Abstract
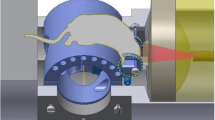
Blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging of the human brain requires bulky equipment for the generation of magnetic fields. Photoacoustic computed tomography obviates the need for magnetic fields by using light and sound to measure deoxyhaemoglobin and oxyhaemoglobin concentrations to then quantify oxygen saturation and blood volumes. Yet, the available imaging speeds, fields of view (FOV), sensitivities and penetration depths have been insufficient for functional imaging of the human brain. Here, we show that massively parallel ultrasonic transducers arranged hemispherically around the human head can produce tomographic images of the brain with a 10-cm-diameter FOV and spatial and temporal resolutions of 350 µm and 2 s, respectively. In patients who had a hemicraniectomy, a comparison of functional photoacoustic computed tomography and 7 T BOLD functional magnetic resonance imaging showed a strong spatial correspondence in the same FOV and a high temporal correlation between BOLD signals and photoacoustic signals, with the latter enabling faster detection of functional activation. Our findings establish the use of photoacoustic computed tomography for human brain imaging.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. 3D functional image stacks of fMRI and fPACT for participant 3 (FT and LP session 1) are available online (https://doi.org/10.5061/dryad.sxksn0310). Other data are too large to be publicly shared, yet they are available for research purposes from the corresponding authors on reasonable request.
Code availability
The fMRI data were processed using the freely available software package SPM12. The codes used to extract the fPACT function are available online (https://doi.org/10.5281/zenodo.4615721). The reconstruction codes based on the universal backprojection algorithm, the system control software and the data collection software are proprietary and used in licensed technologies, yet they are available from the corresponding authors on reasonable request.
References
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008).
Ogawa, S., Lee, T.-M., Kay, A. R. & Tank, D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl Acad. Sci. USA 87, 9868–9872 (1990).
Pfeuffer, J. et al. Zoomed functional imaging in the human brain at 7 Tesla with simultaneous high spatial and high temporal resolution. Neuroimage 17, 272–286 (2002).
Nowogrodzki, A. The world’s strongest MRI machines are pushing human imaging to new limits. Nature 563, 24–26 (2018).
Schenck, J. F. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 23, 815–850 (1996).
Price, D. L., De Wilde, J. P., Papadaki, A. M., Curran, J. S. & Kitney, R. I. Investigation of acoustic noise on 15 MRI scanners from 0.2 T to 3 T. J. Magn. Reson. Imaging 13, 288–293 (2001).
Rahmim, A. & Zaidi, H. PET versus SPECT: strengths, limitations and challenges. Nucl. Med. Commun. 29, 193–207 (2008).
Darvas, F., Pantazis, D., Kucukaltun-Yildirim, E. & Leahy, R. M. Mapping human brain function with MEG and EEG: methods and validation. NeuroImage 23, S289–S299 (2004).
Eggebrecht, A. T. et al. Mapping distributed brain function and networks with diffuse optical tomography. Nat. Photon. 8, 448–454 (2014).
Demene, C. et al. Functional ultrasound imaging of brain activity in human newborns. Sci. Transl. Med. 9, eaah6756 (2017).
Wang, X. et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 21, 803–806 (2003).
Wang, L. V. & Hu, S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science 335, 1458–1462 (2012).
Li, L. et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 1, 0071 (2017).
Wray, P., Lin, L., Hu, P. & Wang, L. V. Photoacoustic computed tomography of human extremities. J. Biomed. Opt. 24, 026003 (2019).
Lin, L. et al. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 9, 2352 (2018).
Jathoul, A. P. et al. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photon. 9, 239–246 (2015).
Wu, Z. et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 4, eaax0613 (2019).
Lin, H.-C. A. et al. Ultrafast volumetric optoacoustic imaging of whole isolated beating mouse heart. Sci. Rep. 8, 14132 (2018).
Nasiriavanaki, M. et al. High-resolution photoacoustic tomography of resting-state functional connectivity in the mouse brain. Proc. Natl Acad. Sci. USA 111, 21–26 (2014).
Gottschalk, S. et al. Rapid volumetric optoacoustic imaging of neural dynamics across the mouse brain. Nat. Biomed. Eng. 3, 392–401 (2019).
Ravina, K. et al. Prospects of photo-and thermoacoustic imaging in neurosurgery. Neurosurgery 87, 11–24 (2020).
Nie, L. et al. Photoacoustic tomography through a whole adult human skull with a photon recycler. J. Biomed. Opt. 17, 110506 (2012).
Xiang, L., Wang, B., Ji, L. & Jiang, H. 4-D photoacoustic tomography. Sci. Rep. 3, 1113 (2013).
Deán-Ben, X. L. et al. Functional optoacoustic neuro-tomography for scalable whole-brain monitoring of calcium indicators. Light Sci. Appl. 5, e16201 (2016).
Deán-Ben, X. L., Fehm, T. F., Ford, S. J., Gottschalk, S. & Razansky, D. Spiral volumetric optoacoustic tomography visualizes multi-scale dynamics in mice. Light Sci. Appl. 6, e16247 (2017).
Matsumoto, Y. et al. Visualising peripheral arterioles and venules through high-resolution and large-area photoacoustic imaging. Sci. Rep. 8, 14930 (2018).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA 97, 11050–11055 (2000).
Kern, M., Bert, S., Glanz, O., Schulze-Bonhage, A. & Ball, T. Human motor cortex relies on sparse and action-specific activation during laughing, smiling and speech production. Commun. Biol. 2, 118 (2019).
Friston, K. J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 (1994).
Strangman, G., Culver, J. P., Thompson, J. H. & Boas, D. A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 17, 719–731 (2002).
Pujol, J., Deus, J., Losilla, J. M. & Capdevila, A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology 52, 1038–1038 (1999).
Brown, S., Ngan, E. & Liotti, M. A larynx area in the human motor cortex. Cereb. Cortex 18, 837–845 (2008).
Price, C. J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 1191, 62–88 (2010).
American National Standards Institute American National Standard for the Safe Use of Lasers ANSI z136.1–2007 (Laser Institute of America, 2007).
Zhang, D. & Raichle, M. E. Disease and the brain’s dark energy. Nat. Rev. Neurol. 6, 15–28 (2010).
Na, S. et al. Transcranial photoacoustic computed tomography based on a layered back-projection method. Photoacoustics 20, 100213 (2020).
Shnaiderman, R. et al. A submicrometre silicon-on-insulator resonator for ultrasound detection. Nature 585, 372–378 (2020).
Poudel, J., Na, S., Wang, L. V. & Anastasio, M. A. Iterative image reconstruction in transcranial photoacoustic tomography based on the elastic wave equation. Phys. Med. Biol. 65, 055009 (2020).
Mitsuhashi, K. et al. A forward-adjoint operator pair based on the elastic wave equation for use in transcranial photoacoustic computed tomography. SIAM J. Imaging Sci. 10, 2022–2048 (2017).
Javaherian, A. & Holman, S. A continuous adjoint for photo-acoustic tomography of the brain. Inverse Probl. 34, 085003 (2018).
Guasch, L., Agudo, O. C., Tang, M.-X., Nachev, P. & Warner, M. Full-waveform inversion imaging of the human brain. NPJ Digit. Med. 3, 28 (2020).
Ulyanov, D., Vedaldi, A. & Lempitsky, V. Deep image prior. In Proc. IEEE Conference on Computer Vision and Pattern Recognition (eds Mortensen, E. & Brendel, W.) 9446–9454 (IEEE, 2018).
Heckel, R. & Hand, P. Deep decoder: concise image representations from untrained non-convolutional networks. Preprint at https://arxiv.org/abs/1810.03982 (2018).
Bostan, E., Heckel, R., Chen, M., Kellman, M. & Waller, L. Deep phase decoder: self-calibrating phase microscopy with an untrained deep neural network. Optica 7, 559–562 (2020).
He, Y., Shi, J., Maslov, K. I., Cao, R. & Wang, L. V. Wave of single-impulse-stimulated fast initial dip in single vessels of mouse brains imaged by high-speed functional photoacoustic microscopy. J. Biomed. Opt. 25, 066501 (2020).
Hu, P., Li, L., Lin, L. & Wang, L. V. Spatiotemporal antialiasing in photoacoustic computed tomography. IEEE Trans. Med. Imaging 39, 3535–3247 (2020).
Xu, Y., Wang, L. V., Ambartsoumian, G. & Kuchment, P. Reconstructions in limited‐view thermoacoustic tomography. Med. Phys. 31, 724–733 (2004).
Kedenburg, S., Vieweg, M., Gissibl, T. & Giessen, H. Linear refractive index and absorption measurements of nonlinear optical liquids in the visible and near-infrared spectral region. Opt. Mater. Express 2, 1588–1611 (2012).
Ashburner, J. et al. SPM12 Manual (Wellcome Centre for Human Neuroimaging, Institute of Neurology, UCL, London, UK, 2014).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Acknowledgements
We thank G. Corral-Leyva for patient care and Y. Luo for discussion on the potential of artificial intelligence in advancing PACT. This work was sponsored by the US National Institutes of Health (NIH) grants R35 CA220436 (Outstanding Investigator Award), U01 NS099717 (BRAIN Initiative), R01 NS102213, R01 NS114382 and R01 EB028297, and by Caltech internal funds (PPF0021).
Author information
Authors and Affiliations
Contributions
L.V.W., C.Y.L., S.N. and J.J.R. conceived the project. S.N., J.J.R., C.Y.L. and L.V.W. designed the study. L.L., S.N., K.M. and J.S. built the system hardware. S.N. developed the system software. S.N., X.Y. and J.J.R. performed the PACT experiments. K.B.J. and L.Y. performed the MRI experiments. P.H., X.Y. and S.N. developed the reconstruction algorithms. X.Y., S.N., K.B.J., J.J.R., C.Y.L., D.J.W. and L.V.W. analysed and interpreted the data. J.J.R. and C.Y.L. recruited the participants. S.N., J.J.R. and X.Y. wrote the manuscript with input from all of the authors. L.V.W., C.Y.L. and D.J.W. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
L.V.W. has a financial interest in Microphotoacoustics Inc., CalPACT LLC and Union Photoacoustic Technologies Ltd., which, however, did not support this work. K.M. has a financial interest in Microphotoacoustics, Inc. The other authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables, video captions and references.
Supplementary Video 1
Comparisons of the angiographic structures and functional maps acquired on 7 T MRI and the 1K3D-fPACT for participant 1.
Supplementary Video 2
Comparisons of the angiographic structures and functional maps acquired on 7 T MRI and the 1K3D-fPACT for participant 3.
Supplementary Video 3
Participant 1 being imaged by the 1K3D-fPACT when performing the finger-tapping task.
Rights and permissions
About this article
Cite this article
Na, S., Russin, J.J., Lin, L. et al. Massively parallel functional photoacoustic computed tomography of the human brain. Nat. Biomed. Eng 6, 584–592 (2022). https://doi.org/10.1038/s41551-021-00735-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00735-8
This article is cited by
-
Advancing insights into in vivo meningeal lymphatic vessels with stereoscopic wide-field photoacoustic microscopy
Light: Science & Applications (2024)
-
Advances in photoacoustic imaging aided by nano contrast agents: special focus on role of lymphatic system imaging for cancer theranostics
Journal of Nanobiotechnology (2023)
-
Functional photoacoustic imaging: from nano- and micro- to macro-scale
Nano Convergence (2023)
-
Parallel interrogation of the chalcogenide-based micro-ring sensor array for photoacoustic tomography
Nature Communications (2023)
-
Photoacoustic vector tomography for deep haemodynamic imaging
Nature Biomedical Engineering (2023)