Abstract
A growing body of evidence indicates that gut microbiota influence brain function and behaviour. However, the molecular basis of how gut bacteria modulate host nervous system function is largely unknown. Here we show that vitamin B12-producing bacteria that colonize the intestine can modulate excitatory cholinergic signalling and behaviour in the host Caenorhabditis elegans. Here we demonstrate that vitamin B12 reduces cholinergic signalling in the nervous system through rewiring of the methionine (Met)/S-adenosylmethionine cycle in the intestine. We identify a conserved metabolic crosstalk between the methionine/S-adenosylmethionine cycle and the choline-oxidation pathway. In addition, we show that metabolic rewiring of these pathways by vitamin B12 reduces cholinergic signalling by limiting the availability of free choline required by neurons to synthesize acetylcholine. Our study reveals a gut–brain communication pathway by which enteric bacteria modulate host behaviour and may affect neurological health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data files from this study have been uploaded to figshare and are freely available at https://doi.org/10.6084/m9.figshare.21197953. Source data are provided with this paper. All other data and reagents generated in this study are available from the corresponding author on reasonable request.
Code availability
The MATLAB scripts used in this study are available at https://github.com/jeremyflorman/Tracker_GUI.
References
Cryan, J. F. et al. The microbiota–gut–brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Zhu, S. et al. The progress of gut microbiome research related to brain disorders. J. Neuroinflammation 17, 25 (2020).
Sandhu, K. V. et al. Feeding the microbiota–gut–brain axis: diet, microbiome, and neuropsychiatry. Transl. Res. 179, 223–244 (2017).
Mohajeri, M. H., La Fata, G., Steinert, R. E. & Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 76, 481–496 (2018).
Mitrea, L., Nemes, S. A., Szabo, K., Teleky, B. E. & Vodnar, D. C. Guts imbalance imbalances the brain: a review of gut microbiota association with neurological and psychiatric disorders. Front. Med. 9, 813204 (2022).
Buffington, S. A. et al. Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell 184, 1740–1756 (2021).
Org, E. et al. Genetic and environmental control of host–gut microbiota interactions. Genome Res. 25, 1558–1569 (2015).
Montgomery, T. L. et al. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc. Natl Acad. Sci. USA 117, 27516–27527 (2020).
Karl, J. P. et al. Effects of psychological, environmental and physical stressors on the gut microbiota. Front. Microbiol. 9, 2013 (2018).
Ezra-Nevo, G., Henriques, S. F. & Ribeiro, C. The diet–microbiome tango: how nutrients lead the gut brain axis. Curr. Opin. Neurobiol. 62, 122–132 (2020).
Lloyd-Price, J., Abu-Ali, G. & Huttenhower, C. The healthy human microbiome. Genome Med. 8, 51 (2016).
Chow, J. & Mazmanian, S. K. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 7, 265–276 (2010).
Fung, T. C., Olson, C. A. & Hsiao, E. Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 20, 145–155 (2017).
Fischbach, M. A. Microbiome: focus on causation and mechanism. Cell 174, 785–790 (2018).
Walter, J., Armet, A. M., Finlay, B. B. & Shanahan, F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell 180, 221–232 (2020).
Berg, M. et al. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. ISME J. 10, 1998–2009 (2016).
Samuel, B. S., Rowedder, H., Braendle, C., Felix, M. A. & Ruvkun, G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proc. Natl Acad. Sci. USA 113, E3941–E3949 (2016).
Dirksen, P. et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host–microbiome model. BMC Biol. 14, 38 (2016).
Watson, E. et al. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell 156, 1336–1337 (2014).
Zhang, J., Holdorf, A. D. & Walhout, A. J. C. elegans and its bacterial diet as a model for systems-level understanding of host–microbiota interactions. Curr. Opin. Biotechnol. 46, 74–80 (2017).
Seth, P. et al. Regulation of microRNA machinery and development by interspecies S-nitrosylation. Cell 176, 1014–1025 (2019).
Donato, V. et al. Bacillus subtilis biofilm extends Caenorhabditis elegans longevity through downregulation of the insulin-like signalling pathway. Nat. Commun. 8, 14332 (2017).
Smolentseva, O. et al. Mechanism of biofilm-mediated stress resistance and lifespan extension in C. elegans. Sci. Rep. 7, 7137 (2017).
O’Donnell, M. P., Fox, B. W., Chao, P. H., Schroeder, F. C. & Sengupta, P. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 583, 415–420 (2020).
Goya, M. E. et al. Probiotic Bacillus subtilis protects against α-synuclein aggregation in C. elegans. Cell Rep. 30, 367–3807 (2020).
MacNeil, L. T., Watson, E., Arda, H. E., Zhu, L. J. & Walhout, A. J. Diet-induced developmental acceleration independent of TOR and insulin in C. elegans. Cell 153, 240–252 (2013).
Coolon, J. D., Jones, K. L., Todd, T. C., Carr, B. C. & Herman, M. A. Caenorhabditis elegans genomic response to soil bacteria predicts environment-specific genetic effects on life history traits. PLoS Genet. 5, e1000503 (2009).
Huang, Y. C. et al. Gain-of-function mutations in the UNC-2/CaV2à channel lead to excitation-dominant synaptic transmission in Caenorhabditis elegans. eLife 8, e45905 (2019).
Arzani, M. et al. Gut–brain axis and migraine headache: a comprehensive review. J. Headache Pain 21, 15 (2020).
Dahlin, M. & Prast-Nielsen, S. The gut microbiome and epilepsy. EBioMedicine 44, 741–746 (2019).
Golubeva, A. V. et al. Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 24, 166–178 (2017).
van Hemert, S. et al. Migraine associated with gastrointestinal disorders: review of the literature and clinical implications. Front. Neurol. 5, 241 (2014).
Lum, G. R., Olson, C. A. & Hsiao, E. Y. Emerging roles for the intestinal microbiome in epilepsy. Neurobiol. Dis. 135, 104576 (2020).
Sharon, G. et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618 (2019).
Swierczek, N. A., Giles, A. C., Rankin, C. H. & Kerr, R. A. High-throughput behavioral analysis in C. elegans. Nat. Methods 8, 592–598 (2011).
Estes, K. A., Dunbar, T. L., Powell, J. R., Ausubel, F. M. & Troemel, E. R. bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 107, 2153–2158 (2010).
Martens, J. H., Barg, H., Warren, M. J. & Jahn, D. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 58, 275–285 (2002).
Green, R. et al. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 3, 17040 (2017).
Arda, H. E. et al. Functional modularity of nuclear hormone receptors in a Caenorhabditis elegans metabolic gene regulatory network. Mol. Syst. Biol. 6, 367 (2010).
Bender, D. A. Nutritional Biochemistry of the Vitamins (Cambridge:Cambridge University Press) (2003).
LeBlanc, J. G. et al. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24, 160–168 (2013).
Watanabe, F. & Bito, T. Vitamin B12 sources and microbial interaction. Exp. Biol. Med. 243, 148–158 (2018).
Bito, T., Matsunaga, Y., Yabuta, Y., Kawano, T. & Watanabe, F. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio 3, 112–117 (2013).
Nguyen, M., Alfonso, A., Johnson, C. D. & Rand, J. B. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 140, 527–535 (1995).
Kennedy, D. O. B vitamins and the brain: mechanisms, dose and efficacy–a review. Nutrients 8, 68 (2016).
Duerr, J. S. et al. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J. Neurosci. 19, 72–84 (1999).
Culotti, J. G., Von Ehrenstein, G., Culotti, M. R. & Russell, R. L. A second class of acetylcholinesterase-deficient mutants of the nematode Caenorhabditis elegans. Genetics 97, 281–305 (1981).
Jospin, M. et al. A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biol. 7, e1000265 (2009).
Becchetti, A., Aracri, P., Meneghini, S., Brusco, S. & Amadeo, A. The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front. Physiol. 6, 22 (2015).
Florman, J. T. & Alkema, M. J. Co-transmission of neuropeptides and monoamines choreograph the C. elegans escape response. PLoS Genet. 18, e1010091 (2022).
Richmond, J. E. & Jorgensen, E. M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 2, 791–797 (1999).
Qian, K. Y. et al. Male pheromones modulate synaptic transmission at the C. elegans neuromuscular junction in a sexually dimorphic manner. eLife 10, e67170 (2021).
Pierce-Shimomura, J. T. et al. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc. Natl Acad. Sci. USA 105, 20982–20987 (2008).
Ghosh, R. & Emmons, S. W. Episodic swimming behavior in the nematode C. elegans. J. Exp. Biol. 211, 3703–3711 (2008).
Giese, G. E. et al. Caenorhabditis elegans methionine/S-adenosylmethionine cycle activity is sensed and adjusted by a nuclear hormone receptor. eLife 9, e60259 (2020).
Li, Y., Na, K., Lee, H. J., Lee, E. Y. & Paik, Y. K. Contribution of sams-1 and pmt-1 to lipid homoeostasis in adult Caenorhabditis elegans. J. Biochem. 149, 529–538 (2011).
Walker, A. K. et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell 147, 840–852 (2011).
Moustafa, A. A., Hewedi, D. H., Eissa, A. M., Frydecka, D. & Misiak, B. Homocysteine levels in schizophrenia and affective disorders-focus on cognition. Front. Behav. Neurosci. 8, 343 (2014).
Puig-Alcaraz, C., Fuentes-Albero, M., Calderon, J., Garrote, D. & Cauli, O. Increased homocysteine levels correlate with the communication deficit in children with autism spectrum disorder. Psychiatry Res. 229, 1031–1037 (2015).
Obeid, R., McCaddon, A. & Herrmann, W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin. Chem. Lab. Med. 45, 1590–1606 (2007).
Finkelstein, J. D. The metabolism of homocysteine: pathways and regulation. Eur. J. Pediatr. 157, S40–S44 (1998).
Shinohara, Y., Hasegawa, H., Ogawa, K., Tagoku, K. & Hashimoto, T. Distinct effects of folate and choline deficiency on plasma kinetics of methionine and homocysteine in rats. Metabolism 55, 899–906 (2006).
Mato, J. M., Corrales, F. J., Lu, S. C. & Avila, M. A. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 16, 15–26 (2002).
Xue, G. P. & Snoswell, A. M. Comparative studies on the methionine synthesis in sheep and rat tissues. Comp. Biochem. Physiol. B 80, 489–494 (1985).
Ueland, P. M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 34, 3–15 (2011).
da Costa, K. A., Gaffney, C. E., Fischer, L. M. & Zeisel, S. H. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am. J. Clin. Nutr. 81, 440–444 (2005).
Birks, R. I. & Fitch, J. G. Storage and release of acetylcholine in a sympathetic ganglion. J. Physiol. 240, 125–134 (1974).
Birks, R. I. & Macintosh, F. C. Acetylcholine metabolism at nerve-endings. Br. Med. Bull. 13, 157–161 (1957).
Evans, J. C. et al. Betaine-homocysteine methyltransferase: zinc in a distorted barrel. Structure 10, 1159–1171 (2002).
Wasmuth, J., Schmid, R., Hedley, A. & Blaxter, M. On the extent and origins of genic novelty in the phylum Nematoda. PLoS Negl. Trop. Dis. 2, e258 (2008).
Schwahn, B. C. et al. Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem. J. 382, 831–840 (2004).
Liu, R. et al. MTHFR C677T polymorphism and migraine risk: a meta-analysis. J. Neurol. Sci. 336, 68–73 (2014).
Azimova, J. E. et al. Effects of MTHFR gene polymorphism on the clinical and electrophysiological characteristics of migraine. BMC Neurol. 13, 103 (2013).
Maydan, J. S. et al. Efficient high-resolution deletion discovery in Caenorhabditis elegans by array comparative genomic hybridization. Genome Res. 17, 337–347 (2007).
Peden, A. S. et al. Betaine acts on a ligand-gated ion channel in the nervous system of the nematode C. elegans. Nat. Neurosci. 16, 1794–1801 (2013).
Hardege, I. et al. Neuronally produced betaine acts via a ligand-gated ion channel to control behavioral states. Proc. Natl Acad. Sci. USA 119, e2201783119 (2022).
Hansen, T. V. A. et al. The Caenorhabditis elegans DEG-3/DES-2 channel is a betaine-gated receptor insensitive to monepantel. Molecules 27, 312 (2022).
Rosas-Rodriguez, J. A. & Valenzuela-Soto, E. M. The glycine betaine role in neurodegenerative, cardiovascular, hepatic, and renal diseases: insights into disease and dysfunction networks. Life Sci. 285, 119943 (2021).
Simon, J. R. & Kuhar, M. G. Impulse-flow regulation of high affinity choline uptake in brain cholinergic nerve terminals. Nature 255, 162–163 (1975).
Matthies, D. S., Fleming, P. A., Wilkes, D. M. & Blakely, R. D. The Caenorhabditis elegans choline transporter CHO-1 sustains acetylcholine synthesis and motor function in an activity-dependent manner. J. Neurosci. 26, 6200–6212 (2006).
Mullen, G. P. et al. Choline transport and de novo choline synthesis support acetylcholine biosynthesis in Caenorhabditis elegans cholinergic neurons. Genetics 177, 195–204 (2007).
Kon, S. K. & Porter, J. W. The intestinal synthesis of vitamins in the ruminant. Vitam. Horm. 12, 53–68 (1954).
McDonagh, A., Crew, J. & van der Linden, A. M. Dietary vitamin B12 regulates chemosensory receptor gene expression via the MEF2 transcription factor in Caenorhabditis elegans. G3 12, jkac107 (2022).
Wei, W. & Ruvkun, G. Lysosomal activity regulates Caenorhabditis elegans mitochondrial dynamics through vitamin B12 metabolism. Proc. Natl Acad. Sci. USA 117, 19970–19981 (2020).
Akduman, N. et al. Bacterial vitamin B12 production enhances nematode predatory behavior. ISME J. 14, 1494–1507 (2020).
Albert, M. J., Mathan, V. I. & Baker, S. J. Vitamin B12 synthesis by human small intestinal bacteria. Nature 283, 781–782 (1980).
Baker, S. J. Contribution of the microflora of the small intestine to the vitamin B12 nutriture of man. Nutr. Rev. 39, 147–148 (1981).
Hill, M. J. Intestinal flora and endogenous vitamin synthesis. Eur. J. Cancer Prev. 6, S43–S45 (1997).
Kolhouse, J. F. & Allen, R. H. Recognition of two intracellular cobalamin binding proteins and their identification as methylmalonyl-CoA mutase and methionine synthetase. Proc. Natl Acad. Sci. USA 74, 921–925 (1977).
Evans, J. C. et al. Structures of the N-terminal modules imply large domain motions during catalysis by methionine synthase. Proc. Natl Acad. Sci. USA 101, 3729–3736 (2004).
Jope, R. S. High affinity choline transport and acetylCoA production in brain and their roles in the regulation of acetylcholine synthesis. Brain Res. 180, 313–344 (1979).
Ferguson, S. M. et al. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc. Natl Acad. Sci. USA 101, 8762–8767 (2004).
Hartmann, J., Kiewert, C., Duysen, E. G., Lockridge, O. & Klein, J. Choline availability and acetylcholine synthesis in the hippocampus of acetylcholinesterase-deficient mice. Neurochem. Int. 52, 972–978 (2008).
Koppen, A., Klein, J., Erb, C. & Loffelholz, K. Acetylcholine release and choline availability in rat hippocampus: effects of exogenous choline and nicotinamide. J. Pharmacol. Exp. Ther. 282, 1139–1145 (1997).
Cohen, E. L. & Wurtman, R. J. Brain acetylcholine: control by dietary choline. Science 191, 561–562 (1976).
Molloy, A. M. et al. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 123, 917–923 (2009).
Dror, D. K. & Allen, L. H. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr. Rev. 66, 250–255 (2008).
Shaik, M. M., Tan, H. L., Kamal, M. A. & Gan, S. H. Do folate, vitamins B6 and B12 play a role in the pathogenesis of migraine? The role of pharmacoepigenomics. CNS Neurol. Disord. Drug Targets 13, 828–835 (2014).
Mitra, S., Natarajan, R., Ziedonis, D. & Fan, X. Antioxidant and anti-inflammatory nutrient status, supplementation, and mechanisms in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 78, 1–11 (2017).
Pineles, S. L., Avery, R. A. & Liu, G. T. Vitamin B12 optic neuropathy in autism. Pediatrics 126, e967–e970 (2010).
Nelson, S. B. & Valakh, V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87, 684–698 (2015).
Vecchia, D. & Pietrobon, D. Migraine: a disorder of brain excitatory–inhibitory balance? Trends Neurosci. 35, 507–520 (2012).
Eichler, S. A. & Meier, J. C. E–I balance and human diseases—from molecules to networking. Front Mol. Neurosci. 1, 2 (2008).
Weisburg, W. G., Barns, S. M., Pelletier, D. A. & Lane, D. J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703 (1991).
Edelstein, A. D. et al. Advanced methods of microscope control using muManager software. J. Biol. Methods 1, e10 (2014).
Kamath, R. S. & Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30, 313–321 (2003).
Hulo, N. et al. The PROSITE database. Nucleic Acids Res. 34, D227–D230 (2006).
Lemoine, F. et al. NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res. 47, W260–W265 (2019).
Yamada, K., Yamada, S., Tobimatsu, T. & Toraya, T. Heterologous high level expression, purification, and enzymological properties of recombinant rat cobalamin-dependent methionine synthase. J. Biol. Chem. 274, 35571–35576 (1999).
Garrow, T. A. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J. Biol. Chem. 271, 22831–22838 (1996).
Acknowledgements
We thank the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440), for some of the worm and bacterial strains. We thank B. Samuel, H. Schulenburg, M. Shapira, V. Ambros and M. Treinin for bacterial strains; V. Budnik, A. Byrne, A. Walker and C. Vance for helpful discussions; and W. Joyce for technical support. This work was supported in part by National Institutes of Health grant numbers R01NS107475 and R01GM140480 (M.J.A.), DK068429 (A.J.M.W.), 1R35GM131877 (F.C.S.) and a grant from the Riccio Fund for Neuroscience (M.J.A. and A.J.M.W.). F.C.S. is a Faculty Scholar of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
W.K.K., J.T.F., B.W.F., A.A. and A.T. performed the experiments and analysed the data. W.K.K., J.T.F., B.W.F., F.C.S., A.J.M.W. and M.J.A. designed the experiments. W.K.K. and M.J.A. conceived the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks Mauro Costa-Mattioli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
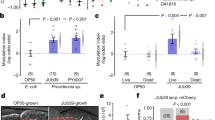
Extended Data Fig. 1 Effects of different bacterial diets on growth, immune response and acdh-1 expression of C. elegans.
a, Growth rate as indicated by body length of unc-2(gof) mutants grown on the indicated bacterial strains for 24 h (mean ± s.e.m., n = 3, one-way ANOVA with Dunnett’s multiple comparison). b, Fluorescence values of immune reporter Pirg-1::GFP after 24 h exposure to indicated bacterial strains. Pathogenic strain P. aeruginosa 14 was included as a positive control (mean ± s.e.m., n = 3, one-way ANOVA with Dunnett’s multiple comparison). c, Representative DIC and fluorescence images of Pacdh-1::GFP animals fed different bacterial diets for 24 h. Shades of green represent relative GFP expression levels, ‘High’ indicates strong fluorescence throughout the intestine as in the OP50 shown, ‘Low’ indicates barely detectable fluorescence as in the Comamonas shown, ‘Moderate’ indicates visible fluorescence but weaker compared to the GFP signal on OP50 (n = 3 biologically independent samples with similar results). Scale bar, 300 µm. d, Growth rate as indicated by body length of wild-type and unc-2(gof) mutants fed OP50, OP50 with 64 nM B12, Comamonas, or Comamonas cbiAΔ for 24 h (mean ± s.e.m., n = 3, one-way ANOVA with Dunnett’s multiple comparison). e, Reversals of wild-type and unc-2(gof) mutants fed OP50, Comamonas, Comamonas cbiAΔ, or Comamonas cbiAΔ with 64 nM B12 for 24 h (mean ± s.e.m., one-way ANOVA with Dunnett’s multiple comparison). f, Growth rate as indicated by body length of wild-type and unc-2(gof) mutants fed live or heat-killed OP50 ± 64 nM B12 for 24 h (mean ± s.e.m., n = 3, one-way ANOVA with Tukey’s multiple comparison). Source numerical data are provided.
Extended Data Fig. 2 Antibiotic susceptibility for C. aquatica gut bacteria elimination.
a, Bacterial CFU per animal from unc-2(gof) mutants grown on Comamonas treated with the indicated concentrations of kanamycin for 24 h (mean ± s.e.m., n = 3, one-way ANOVA with Dunnett’s multiple comparison). b, Reversal frequency of unc-2(gof) mutants grown on OP50 with indicated concentrations of kanamycin for 24 h (mean ± s.e.m., one-way ANOVA with Dunnett’s multiple comparison). c, Bacterial colonies of OP50 and Comamonas isolated the worm gut. Bacterial colonies were isolated and identified by 16 S rRNA gene using 27 f and 1492r primers (Methods) (n = 21 independent experiments with similar results). Source numerical data are provided.
Extended Data Fig. 3 B12 reduces cholinergic signalling especially under conditions of increased acetylcholine release.
a, Reversal frequency of unc-2(gof) and cat-1(ok411); unc-2(gof) mutants fed OP50 ± 64 nM B12 (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). b, Quantification of locomotion speed of wild-type and ace-1(p1000); ace-2(g72) mutants fed OP50, OP50 with 64 nM B12, Comamonas, or Comamonas cbiAΔ for 24 h (mean ± s.e.m., n = 4, two-way ANOVA with Tukey’s multiple comparison). c-f, Quantification of reversal frequency (c), locomotion speed (d), head bending (e), and body bending (f) of wild-type and unc-2(gof) mutants fed OP50 ± 64 nM B12 for 24 h (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). g, Quantification of paralysis percentage of wild-type animals fed OP50 on 1 mM aldicarb-containing NGM agar plates or M9 liquid buffer (mean ± s.e.m., n = 4 (crawling on agar), n = 5 (swimming on liquid), two-way ANOVA with Tukey’s multiple comparison). Source numerical data are provided.
Extended Data Fig. 4 B12 regulates C. elegans behaviour and growth through Met/SAM cycle.
a, Reversals of unc-2(gof), sams-1(ok2946); unc-2(gof), cbs-2(ok666); unc-2(gof), pcca-1(ok2282) unc-2(gof), or mce-1(ok243); unc-2(gof) mutants fed OP50 ± B12 for 24 h (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). b, Growth rate of unc-2(gof), mmcm-1(ok1637); unc-2(gof), metr-1(ok521); unc-2(gof), sams-1(ok2946); unc-2(gof) mutants fed OP50 ± B12 for 24 h (mean ± s.e.m., n = 3, two-way ANOVA with Tukey’s multiple comparison). c, Quantification of paralysis percentage of unc-2(gof) and sams-1(ok2946); unc-2(gof) mutants fed OP50 ± B12 on 1 mM aldicarb (mean ± s.e.m., n = 4, two-way ANOVA with Tukey’s multiple comparison). d, Quantification of acetylcholine in unc-2(gof) mutants fed OP50 ± B12 for 24 h (mean ± s.e.m., one-way ANOVA with Dunnett’s multiple comparison). e, Quantification of acetylcholine in WT and metr-1(ok521) mutants fed OP50 ± B12 for 24 h (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). f, Quantification of methionine (Met) in wild-type, metr-1(ok521), unc-2(gof), metr-1(ok521); unc-2(gof) mutants fed OP50 ± B12 for 24 h (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). g, Growth rate of metr-1(ok521); unc-2(gof) mutants expressing metr-1 cDNA driven by the indicated tissue-specific promoter fed OP50 ± B12 for 24 h (mean ± s.e.m., n = 3, two-way ANOVA with Tukey’s multiple comparison). h-i, Growth rate (h) and reversals (i) of unc-2(gof) mutants fed OP50 with the indicated metabolites for 24 h (mean ± s.e.m., n = 3 (h), n indicated (i), one-way ANOVA with Dunnett’s multiple comparison). j,k, Quantification of homocysteine (Hcy) (j), S-adenosylhomocysteine (SAH) (k) in wild-type, metr-1(ok521), unc-2(gof), metr-1(ok521); unc-2(gof) mutants fed OP50 ± B12 for 24 h (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). Source numerical data are provided.
Extended Data Fig. 5 Choline metabolism is linked to the Met/SAM cycle.
a, Growth rate as indicated by body length of unc-2(gof) mutants fed OP50 ± 64 nM B12 with 30 mM choline for 24 h (mean ± s.e.m., n = 3, two-way ANOVA with Tukey’s multiple comparison). b, Expression pattern of Palh-9::GFP in the intestine, hypodermis, and RIM neurons (n = 3 biologically independent samples with similar results). Scale bar, 100 µm. c, Growth rate as indicated by body length of unc-2(gof) mutants subjected to RNAi knockdown of chdh-1 or alh-9 fed OP50 ± 64 nM B12 for 24 h (mean ± s.e.m., n = 3, two-way ANOVA with Tukey’s multiple comparison). Source numerical data are provided.
Extended Data Fig. 6 In vitro activity assay of METR-1.
a, SDS–PAGE of purified METR-1 proteins. Purity of immunopurified GFP-tagged METR-1 protein was analysed with 4–10% polyacrylamide gels electrophoresis under denaturing conditions. Panels show silver staining (left) and western blot stained with a GFP antibody (right). Arrowhead indicates METR-1::GFP protein (molecular weight ~ 166 kDa). (n = 3 independent experiments with similar results) b, Methionine synthase activity of the purified METR-1::GFP protein was assayed in the presence of 5-methyltetrahydrofolate (5-meTHF) or betaine as a methyl donor ± SAM and DTT. Enzyme reaction was performed in 50 mM Tris-HCl buffer (pH 7.5) at 25 °C for 6 h (Methods) (mean ± s.e.m., one-way ANOVA with Dunnett’s multiple comparison). Source numerical data and source blot images are provided.
Extended Data Fig. 7 A neuronal choline transporter is required to mediate the effect of B12 on excitatory transmission.
a, Reversal frequency of unc-2(gof), acr-23(ok2804); unc-2(gof), or lgc-41(sy1494) unc-2(gof) mutants fed OP50 ± 64 nM B12 for 24 h (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). b, Reversal frequency of unc-2(gof) or deg-3(u701); unc-2(gof) mutants fed OP50 ± 64 nM B12 for 24 h (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). c, Reversal frequency of unc-2(gof) mutants fed OP50, OP50 with B12 (64 nM), OP50 with betaine (75 mM), or OP50 with both B12 and betaine (mean ± s.e.m., two-way ANOVA with Tukey’s multiple comparison). d, Growth rate as indicated by body length of unc-2(gof) and cho-1(tm373); unc-2(gof) mutants fed OP50 ± 64 nM B12 for 24 h (mean ± s.e.m., n = 3, two-way ANOVA with Tukey’s multiple comparison). Source numerical data are provided.
Supplementary information
Source data
Source Data Fig. 1
Source numerical data.
Source Data Fig. 2
Source numerical data.
Source Data Fig. 3
Source numerical data.
Source Data Fig. 4
Source numerical data.
Source Data Fig. 5
Source numerical data.
Source Data Fig. 6
Source numerical data.
Source Data Fig. 7
Source numerical data.
Source Data Extended Data Fig. 1
Source numerical data.
Source Data Extended Data Fig. 2
Source numerical data.
Source Data Extended Data Fig. 3
Source numerical data.
Source Data Extended Data Fig. 4
Source numerical data.
Source Data Extended Data Fig. 5
Source numerical data.
Source Data Extended Data Fig. 6
Source numerical data, and unprocessed gel and blot.
Source Data Extended Data Fig. 7
Source numerical data.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6a, and unprocessed gel and blot
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, W.K., Florman, J.T., Araya, A. et al. Vitamin B12 produced by gut bacteria modulates cholinergic signalling. Nat Cell Biol 26, 72–85 (2024). https://doi.org/10.1038/s41556-023-01299-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-023-01299-2