Abstract
Coat proteins have a central role in vesicular transport by binding to cargoes for their sorting into intracellular pathways. Cargo recognition is mediated by components of the coat complex known as adaptor proteins1,2,3. We previously showed that Arf-GAP with coil-coil, ANK repeat and PH domain-containing protein 1 (ACAP1) functions as an adaptor for a clathrin coat complex that has a function in endocytic recycling4,5,6. Here, we show that the protein kinase Akt acts as a co-adaptor in this complex, and is needed in conjunction with ACAP1 to bind to cargo proteins to promote their recycling. In addition to advancing the understanding of endocytic recycling, we uncover a fundamentally different function in which a kinase acts, as Akt in this case is an effector rather than a regulator in a cellular event.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Robinson, M. S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174 (2004).
Traub, L. M. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 10, 583–596 (2009).
Miller, E. A. & Barlowe, C. Regulation of coat assembly—sorting things out at the ER. Curr. Opin. Cell Biol. 22, 447–453 (2010).
Dai, J. et al. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev. Cell 7, 771–776 (2004).
Li, J. et al. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin β1 to control cell migration. Dev. Cell 9, 663–673 (2005).
Li, J. et al. An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol. 178, 453–464 (2007).
Caswell, P. & Norman, J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 18, 257–263 (2008).
Grant, B. D. & Donaldson, J. G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 (2009).
Hsu, V. W., Bai, M. & Li, J. Getting active: protein sorting in endocytic recycling. Nat. Rev. Mol. Cell Biol. 13, 323–328 (2012).
Irannejad, R. & von Zastrow, M. GPCR signaling along the endocytic pathway. Curr. Opin. Cell Biol. 27, 109–116 (2014).
Rodriguez-Boulan, E. & Macara, I. G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225–242 (2014).
Cullen, P. J. & Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018).
Bai, M. et al. Mechanistic insights into regulated cargo binding by ACAP1 protein. J. Biol. Chem. 287, 28675–28685 (2012).
Bonifacino, J. S. & Traub, L. M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev. Biochem 72, 395–447 (2003).
Manning, B. D. & Toker, A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017).
Alessi, D. R. et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 (1996).
Oh, S. J. & Santy, L. C. Differential effects of cytohesins 2 and 3 on β1 integrin recycling. J. Biol. Chem. 285, 14610–14616 (2010).
Rosselli-Murai, L. K. et al. Loss of PTEN promotes formation of signaling-capable clathrin-coated pits. J. Cell Sci. 131, jcs208926 (2018).
Cao, T. T., Deacon, H. W., Reczek, D., Bretscher, A. & von Zastrow, M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature 401, 286–290 (1999).
Conner, S. D. & Schmid, S. L. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J. Cell Biol. 156, 921–929 (2002).
Kuroda, F., Moss, J. & Vaughan, M. Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1γ. Proc. Natl Acad. Sci. USA 104, 3201–3206 (2007).
Liberali, P. et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 27, 970–981 (2008).
Weller, S. G. et al. Src kinase regulates the integrity and function of the Golgi apparatus via activation of dynamin 2. Proc. Natl Acad. Sci. USA 107, 5863–5868 (2010).
Ren, J. & Guo, W. ERK1/2 regulate exocytosis through direct phosphorylation of the exocyst component Exo70. Dev. Cell 22, 967–978 (2012).
Luo, G., Zhang, J., Luca, F. C. & Guo, W. Mitotic phosphorylation of Exo84 disrupts exocyst assembly and arrests cell growth. J. Cell Biol. 202, 97–111 (2013).
Xiao, G. Y., Mohanakrishnan, A. & Schmid, S. L. Role for ERK1/2-dependent activation of FCHSD2 in cancer cell-selective regulation of clathrin-mediated endocytosis. Proc. Natl Acad. Sci. USA 115, E9570–E9579 (2018).
Yang, J. S. et al. GAPDH inhibits intracellular pathways during starvation for cellular energy homeostasis. Nature 561, 263–267 (2018).
Powelka, A. M. et al. Stimulation-dependent recycling of integrin β1 regulated by ARF6 and Rab11. Traffic 5, 20–36 (2004).
Acknowledgements
We thank S.-Y. Park for advice and discussion, and staff at the Center for Macromolecular Interactions (CMI) in the department of Biological Chemistry and Molecular Pharmacology at Harvard Medical School for access to Octet RED384. This research is supported by grants from the National Institutes of Health to V.W.H. (GM115683), M.J.E. (CA116020) and P.A.C. (CA74305).
Author information
Authors and Affiliations
Contributions
J.-W.H., M.B., J.L., K.L., J.-S.Y. and N.C. performed experiments. J.-W.H., M.B., J.L., J.-S.Y., K.L., N.C., P.A.C., M.J.E. and V.W.H. participated in designing experiments and analysing the results. V.W.H. supervised the research with help from J.L. V.W.H. wrote the manuscript with help from J.-W.H.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
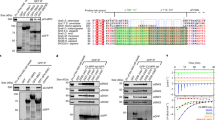
Extended Data Fig. 1 Further characterizing cargo binding interactions.
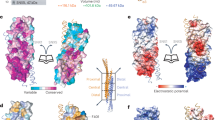
a, b, c, Pulldown interactions from 2 independent experiments are quantified for studies shown in Fig. 1a (a), in Fig. 1b (b), and in Fig. 1c (c). Data available in Source Data Extended Data Fig. 1a–c. d, e, Pulldown studies followed by Coomassie staining to detect soluble forms Akt bound to GST-α5 on beads (d) or soluble Akt and ACAP1 bound to GST-α5 on beads (e); n = 2 independent experiments. Input shows 10% of soluble components used in the incubation. Data available in Unprocessed Blots Extended Data Fig. 1d, e. f, A soluble complex containing GST-α5, Akt, and ACAP1 is detected by incubating the three components in solution, followed by isolation using glutathione beads, and then Coomassie staining to assess complex formation; n = 2 independent experiments. Input shows 10% of soluble components used for the incubation. Data available in Unprocessed Blots Extended Data Fig. 1f. g, h, i, Pulldown interactions from 2 independent experiments are quantified for studies shown in Fig. 1d (g), in Fig. 1e (h), and in Fig. 1f (i). Data available in Source Data Extended Data Fig. 1g–i. j, k, Primary data for biolayer interferometry measurements that quantify the interaction between GST-α5 and the Akt kinase domain shown in Fig. 1g (j), and between the Akt kinase domain and the carboxyl portion of ACAP1 shown in Fig. 1h (k); n = 2 independent experiments. l, A model for how Akt and ACAP1 act as co-adaptors in binding to the α5β1 integrin heterodimer.
Extended Data Fig. 2 Akt having a non-kinase role in integrin recycling.
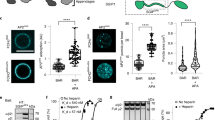
a, Efficiency of siRNA against Akt and its rescues in HeLa cells that express the ACAP1 mutant (S554D), as detected by immunoblotting of whole cell lysates; n = 2 independent experiments. Data available in Unprocessed Blots Extended Data Fig. 2a. b, Representative primary images from the integrin recycling assay shown in Fig. 1i; n = 3 independent experiments. The colocalization of endosomal β1 with Rab11, a marker of the recycling endosome, is assessed; β1 (red), Rab11 (green), bar = 10 um.
Extended Data Fig. 3 Further supporting a non-kinase role of Akt in integrin recycling.
a, Cell migration of the S554D-expressing HeLa cells as assessed through the transwell-based assay; n = 3 independent experiments, with each experiment examining three fields of transwell membranes. Primary images are shown on left, bar = 200 um. Quantitation is shown on right, mean ± SD, *p = 1.57 × 10−20, NS p = 0.125, paired two-tailed student’s t-test. Data available in Source Data Extended Data Fig. 3a. b, c, Integrin recycling assay assessing the effect of treating the S554D-expressing HeLa cells (b), or control HeLa cells (c), with the Akt kinase inhibitor GDC0068; n = 3 independent experiments. Quantitation is shown on left, mean ± SD, with statistics performed for the 5-minute time point, NS p = 0.248 (for analysis in b), *p = 1.54 × 10−25 (for analysis in c), paired two-tailed student’s t-test. Primary images, assessing the colocalization of endosomal β1 with cellubrevin (a marker of the recycling endosome), are shown on right, β1 (red), Cbv (green), bar = 10 um. Data available in Source Data Extended Data Fig. 3b, c.
Extended Data Fig. 4 Further characterizing the effect of expressing a mutant α5 integrin.
a, Primary images from the integrin recycling assay shown in Fig. 2d; n = 3 independent experiments. The colocalization of endosomal β1 with Rab11 is assessed; β1 (red), Rab11 (green), bar = 10 um. b, Cell migration of HeLa cells that express different α5 forms as assessed through the transwell-based assay; n = 3 independent experiments, with each experiment examining three fields of transwell membranes. Primary images are shown on left, bar = 200 um. Quantitation is shown on right, mean ± SD, *p = 2.08 × 10−24, paired two-tailed student’s t-test. Data available in Source Data Extended Data Fig. 4b. c, Representative primary images for the colocalization study shown in Fig. 2g; n = 3 independent experiments. The colocalization of endosomal β1 with Rab11 is assessed, β1 (red), Rab11 (green), bar = 10 um.
Extended Data Fig. 5 Further supporting a non-kinase role of Akt in TfR recycling.
a, Primary images from the TfR recycling assay shown in Fig. 3a; n = 3 independent experiments. The colocalization of endosomal Tf with Rab11 is assessed; Tf (red), Rab11 (green), bar = 10 um. b, TfR recycling assay examining the effect of siRNA against Akt, and also rescue using wild-type (WT) or kinase-dead (K179M) Akt in HEK293 cells; n = 3 independent experiments, with each experiment examining 10 cells. Quantitation is shown above, mean ± SD, with statistics performed on the 25-minute time point, *p = 8.86 × 10−27, NS p = 0.557, paired two-tailed student’s t-test. Primary images along with line scans are shown below, Tf (red), cellubrevin (green), bar = 10 um. Data available in Source Data Extended Data Fig. 5b.
Extended Data Fig. 6 Further characterizing how Akt acts in the endocytic transport of TfR.
a, TfR internalization assay examining the effect of siRNA against Akt, n = 3 independent experiments, with each experiment examining 10 cells. Quantitation is shown above, mean ± SD, with statistics performed for the 5-minute time point, NS p = 0.538, paired two-tailed student’s t-test. Primary images are shown below, Tf (red), EEA1 (a marker of the early endosome, green), bar = 10 um. Data available in Source Data Extended Data Fig. 6a. b, TfR recycling assay examining the effect of siRNA against Akt, and also rescues using various forms of Akt in HeLa cells; n = 3 independent experiments, with each experiment examining 10 cells. Quantitation is shown above, mean ± SD, with statistics performed on the 25-minute time point, *p = 2.68 × 10−27, NS p = 0.303, paired two-tailed student’s t-test. Primary images along with line scans are shown below, Tf (red), Rab11 (green), bar = 10 um. Data available in Source Data Extended Data Fig. 6b.
Extended Data Fig. 7 Further characterizing how Akt acts in TfR recycling.
a, TfR recycling assay examining the effect of treating HeLa cells with the Akt kinase inhibitor GDC0068; n = 3 independent experiments, with each experiment examining 10 cells. Quantitation is shown on left, mean ± SD with statistics performed on the 30-minute time point, NS p = 0.222, paired two-tailed student’s t-test. Primary images along with line scans are shown on right; Tf (red), cellubrevin (green), bar = 10 um. Data available in Source Data Extended Data Fig. 7a. b, Efficiency of siRNA against Akt1 and siRNA against Akt2 in HeLa cells, as assessed by immunoblotting of whole cell lysates; n = 2 independent experiments. Actin level confirms similar levels of cells examined. Data available in Unprocessed Blots Extended Data Fig. 7b. c, d, TfR recycling assay examining the effect of siRNA against different isoforms of Akt in HeLa cells; n = 3 independent experiments, with each experiment examining 10 cells. Quantitation is shown in (c), mean ± SD, with statistics performed on the 25-minute time point, *p = 1.72 × 10−24 (control vs si-Akt1), *p = 1.53 × 10−30 (control vs si-Akt2), *p = 1.78 × 10−43 (control vs si-Akt1/si-Akt2), paired two-tailed student’s t-test. Data available in Source Data Extended Data Fig. 7c. Primary images along with line scans are shown in (d), Tf (red), Rab11 (green), bar = 10 um.
Extended Data Fig. 8 Further supporting Akt acts as a co-adaptor with ACAP1 in cargo binding.
a, Schematic showing the sequence of the TfR cytoplasmic domain, and the portions covered by the N19 and the NΔ19 constructs. b, Pulldown studies titrating increasing level of different fusion proteins of Akt and ACAP1 for their binding to the TfR cytoplasmic domain; n = 2 independent experiments. Left panel compares binding by Akt-ACAP1 heterodimer and Akt homodimer. Right panel compares binding by Akt-ACAP1 heterodimer and ACAP1 homodimer. Data available in Unprocessed Blots Extended Data Fig. 8b. c, d, Co-precipitation studies examining the effect of siRNA against Akt on the association of endosomal TfR with ACAP1 and Akt in HeLa cells (c), and siRNA against ACAP1 on the association of endosomal TfR with Akt and ACAP1 in HeLa cells (d); n = 2 independent experiments. Biotin-labeled Tf was internalized for 2 hours to label the endosomal pool of TfR. Immunoblotting of whole cell lysates confirms the efficiency of siRNA treatment. Data available in Unprocessed Blots Extended Data Fig. 8c, d.
Extended Data Fig. 9 Further characterizing membrane recruitment of Akt.
a, Isolating a membrane fraction from HeLa cells enriched for the recycling endosome using a sucrose gradient established through equilibrium centrifugation; n = 2 independent experiments. Fractions enriched for the recycling endosome were identified by tracking cellubrevin and internalized Tf (which bound to endosomal TfR), and not surface Tf (which bound to surface TfR). Data available in Unprocessed Blots Extended Data Fig. 9a. b, c, d, Recruitment studies showing that Akt alone cannot be recruited to endosomal membrane (b), while ARF6 alone in its activate form can be recruited to endosomal membrane (c), and clathrin recruitment to endosomal membrane requires ARF6 with either Akt or ACAP1 (d); n = 2 independent experiments. Cellubrevin tracks endosomal membrane. Data available in Unprocessed Blots Extended Data Fig. 9b–d. e, Recruitment study examining the relative levels of ARF6, Akt, ACAP1, and clathrin recruited to liposomes; n = 2 independent experiments. Input shows the total amount of each component added for the incubation. Data available in Unprocessed Blots Extended Data Fig. 9e.
Extended Data Fig. 10 Further characterizing endogenous Akt at the recycling endosome.
a, Confocal microscopy examining the colocalization of endogenous Akt with endosomal Tf in HeLa cells; n = 3 independent experiments, with each experiment examining 10 cells. Primary images are shown, Akt (green), Tf (red), bar = 10 um. b, Confocal microscopy examining the effect of a more denaturing fixative (containing methanol and acetone) on the ability to detect endogenous clathrin at the recycling endosome in HeLa cells; n = 3 independent experiments, with each experiment examining 10 cells. Primary images along with line scan are shown on left for the colocalization of clathrin with endosomal Tf, clathrin (green), Tf (red), bar = 10 um. Quantitation is shown on right, mean ± SD, NS p = 0.106, paired two-tailed student’s t-test. Data available in Source Data Extended Data Fig. 10b. c, Confocal microscopy examining the colocalization of different forms of ARNO with endosomal Tf in HeLa cells; n = 3 independent experiments, with each experiment examining 10 cells. Primary images along with line scan are shown on left for the colocalization of endogenous Akt with endosomal Tf, ARNO (green), Tf (red), bar = 10 um. Quantitation is shown on right, mean ± SD, NS p = 0.686, paired two-tailed student’s t-test. Data available in Source Data Extended Data Fig. 10c. d, Confocal microscopy examining the effect of serum stimulation on Akt localization at the recycling endosome in HeLa cells; n = 3 independent experiments, with each experiment examining 10 cells. Primary images along with line scan are shown above for the colocalization of endogenous Akt with endosomal Tf, Akt (red), Tf (green), bar = 10 um. Quantitation is shown below, mean ± SD, NS p = 0.325, paired two-tailed student’s t-test. Data available in Source Data Extended Data Fig. 10d.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots and/or gels.
Source Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 9
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Hsu, JW., Bai, M., Li, K. et al. The protein kinase Akt acts as a coat adaptor in endocytic recycling. Nat Cell Biol 22, 927–933 (2020). https://doi.org/10.1038/s41556-020-0530-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-020-0530-z
This article is cited by
-
Phosphoglycerate kinase 1 acts as a cargo adaptor to promote EGFR transport to the lysosome
Nature Communications (2024)
-
Integrating intracellular nanovesicles into integrin trafficking pathways and beyond
Cellular and Molecular Life Sciences (2022)