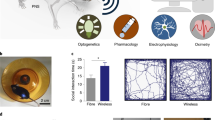
Abstract
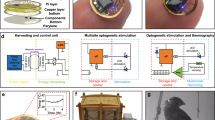
Fully implantable wireless systems for the recording and modulation of neural circuits that do not require physical tethers or batteries allow for studies that demand the use of unconstrained and freely behaving animals in isolation or in social groups. Moreover, feedback-control algorithms that can be executed within such devices without the need for remote computing eliminate virtual tethers and any associated latencies. Here we report a wireless and battery-less technology of this type, implanted subdermally along the back of freely moving small animals, for the autonomous recording of electroencephalograms, electromyograms and body temperature, and for closed-loop neuromodulation via optogenetics and pharmacology. The device incorporates a system-on-a-chip with Bluetooth Low Energy for data transmission and a compressed deep-learning module for autonomous operation, that offers neurorecording capabilities matching those of gold-standard wired systems. We also show the use of the implant in studies of sleep–wake regulation and for the programmable closed-loop pharmacological suppression of epileptic seizures via feedback from electroencephalography. The technology can support a broader range of applications in neuroscience and in biomedical research with small animals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. Source data for Figs. 2, 3, 4e–h and 6 are provided with this paper. Source data for Figs. 4i,j and 5 and the raw data are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request.
Code availability
The custom code for analysing electroencephalography and electromyography data is available at https://github.com/wouyanglv/Wireless_EEG.
References
Amiri, S., Fazel-Rezai, R. & Asadpour, V. A review of hybrid brain-computer interface systems. Adv. Hum. Comput. Interact. https://doi.org/10.1155/2013/187024 (2013).
Teplan, M. Fundamentals of EEG measurement. Meas. Sci. Rev. 2, 1–11 (2002).
Tagluk, M. E., Sezgin, N. & Akin, M. Estimation of sleep stages by an artificial neural network employing EEG, EMG and EOG. J. Med. Syst. 34, 717–725 (2010).
Won, S. M., Cai, L., Gutruf, P. & Rogers, J. A. Wireless and battery-free technologies for neuroengineering. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-021-00683-3 (2021).
Srinivasan, N. Cognitive neuroscience of creativity: EEG based approaches. Methods 42, 109–116 (2007).
Onton, J., Delorme, A. & Makeig, S. Frontal midline EEG dynamics during working memory. Neuroimage 27, 341–356 (2005).
Mirowski, P., Madhavan, D., LeCun, Y. & Kuzniecky, R. Classification of patterns of EEG synchronization for seizure prediction. Clin. Neurophysiol. 120, 1927–1940 (2009).
Eban-Rothschild, A., Appelbaum, L. & de Lecea, L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology 43, 937–952 (2018).
Fuller, P. M., Gooley, J. J. & Saper, C. B. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythms 21, 482–493 (2006).
Yang, Y. et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat. Neurosci. 24, 1035–1045 (2021).
Zayachkivsky, A., Lehmkuhle, M. J. & Dudek, F. E. Long-term continuous EEG monitoring in small rodent models of human disease using the epoch wireless transmitter system. J. Vis. Exp. https://doi.org/10.3791/52554 (2015).
Park, S. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 20, 612–619 (2017).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. https://doi.org/10.1038/natrevmats.2016.93 (2017).
Afshar, P. et al. A translational platform for prototyping closed-loop neuromodulation systems. Front. Neural Circuits 6, 117 (2013).
Mirza, K. B., Golden, C. T., Nikolic, K. & Toumazou, C. Closed-loop implantable therapeutic neuromodulation systems based on neurochemical monitoring. Front. Neurosci. 13, 808 (2019).
Roy, Y. et al. Deep learning-based electroencephalography analysis: a systematic review. J. Neural Eng. 16, 51001 (2019).
Baldassano, S. et al. Cloud computing for seizure detection in implanted neural devices. J. Neural Eng. 16, 26016 (2019).
Wang, F., Kaushal, R. & Khullar, D. Should health care demand interpretable artificial intelligence or accept “black box” medicine? Ann. Intern. Med. 172, 59–60 (2020).
Liu, X. & Richardson, A. G. Edge deep learning for neural implants: a case study of seizure detection and prediction. J. Neural Eng. 18, 46034 (2021).
Ahmad, I. et al. EEG-based epileptic seizure detection via machine/deep learning approaches: a systematic review. Comput. Intell. Neurosci. 2022, 6486570 (2022).
Hügle, M. et al. Early seizure detection with an energy-efficient convolutional neural network on an implantable microcontroller. In 2018 International Joint Conference on Neural Networks (IJCNN) 1–7 (IEEE, 2018).
Bahr, A. et al. Epileptic seizure detection on an ultra-low-power embedded RISC-V processor using a convolutional neural network. Biosensors 11, 203 (2021).
David, R. et al. Tensorflow lite micro: embedded machine learning on tinyml systems. In Proc. Machine Learning and Systems 3 (eds Smola, A., Dimakis, A. & Stoica, I.) 800–811 (MLSys, 2021).
Pinnell, R. C., Almajidy, R. K., Kirch, R. D., Cassel, J. C. & Hofmann, U. G. A wireless EEG recording method for rat use inside the water maze. PLoS ONE 11, e0147730 (2016).
Jia, Y. et al. A software-defined radio receiver for wireless recording from freely behaving animals. IEEE Trans. Biomed. Circuits Syst. 13, 1645–1654 (2019).
Zayachkivsky, A., Lehmkuhle, M. J., Fisher, J. H., Ekstrand, J. J. & Dudek, F. E. Recording EEG in immature rats with a novel miniature telemetry system. J. Neurophysiol. 109, 900–911 (2013).
Chang, P., Hashemi, K. S. & Walker, M. C. A novel telemetry system for recording EEG in small animals. J. Neurosci. Methods 201, 106–115 (2011).
Jung, Y. H. et al. Stretchable twisted‐pair transmission lines for microwave frequency wearable electronics. Adv. Funct. Mater. 26, 4635–4642 (2016).
Daniel, Ţ. D. & Neagu, M. in Compendium of New Techniques in Harmonic Analysis (ed. Lamchich, M. T.) Ch. 2 (IntechOpen, 2018).
Usakli, A. B. Improvement of EEG signal acquisition: an electrical aspect for state of the art of front end. Comput. Intell. Neurosci. 2010, 630649 (2010).
Miladinović, Đ. et al. SPINDLE: end-to-end learning from EEG/EMG to extrapolate animal sleep scoring across experimental settings, labs and species. PLoS Comput. Biol. 15, e1006968 (2019).
Tsimbalo, E., Fafoutis, X. & Piechocki, R. Fix it, don’t bin it! - CRC error correction in Bluetooth low energy. In 2015 IEEE 2nd World Forum on Internet of Things (WF-IoT) 286–290 (IEEE, 2015).
Wolf, P. D. in Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment (ed. Reichert, W. M.) Ch. 3 (CRC Press/Taylor & Francis, 2008).
Kadam, S. D. et al. Methodological standards and interpretation of video‐electroencephalography in adult control rodents. A TASK 1‐WG 1 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia 58, 10–27 (2017).
Gage, G. J. et al. Surgical implantation of chronic neural electrodes for recording single unit activity and electrocorticographic signals. J. Vis. Exp. https://doi.org/10.3791/3565 (2012).
Kim, R. & Nam, Y. Novel platinum black electroplating technique improving mechanical stability. In 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 184–187 (IEEE, 2013).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Aravanis, A. M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Zhang, Y. et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Sci. Adv. 5, eaaw5296 (2019).
Hodor, A., Palchykova, S., Gao, B. & Bassetti, C. L. Baclofen and gamma-hydroxybutyrate differentially altered behavior, EEG activity and sleep in rats. Neuroscience 284, 18–28 (2015).
Sweeney-Reed, C. M., Nasuto, S. J., Vieira, M. F. & Andrade, A. O. Empirical mode decomposition and its extensions applied to EEG analysis: a review. Adv. Data Sci. Adapt. Anal. 10, 1840001 (2018).
Budd, T. W. et al. Repetition suppression of the rat auditory evoked potential at brief stimulus intervals. Brain Res. 1498, 59–68 (2013).
Nir, Y., Vyazovskiy, V. V., Cirelli, C., Banks, M. I. & Tononi, G. Auditory responses and stimulus-specific adaptation in rat auditory cortex are preserved across NREM and REM sleep. Cereb. Cortex 25, 1362–1378 (2015).
Walsh, R. N. & Cummins, R. A. The open-field test: a critical review. Psychol. Bull. 83, 482–504 (1976).
Díaz-Morán, S. et al. Relationships of open-field behaviour with anxiety in the elevated zero-maze test: focus on freezing and grooming. World J. Neurosci. https://doi.org/10.4236/wjns.2014.41001 (2014).
Sturman, O., Germain, P.-L. & Bohacek, J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress 21, 443–452 (2018).
Seibenhener, M. L. & Wooten, M. C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. https://doi.org/10.3791/52434 (2015).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Silva‐Pérez, M., Sánchez‐López, A., Pompa‐del‐Toro, N. & Escudero, M. Identification of the sleep–wake states in rats using the high‐frequency activity of the electroencephalogram. J. Sleep Res. 30, e13233 (2020).
Brankačk, J., Kukushka, V. I., Vyssotski, A. L. & Draguhn, A. EEG gamma frequency and sleep–wake scoring in mice: comparing two types of supervised classifiers. Brain Res. 1322, 59–71 (2010).
Briese, E. Normal body temperature of rats: the setpoint controversy. Neurosci. Biobehav. Rev. 22, 427–436 (1998).
Chen, M. C. et al. Anterior insula regulates multiscale temporal organization of sleep and wake activity. J. Biol. Rhythms 31, 182–193 (2016).
Machado, R. B., Tufik, S. & Suchecki, D. Role of corticosterone on sleep homeostasis induced by REM sleep deprivation in rats. PLoS ONE 8, e63520 (2013).
Zhang, H. et al. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 5, eaaw0873 (2019).
Kim, S. et al. Soft, skin-interfaced microfluidic systems with integrated immunoassays, fluorometric sensors, and impedance measurement capabilities. Proc. Natl Acad. Sci. USA 117, 27906–27915 (2020).
Burton, A. et al. Wireless, battery-free, and fully implantable electrical neurostimulation in freely moving rodents. Microsyst. Nanoeng. https://doi.org/10.1038/s41378-021-00294-7 (2021).
Mickle, A. D. et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019).
Lee, K. H. et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 4, 148–158 (2020).
Won, S. M., Song, E., Reeder, J. T. & Rogers, J. A. Emerging modalities and implantable technologies for neuromodulation. Cell 181, 115–135 (2020).
Borgerding, M. kissfft (GitHub, 2022); https://github.com/mborgerding/kissfft
Acknowledgements
We acknowledge financial support from the Querrey Simpson Institute for Bioelectronics at Northwestern University and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (grant number R44NS107142). This work made use of the NUFAB facility of Northwestern University’s NUANCE Center, which has received support from the Soft and Hybrid Nanotechnology Experimental Resource (grant number NSF ECCS1542205), the Materials Research Science and Engineering Center (grant number DMR1720139), the State of Illinois and Northwestern University. We acknowledge surgical and imaging work performed by the Developmental Therapeutics Core and the Center for Advanced Molecular Imaging at Northwestern University, which have received support from the Robert H Lurie Comprehensive Cancer Center (grant number NCI CCSG 773 P30 CA060553). From the US Army MRICD, we thank J. Abraham for his graphics assistance. C.H.G. was supported by the LUCI programme sponsored by the OASD R&E. K. Kwon acknowledges support by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science, ICT & Future Planning (MSIP); No. 2021R1F1A106387111, No. 2022R1C1C1010555, No. 2020R1A5A8018367 and BK21).
Author information
Authors and Affiliations
Contributions
J.A.R., C.H.G., T.C.J., A.R.B., R.G., W.O., W.L. and Y.Z. conceived the project and designed the research; W.O., W.L., Y.Z., T.C.J., C.H.G. and J.A.R. analysed the data and wrote the paper; W.O., W.L., S.P.L. and K. Kwon designed the electronics; W.O., W.L., J.T. and K. Kwon designed the firmware and software; W.O. designed the AI algorithms, Y.Z. and J.W. designed and manufactured the drug-delivery module; J.U.K., W.O., G.M. and T.K. designed and manufactured the soft epidural electrodes; W.O., W.L., Y. Liu, H.S. and Y. Yang designed the optogenetic probes; W.O., Y. Liu, Y. Lu and Yunyun Wu designed and manufactured the serpentine and twisted-pair interconnects; H.L., Z.X. and Y.H. conducted finite-element analyses; W.O., W.L., Y. Liu, J.U.K., H.S., Yunyun Wu, Y. Yang and S.M.W. developed the encapsulation method; W.O., W.L., Y.Z., Y. Liu, H.S., Yunyun Wu, Y. Lu, J.W., Y. Yang, Yixin Wu, C.W., W.B., H.G., T.L., H.B., G.M., J.Z., S.R.M., Y. Yu and M.S. fabricated the devices and tested them in animal models; C.H.G., T.C.J., A.J.W., J.A.M., K. Kilner, W.O., W.L., Y.Z., E.M.H.-D., I.S., N.G.-H. and C.R.H. designed the animal-model studies, and performed device implantation and computed tomography; Y. Liu, J.U.K. and H.S. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
J.A.R. and A.R.B. are co-founders of NeuroLux Inc., which has potential commercial interest in the technology described in this work. C.H.G., R.G., S.P.L., K. Kilner, Y. Yu and M.S. are employees of NeuroLux Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Max Ortiz-Catalan, Silvestro Micera and Thomas Stieglitz for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Power consumption profiles of the device under different configurations.
a, Testing scheme in which a sourcemeter is used as a power source instead of the NFC power. (b, c) Power profiles with the optogenetics module inactivated (b) without and (c) with supercapacitors. The supercapacitors effectively mitigate the power surges during BLE data transceiving (Tx/Rx). The tables summarize the current consumption in different operating stages. (d, e) Power profiles with the optogenetics module activated (d) without and (e) with supercapacitors. The supercapacitors mitigate power surges during active LEDs and active BLE Rx/Tx and are recharged during lower power consumptions. The tables summarize the current consumption in different operating stages.
Extended Data Fig. 2 Characterization of the wireless power transfer performance.
a, A device mounted on an agarose gel rat model for measuring the effect of rat rearing angle on the wireless power transfer and device survival time. b, Experiment setup simulating the rearing of a rat at the corner of the cage. c, Experiment setup simulating the rearing of a rat at the center of the cage. d, The power received by the device at different heights and locations of the cage under an input RF power of 2 W. e, The power received by the device when the rat rears with different angles at the center and corner of the cage under an input RF power of 2 W. f, Device survival time (that is device working time before it loses power to support its operation) at different rat rearing angles. The device is started normally and then the rat holds a rearing angle for an extended period of time at the center and corner of the cage under an input RF power of 2 W. g, The power received by the device at different heights and locations of the cage under an input RF power of 6 W. h, The power received by the device when the rat rears with different angles at the center and corner of the cage under an input RF power of 6 W. i, Device survival time at different rat rearing angles under an input RF power of 6 W.
Extended Data Fig. 3 Acute in vivo comparison of EEG signals measured by the PtBk-plated EEG electrodes and stainless steel screws.
a, EEG spectrograms during slow wave sleep and awake states in a rat show good correlation between the PtBk-plated EEG electrodes and stainless steel screws. (b-e) Comparison of time-domain EEG signals (left) and EEG power spectra (right) measured by of the PtBk-plated EEG electrodes and stainless steel screws during (b) awake, (c) slow wave sleep, (d) fatal plus onset, and (e) euthanized states and their correlations.
Extended Data Fig. 4 Benchtop validation of simultaneous optogenetic stimulation and electrical recording in PBS.
a, Experimental setup in which the device was submerged in PBS to simulate the electrical conduction of the body of a rat. The device performed simultaneous bilateral optical stimulation at 5 Hz (50% duty cycle) and electrical recording. The electrical signal was fed through PBS by a function generator that performed a cyclic frequency sweep (0.5 → 100 → 0.5 Hz) with a constant amplitude. b, Spectrogram showing recorded signal from the test. The signal showed clean frequency characteristics without interference from the μ-ILEDs, which would show as a horizontal band at 5 Hz if it existed. The video recording of the experiment is provided as Supplementary Video 1.
Extended Data Fig. 5 Characterization of drug-release rate.
a, UV-Vis spectra of different concentrations of Rhodamine B. b, Calibration curve of absorption at 550 nm v.s. concentration of Rhodamine B. c, UV-Vis spectra of PBS containing Rhodamine B released from the drug delivery vehicle at different time since electrolysis was triggered.
Extended Data Fig. 6 Additional data on validating the wireless EEG system against the conventional tethered system.
a, Baseline time-domain EEG data of rat #2 (used for pilocarpine stimulation study). b, Spectrograms of the baseline EEG of rat #2 measured by the tethered and wireless systems, respectively. c, The frequency spectra of EEG data measured by the tethered and wireless systems at the baseline and after baclofen stimulation. d, The frequency spectra of EEG data measured by the tethered and wireless systems at the baseline and after pilocarpine stimulation.
Extended Data Fig. 7 Validation of the wireless neurorecording functionality in its final intended, fully implanted configuration using the pilocarpine-induced seizure model.
a, EEG data measured by a fully implanted device in a rat under the stimulation of Pilocarpine (400 mg/kg). b, The corresponding frequency spectra of EEG at different conditions (baseline, drug onset, and seizure).
Extended Data Fig. 8 Characteristics of different sleep stages measured by the fully implanted, wireless device.
a, Time-domain data of EEG and EMG during wakefulness. b, Power spectrum of EEG during wakefulness showing medium powers in the delta band (0.5–4 Hz) and theta band (6–9 Hz). c, Time-domain data of EEG and EMG during NREM sleep. d, Power spectrum of EEG during NREM sleep showing a high power in the delta band (0.5–4 Hz) and a low power in the theta band (6–9 Hz). e, Time-domain data of EEG and EMG during REM sleep. f, Power spectrum of EEG during REM sleep showing a low power in the delta band (0.5–4 Hz) and a high power in the theta band (6–9 Hz).
Extended Data Fig. 9 Empirical mode decomposition of representative signals at different sleep stages.
a, EMD of EEG during wakefulness. b, Hilbert-Huang transform of EMD during wakefulness. c, EMD of EEG during NREM. d, Hilbert-Huang transform of EMD during NREM. e, EMD of EEG during rem. f, Hilbert-Huang transform of EMD during REM. The scale bar represents 100 μV.
Extended Data Fig. 10 Metrics of training the CNN model.
a, Training and validation loss. b, Training and validation accuracy.
Supplementary information
Supplementary Information
Supplementary notes, figures, tables and references.
Supplementary Video
Benchtop validation of simultaneous optogenetic stimulation and electrical recording in phosphate buffered saline.
Source data
Source Data For Fig. 2
Source data.
Source Data For Fig. 3
Source data.
Source Data For Fig. 4
Source data.
Source Data For Fig. 6
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ouyang, W., Lu, W., Zhang, Y. et al. A wireless and battery-less implant for multimodal closed-loop neuromodulation in small animals. Nat. Biomed. Eng 7, 1252–1269 (2023). https://doi.org/10.1038/s41551-023-01029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-023-01029-x
This article is cited by
-
Janus microparticles-based targeted and spatially-controlled piezoelectric neural stimulation via low-intensity focused ultrasound
Nature Communications (2024)
-
Through-polymer, via technology-enabled, flexible, lightweight, and integrated devices for implantable neural probes
Microsystems & Nanoengineering (2024)
-
Motion artefact management for soft bioelectronics
Nature Reviews Bioengineering (2024)
-
Phase-separated porous nanocomposite with ultralow percolation threshold for wireless bioelectronics
Nature Nanotechnology (2024)
-
A Skin-Inspired Self-Adaptive System for Temperature Control During Dynamic Wound Healing
Nano-Micro Letters (2024)