Abstract
Upon endoplasmic reticulum (ER) stress, activation of the ER-resident transmembrane protein kinase/endoribonuclease inositol-requiring enzyme 1 (IRE1) initiates a key branch of the unfolded protein response (UPR) through unconventional splicing generation of the transcription factor X-box-binding protein 1 (XBP1s). Activated IRE1 can form large clusters/foci, whose exact dynamic architectures and functional properties remain largely elusive. Here we report that, in mammalian cells, formation of IRE1α clusters is an ER membrane-bound phase separation event that is coupled to the assembly of stress granules (SGs). In response to different stressors, IRE1α clusters are dynamically tethered to SGs at the ER. The cytosolic linker portion of IRE1α possesses intrinsically disordered regions and is essential for its condensation with SGs. Furthermore, disruption of SG assembly abolishes IRE1α clustering and compromises XBP1 mRNA splicing, and such IRE1α–SG coalescence engenders enrichment of the biochemical components of the pro-survival IRE1α–XBP1 pathway during ER stress. Our findings unravel a phase transition mechanism for the spatiotemporal assembly of IRE1α–SG condensates to establish a more efficient IRE1α machinery, thus enabling higher stress-handling capacity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
For the IDR prediction analysis, protein sequences were obtained from the NCBI protein database with the following accession numbers: NP_001424.3 for hIRE1α, NP_031374.2 for hATF6α and NP_004827.4 for hPERK. For the sequence alignment analysis of IRE1 proteins from multiple species, protein sequences were from the Uniprot database with their Uniprot ID numbers as follows: O75460 for human, A0A2K5X0L5 for macaque, A0A0G2K2H4 for rat, Q9EQY0 for mouse, Q09499 for worm (Caenorhabditis elegans), A8JR46 for fruit fly (Drosophila melanogaster) and P32361 for yeast (Saccharomyces cerevisiae). Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
References
Chen, S., Novick, P. & Ferro-Novick, S. ER structure and function. Curr. Opin. Cell Biol. 25, 428–433 (2013).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Mori, K., Ma, W., Gething, M. J. & Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993).
Cox, J. S., Shamu, C. E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Tirasophon, W., Welihinda, A. A. & Kaufman, R. J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12, 1812–1824 (1998).
Sidrauski, C. & Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90, 1031–1039 (1997).
Shamu, C. E. & Walter, P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028–3039 (1996).
Welihinda, A. A. & Kaufman, R. J. The unfolded protein response pathway in Saccharomyces cerevisiae. Oligomerization and trans-phosphorylation of Ire1p (Ern1p) are required for kinase activation. J. Biol. Chem. 271, 18181–18187 (1996).
Maurel, M., Chevet, E., Tavernier, J. & Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 (2014).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Lu, Y., Liang, F. X. & Wang, X. A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Mol. Cell 55, 758–770 (2014).
Hetz, C., Martinon, F., Rodriguez, D. & Glimcher, L. H. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol. Rev. 91, 1219–1243 (2011).
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Hetz, C., Chevet, E. & Oakes, S. A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 17, 829–838 (2015).
Bettigole, S. E. & Glimcher, L. H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138 (2015).
Huang, S., Xing, Y. & Liu, Y. Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease. J. Biol. Chem. 294, 18726–18741 (2019).
Chen, X. & Cubillos-Ruiz, J. R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 21, 71–88 (2021).
Kimata, Y. et al. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol. 179, 75–86 (2007).
Li, H., Korennykh, A. V., Behrman, S. L. & Walter, P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl Acad. Sci. USA 107, 16113–16118 (2010).
Belyy, V., Tran, N. H. & Walter, P. Quantitative microscopy reveals dynamics and fate of clustered IRE1alpha. Proc. Natl Acad. Sci. USA 117, 1533–1542 (2020).
Ricci, D. et al. Clustering of IRE1α depends on sensing ER stress but not on its RNase activity. FASEB J. 33, 9811–9827 (2019).
Belyy, V., Zuazo-Gaztelu, I., Alamban, A., Ashkenazi, A. & Walter, P. Endoplasmic reticulum stress activates human IRE1α through reversible assembly of inactive dimers into small oligomers. Elife 11, e74342 (2022).
Alberti, S. & Hyman, A. A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213 (2021).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Lyon, A. S., Peeples, W. B. & Rosen, M. K. A framework for understanding the functions of biomolecular condensates across scales. Nat. Rev. Mol. Cell Biol. 22, 215–235 (2021).
Hirose, T., Ninomiya, K., Nakagawa, S. & Yamazaki, T. A guide to membraneless organelles and their various roles in gene regulation. Nat. Rev. Mol. Cell Biol. 24, 288–304 (2022).
Protter, D. S. W. & Parker, R. Principles and properties of stress granules. Trends Cell Biol. 26, 668–679 (2016).
Panas, M. D., Ivanov, P. & Anderson, P. Mechanistic insights into mammalian stress granule dynamics. J. Cell Biol. 215, 313–323 (2016).
Namkoong, S., Ho, A., Woo, Y. M., Kwak, H. & Lee, J. H. Systematic characterization of stress-induced RNA granulation. Mol. Cell 70, 175–187 e178 (2018).
Markmiller, S. et al. Context-dependent and disease-specific diversity in protein interactions within stress granules. Cell 172, 590–604 e513 (2018).
Tourriere, H. et al. The RasGAP-associated endoribonuclease G3BP mediates stress granule assembly. J. Cell Biol. 222 e200212128072023new. (2023)
Gilks, N. et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398 (2004).
Jain, S. et al. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498 (2016).
Yang, P. et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345 e328 (2020).
Sanders, D. W. et al. Competing protein–RNA Interaction networks control multiphase intracellular organization. Cell 181, 306–324 e328 (2020).
Guillen-Boixet, J. et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181, 346–361 e317 (2020).
Wolozin, B. & Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 20, 649–666 (2019).
Riggs, C. L., Kedersha, N., Ivanov, P. & Anderson, P. Mammalian stress granules and P bodies at a glance. J. Cell Sci. 133, jcs242487 (2020).
Wippich, F. et al. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152, 791–805 (2013).
Lee, J. E., Cathey, P. I., Wu, H., Parker, R. & Voeltz, G. K. Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367, eaay7108 (2020).
Wang, T. et al. Intracellular energy controls dynamics of stress-induced ribonucleoprotein granules. Nat. Commun. 13, 5584 (2022).
Ding, Y. et al. Ratiometric biosensors based on dimerization-dependent fluorescent protein exchange. Nat. Methods 12, 195–198 (2015).
Shin, Y. et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168, 159–171 e114 (2017).
Zhou, J. et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl Acad. Sci. USA 103, 14343–14348 (2006).
Karagoz, G. E. et al. An unfolded protein-induced conformational switch activates mammalian IRE1. eLife 6, e30700 (2017).
Kedersha, N. et al. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151, 1257–1268 (2000).
Sidrauski, C., McGeachy, A. M., Ingolia, N. T. & Walter, P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. eLife 4, e05033 (2015).
Hourihan, J. M., Moronetti Mazzeo, L. E., Fernandez-Cardenas, L. P. & Blackwell, T. K. Cysteine sulfenylation directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol. Cell 63, 553–566 (2016).
Cirillo, L. et al. UBAP2L forms distinct cores that act in nucleating stress granules upstream of G3BP1. Curr. Biol. 30, 698–707 e696 (2020).
Fang, M. Y. et al. Small-molecule modulation of TDP-43 recruitment to stress granules prevents persistent TDP-43 accumulation in ALS/FTD. Neuron 103, 802–819 e811 (2019).
Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 12, 51–54 (2015).
van Anken, E. et al. Specificity in endoplasmic reticulum-stress signaling in yeast entails a step-wise engagement of HAC1 mRNA to clusters of the stress sensor Ire1. eLife 3, e05031 (2014).
He, S. et al. IRE1alpha regulates skeletal muscle regeneration through myostatin mRNA decay. J. Clin. Invest. 131, e143737 (2021).
Chen, Y. et al. Adipocyte IRE1α promotes PGC1α mRNA decay and restrains adaptive thermogenesis. Nat. Metab. 4, 1166–1184 (2022).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Kim, W. J., Back, S. H., Kim, V., Ryu, I. & Jang, S. K. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol. Cell. Biol. 25, 2450–2462 (2005).
Qiu, Y. et al. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1α activation in pancreatic beta cells. Sci. Signal 3, ra7 (2010).
Liu, Y. et al. Role for the endoplasmic reticulum stress sensor IRE1α in liver regenerative responses. J. Hepatol. 62, 590–598 (2015).
Hetz, C. & Glimcher, L. H. Fine-tuning of the unfolded protein response: assembling the IRE1α interactome. Mol. Cell 35, 551–561 (2009).
Wu, H., Carvalho, P. & Voeltz, G. K. Here, there, and everywhere: the importance of ER membrane contact sites. Science 361, eaan5835 (2018).
Gwon, Y. et al. Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science 372, eabf6548 (2021).
Li, Y. et al. Phosphorylation at Ser724 of the ER stress sensor IRE1α governs its activation state and limits ER stress-induced hepatosteatosis. J. Biol. Chem. 298, 101997 (2022).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Ishida, T. & Kinoshita, K. PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res. 35, W460–W464 (2007).
Romero, P. et al. Sequence complexity of disordered protein. Proteins 42, 38–48 (2001).
Acknowledgements
We thank P. Li from Tsinghua University for insightful discussions. This work was supported by grants from the Ministry of Science and Technology of China (National Key R&D Program of China 2018YFA0800700) and from the National Natural Science Foundation of China (82230026, 32021003, 92357307, 91857204 and 323B1006) to Y.L. and R.X. This work was also supported by the Fundamental Research Funds for the Central Universities (2042022dx0003) to Y.L.
Author information
Authors and Affiliations
Contributions
S.L., X.Z. and Y.L. conceived and designed the studies. S.L., X.Z. and X.Y. planned and performed most of the experiments and analysed the data. G.W., S.H., P.C., J.C., Z.W. and Y.Z. performed some of the cell line experiments. M.T., K.H. and R.X. assisted with microscopy imaging. K.L., Y.W., L.J., Q.A.W., L.R. and J.L. provided essential reagents and assisted with data analysis. S.L. and Y.L. wrote and edited the original manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Hyung Don Ryoo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
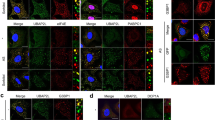
Extended Data Fig. 1 Endogenous IRE1α forms large clusters in response to different types of stress.
a. Representative confocal microscopy images of endogenous IRE1α clusters visualized by immunofluorescent (IF) staining of IRE1α (red) in HeLa cells treated with thapsigargin (Tg) at the indicated concentrations for 3 h. Nucleus was stained with DAPI (blue). Scale bar, 20 μm. Magnification of the demarcated region was shown as the inset for each condition from the merged images. Inset scale bar, 20 μm. Shown also is the quantification of the percentage of cells containing IRE1α clusters and the IRE1α cluster index (mean number in IRE1α cluster-positive cells). Results are presented as mean ± SD, n = 9 image fields analyzed for each condition from three independent experiments. b. Representative IF images of endogenous IRE1α clusters in HeLa cells treated with DMSO (Ctrl), Tg (1 μM, 3 h), sodium arsenite (SA, 500 μM, 1 h) or heat shock (HS, 43 °C, 1 h). Scale bar, 20 μm; Inset scale bar, 20 μm. The percentage of cells containing IRE1α clusters and the IRE1α cluster index are shown as mean ± SD, n = 15 image fields analyzed for each condition from three independent experiments.
Extended Data Fig. 2 IRE1α but not PERK or ATF6 forms SG-colocalized clusters in response to different types of stress.
a-c. HeLa cells were treated with DMSO control (Ctrl), 1 μM thapsigargin (Tg, 3 h), 500 μM sodium arsenite (SA, 1 h) or heat shock (HS, 1 h) at 43 °C. a. Representative confocal microscopy images of IRE1α clusters and stress granules (SGs) visualized by IF staining of IRE1α (red) along with the SG marker TIA1 (green), with enlargement of the indicated inset areas shown from the merged images. Scale bar, 10 μm. The arrow in each enlarged inset indicates the plane for generating line profiles, and the line profiling of fluorescence intensities is shown on the right with Pearson’s correlation coefficient (r) included. Results are representative of three independent experiments for each condition. b. Colocalization of immunofluorescent signal for IRE1α with that for TIA1. Pearson’s correlation coefficient values above the threshold are shown as mean ± SD, n=number of line profiles analyzed from three independent experiments. c. Quantification of the percentage of cells containing colocalized IRE1α-SG clusters and the IRE1α-SG index (the mean colocalized cluster number in IRE1α-SG-positive cells). Data are shown as mean ± SD, n = 16 image fields analyzed for each condition from three independent experiments. d, e. Mouse embryonic fibroblast (MEF) cells (d) or HEK293T cells (e) were treated with DMSO control (Ctrl), 1 μM Tg for 3 h, 500 μM SA for 1 h or HS for 1 h at 43 °C. Representative confocal images of IRE1α clusters (red) and SGs by IF staining of G3BP1 (green). Shown also are magnifications of the indicated areas from the merged images. Scale bar, 10 μm (d, e). f-h. PERK or ATF6α does not form SG-associated clusters. f. Diagram showing the prediction of disordered regions of human PERK and ATF6α protein by PONDR (Predictor of Natural Disordered Regions, VL-XT). g, h. Representative confocal IF images of G3BP1 (green) and C-Myc-tagged PERK (red, g) in transfected HeLa cells, or G3BP1 (green) and endogenous ATF6α (red, h) in HeLa cells, following exposure to the indicated stressors. Shown also on the right are fluorescent intensity profiles of the indicated planes, and results are representative of three independent experiments for each condition. Scale bar, 10 μm.
Extended Data Fig. 3 Analysis of the formation of IRE1α-SG clusters in response to hypoxia and glucose deprivation.
a, b. Representative confocal images by IF staining of IRE1α (red) along with G3BP1 (green) in HeLa cells upon exposure to (a) hypoxia (1% O2) or (b) glucose deprivation (GD) for the indicated time intervals. Scale bar, 20 μm. Inset scale bar, 10 μm. c. Representative confocal IF images of IRE1α (red) along with G3BP1 (green) in HeLa cells cultured in glucose-free medium (GD) without (Ctrl) or with 25 mM 2-deoxy-D-glucose (2-DG) for 1 h. Scale bar, 20 μm. Inset scale bar, 10 μm. Shown also is the quantification of the percentage of cells with IRE1α, SG or colocalized IRE1α-SG clusters. Results are presented as mean ± SD, and n=number of image fields analyzed from three independent experiments.
Extended Data Fig. 4 Coordinated reductions of coalesced IRE1α-SG clusters during stress recovery.
a-c. HeLa cells were treated with (a) 1 μM thapsigargin (Tg, 3 h), (b) 500 μM sodium arsenite (SA, 1 h), or (c) heat shock (HS, 1 h) at 43 °C. Cells were then allowed to recover from stress by culturing in the medium without stressors for the indicated time intervals. Representative confocal images of IRE1α clusters (red) and G3BP1-positive SGs (green) by IF staining. Shown also are merged images with DAPI staining. Scale bar, 20 μm. Shown below the images is quantification of the percentage of cells with IRE1α, SG or colocalized IRE1α-SG clusters at the indicated post-stress time. Results are presented as mean ± SD, and n = 9 image fields analyzed for each condition from three independent experiments.
Extended Data Fig. 5 The ER lumenal domain of IRE1α is also crucial for its clustering with SGs.
a. Representative time-lapse imaging of light-activated clustering of IRE1α-mCherry-CRY2 without (FL) or with deletion of its lumenal domain (ΔLD) in live COS7 cells. Scale bar, 10 μm. Magnifications of images are also shown for the indicated inset regions. Inset scale bar, 5 μm. b. Representative images of live tagBFP-KDEL-expressing COS7 cells transfected with the indicated ddFP constructs following treatment with 1 μM Tg for 30 min. Scale bar, 10 μm. Quantification of the number of ddFP condensates per cell is also shown as mean ± SD, and n=number of cells from three independent experiments. ***P < 0.001 by two-sided unpaired t-test. c. Representative images of IRE1α (green) colocalization with G3BP1 (red) in HeLa cells transfected with the IRE1α-YFP construct or its ER lumenal domain deletion (ΔLD) variant following treatment with 1 μM Tg for 3 h. Scale bar, 20 μm. Inset scale bar, 10 μm. Shown on the right are line profiles of fluorescence intensities of the indicated plane by the white arrow for each merged image, and results are representative of three independent experiments for each condition.
Extended Data Fig. 6 Depletion of IRE1α does not affect SG formation or its tethering connection to the ER.
a. Representative confocal microscopy IF images of TIA1- (green) and G3BP1-positive (red) granules (left panels) as well as G3BP1 (green) and IRE1α (red) (right panels) in WT HeLa cells versus a HeLa cell line with CRISPR-Cas9-based knockout of IRE1α (IRE1α-KO) following treatment with DMSO (Ctrl), Tg (1 μM, 3 h), SA (500 μM, 1 h) or HS (43 °C, 1 h). Shown also are magnifications of the indicated inset areas from the merged images. Scale bar, 20 μm. b. WT versus IRE1α-KO HeLa cells were transfected with tagBFP-KDEL along with CalN-RA and GB-G3BP1 ddFP constructs to monitor the contacts between the ER and SGs. Representative live cell images acquired following DMSO or Tg (1 μM, 30 min) treatment. Scale bar, 20 μm. Inset scale bar, 1 μm. Quantification of Tg-induced ddFP signals per cell is shown as mean ± SD on the right, and n=number of cells from 2 independent experiments. n.s., no significant difference by two-sided unpaired t-test.
Extended Data Fig. 7 Blocking SG formation by cycloheximide abolishes IRE1α enrichment in the RNP granule fractions.
HEK293T cells were treated with DMSO control (-), (a) Tg (1 μM, 3 h), (b) SA (500 μM, 1 h) or (c) HS (43 °C, 1 h) following no cycloheximide (CHX) pretreatment or 10 μg/ml CHX pre-treatment. Immunoblot analysis of the indicated proteins from whole cell lysate (Input), the soluble fraction without nucleus (Soluble), and the insoluble RNP granule (RG) fraction. α-Tubulin was used as the loading control. Relative abundance of the indicated proteins in the RG fraction was quantified and normalized to their input level. Results are shown as mean ± SD, and n = 3 independent experiments for each condition. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparisons test.
Extended Data Fig. 8 Effects of small-molecule protein translation, IRE1α Kinase/RNase or ISR inhibitors upon IRE1α-SG clusters.
a-c. Effects of protein translation or IRE1α Kinase/RNase inhibitors. HeLa cells were pre-treated for 1 h with DMSO, cycloheximide (CHX, 10 μg/ml), emetine (Eme, 10 μg/mL), puromycin (Puro, 10 μg/ml), the IRE1α RNase inhibitor 4μ8C (10 μM) or IRE1α kinase inhibitor KIRA6 (5 μM) before 3 h Tg treatment. a. Representative confocal IF images of IRE1α clusters (red) and G3BP1-positive SGs (green). Scale bar, 10 μm. Shown also is the quantification of the percentage of cells containing colocalized IRE1α-SG clusters and the IRE1α-SG cluster index. b. Representative agarose gel showing RT-PCR analysis of XBP1 mRNA splicing levels. c. Representative immunoblot analysis of IRE1α and the indicated SG marker proteins. d. Effects of ISRIB on the formation of IRE1α-SG clusters. Representative confocal IF images of IRE1α (red) and G3BP1 (green) in HeLa cells that were pre-treated with DMSO control or ISRIB (1 μM) and then treated with Tg (1 μM, 3 h), SA (0.2 mM, 1 h) or HS (43 °C, 1 h). Scale bar, 20 μm. Inset scale bar, 10 μm. e. Quantification of the percentage of cells containing IRE1α clusters, SGs or colocalized IRE1α-SG clusters in (d). Data in a, e are shown as mean ± SD, and n=number of image fields analyzed from three independent experiments for each condition. ***P < 0.001 by two-way ANOVA with Tukey’s multiple comparisons test.
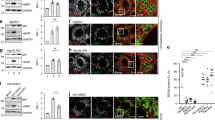
Extended Data Fig. 9 Disruption of SG formation abolishes IRE1α clustering and blunts its functional activity.
a-c. Formation of IRE1α clusters was abrogated in G3BP1/2-deficient cells. a. Representative IF images of IRE1α clusters (red) and TIA1-positive SGs (green) in WT or G3BP1/2-dKO HeLa cells following treatment with DMSO (Ctrl), Tg (1 μM, 3 h), SA (500 μM, 1 h) or HS (43 °C, 1 h). Scale bar, 10 μm. b. Representative IF images of IRE1α clusters (red) and G3BP1-positive SGs (green) in WT or G3BP1/2-dKO #1 and #2 HeLa cells following treatment with DMSO (Ctrl), SA (500 μM, 1 h) or HS (43 °C, 1 h). Scale bar, 10 μm. c. Bar graphs showing quantified percentages of cells containing IRE1α clusters from (b). Data are presented as mean ± SD, n=number of image fields analyzed from three independent experiments for each condition. *** indicates all tested P values are <0.0001 by two-way ANOVA with Tukey’s multiple comparisons test. d-g, Ablation of G3BP1/2 resulted in lower activation of the IRE1α-XBP1 pathway. d, e. Immunoblot analysis of XBP1s and the indicated proteins from lysates of WT or G3BP1/2-dKO HeLa cells following treatment with (d) Tg (1 μM, 3 h) or (e) HS (43 °C, 1 h). The abundance of XBP1s protein was quantified by normalizing to α-Tubulin. f. Quantitative RT-PCR analysis of the mRNA abundance of the indicated XBP1s target genes. Results in d, e and f are presented as mean ± SD, n = 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by two-way ANOVA with Tukey’s multiple comparisons test. g. Immunoblot analysis of the indicated proteins in WT or G3BP1/2-dKO HeLa cells transfected with the empty vector (Vec) or XBP1s construct prior to treatment with the indicated stressors.
Extended Data Fig. 10 Formation of SGs is required for IRE1α clustering and its enhanced RNase output.
a-c. Restored expression of G3BP1/2 normalized the formation of IRE1α-SG clusters in G3BP1/2-deficient cells. a. Representative IF images of IRE1α (red), G3BP1 (green) and G3BP2 (blue) in G3BP1/2-dKO HeLa cells that were transiently transfected with both G3BP1 and G3BP2 constructs prior to treatment with DMSO (Ctrl) or Tg (1 μM, 3 h). Scale bar, 10 μm. b, c. WT and G3BP1/2-dKO HeLa cells were transiently transfected with empty vector (Vec) or the G3BP1/2 constructs for 24 h, followed by treatment with Tg (1 μM, 3 h in b; 10 μM in c). b. Immunoblot analysis of the indicated proteins. c. Trypan blue staining analysis. Quantification of the percentage of dead cells shown as mean ± SD, n = 3 independent experiments for each condition. **P < 0.01, ***P < 0.001, n.s., no significant difference by two-way ANOVA with Tukey’s multiple comparisons test. d, e. HeLa cells were transiently transfected with scramble control RNA or siRNAs against UBAP2/2 L for 48-72 h, followed by treatment with the indicated stressors. d. Quantitative RT-PCR analysis of the mRNA abundance of the indicated genes. Results are presented as mean ± SD, n = 3 independent experiments. ***P < 0.001 by two-way ANOVA with Tukey’s multiple comparisons test. e. Representative confocal IF images of IRE1α (red), UBAP2/2 L (green) and G3BP1 (blue) in HeLa cells. Scale bar, 20 μm. Inset scale bar, 10 μm. f-h. HeLa cells were pre-treated with DMSO or Mitoxantrone (10 μM, 1 h) followed by Tg treatment (1 μM, 3 h). f. Representative confocal IF images of IRE1α (red) and G3BP1 (green). Scale bar, 20 μm. Inset scale bar, 10 μm. g. Quantitative RT-PCR analysis of XBP1 mRNA splicing. Data are presented as mean ± SD, n = 3 independent experiments. ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparisons test. h. Immunoblot analysis of the indicated proteins.
Supplementary information
Supplementary Video 1
Live time-lapse image of an IRE1α–SG cluster undergoing fission at the ER.
Supplementary Video 2
Live time-lapse image of an IRE1α–SG cluster undergoing fusion at the ER.
Supplementary Video 3
Live time-lapse image of light-activated clustering of IRE1α-FL-mCherry-CRY2 versus IRE1α-ΔL-mCherry-CRY2 in the absence of stress.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Zhang, X., Yao, X. et al. Mammalian IRE1α dynamically and functionally coalesces with stress granules. Nat Cell Biol (2024). https://doi.org/10.1038/s41556-024-01418-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41556-024-01418-7