantioselective synthesis of P-stereogenic phosphoramides via kinetic resolution promoted by a chiral bicyclic imidazole nucleophilic catalyst. Tetrahedron Asymmetry 23, 329–332 (2012).
DiRocco, D. A. et al. A multifunctional catalyst that stereoselectively assembles prodrugs. Science 356, 426–430 (2017).
Lee, S. Y., Murphy, J. M., Ukai, A. & Fu, G. C. Nonenzymatic dynamic kinetic resolution of secondary alcohols via enantioselective acylation: synthetic and mechanistic studies. J. Am. Chem. Soc. 134, 15149–15153 (2012).
Joannesse, C. et al. Isothiourea-catalyzed enantioselective carboxy group transfer. Angew. Chem. Int. Ed. 48, 8914–8918 (2009).
McLaughlin, C. & Smith, A. D. Generation and reactivity of C(1)-ammonium enolates by using isothiourea catalysis. Chem. Eur. J. 27, 1533–1555 (2021).
Piotrowski, D. W. et al. Regio- and enantioselective synthesis of azole hemiaminal esters by Lewis base catalyzed dynamic kinetic resolution. J. Am. Chem. Soc. 138, 4818–4823 (2016).
Zhou, M. et al. Chiral bicyclic imidazole-catalyzed acylative dynamic kinetic resolution for the synthesis of chiral phthalidyl esters. Angew. Chem. Int. Ed. 60, 1641–1645 (2021).
Worch, C., Atodiresei, I., Raabe, G. & Bolm, C. Synthesis of enantiopure sulfonimidamides and elucidation of their absolute configuration by comparison of measured and calculated CD spectra and X-ray crystal structure determination. Chem. Eur. J. 16, 677–683 (2009).
Steurer, M. & Bolm, C. Synthesis of amino-functionalized sulfonimidamides and their application in the enantioselective Henry reaction. J. Org. Chem. 75, 3301–3310 (2010).
Acknowledgements
We are grateful to the NSFC (grant nos. 22071046, U22A20378 and 21971056) and the Program for Innovative Research Team in Science and Technology at the University of Henan Province (grant nos. 23IRTSTHN003 and 22IRTSTHN003) for financial support. We also thank the Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, the Henan Key Laboratory of Organic Functional Molecules and Drug Innovation and the NMPA Key Laboratory for Research and Evaluation of Innovative Drug for financial support.
Author information
Authors and Affiliations
Contributions
H.-M.G., M.-S.X. and Y.T. conceived and directed the project. T.W. and H.-L.W. performed the experiments. Y.T. performed the computational studies. H.-M.G. and M.-S.X. supervised the work. M.-S.X. and T.W. co-wrote the original draft of the paper. M.-S.X., Y.T. and H.-M.G. co-wrote the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Choon-Hong Tan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6 and Tables 1–10.
Supplementary Data 1
Cartesian coordinates for the theoretical calculations.
Supplementary Data 2
Crystallographic data for compound 4o; CCDC reference 2285509.
Supplementary Data 3
Crystallographic data for compound 6y; CCDC reference 2285510.
Supplementary Data 4
Structure factors for compound 6y; CCDC reference 2285510.
Supplementary Data 5
Crystallographic data for compound 7d; CCDC reference 2311965.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, T., Wang, HL., Tian, Y. et al. Enantioselective construction of stereogenic-at-sulfur(IV) centres via catalytic acyl transfer sulfinylation. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01522-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01522-z