Abstract
This study developed a postbiotic fermentation solution for fresh oyster preservation with the use of food waste soy whey. Lactiplantibacillus plantarum 299V was able to proliferate in soy whey within 24 h without any supplementation. Pacific oysters (Magallana gigas) were immersed in the postbiotic fermentation solution and stored at 4 °C for 12 days. Pathogenic bacteria Vibrio parahaemolyticus and Salmonella enterica introduced by bioaccumulation were suppressed to levels below the detection limit (<2 log CFU/g) within 4 days. The spoilage-related microbial parameters and chemical parameters were maintained at low levels across the 12 days. Sensory evaluation revealed that the product had a positive effect on most of the participants (>60%). Overall, the postbiotic fermentation solution reported in this study enhanced the shelf life and safety of oysters in a sustainable way and could also be recognized as an innovative probiotic vehicle with potential implications for human health promotion.
Similar content being viewed by others
Introduction
Oysters are renowned as one of the most favoured edible shellfish globally due to their exceptional nutritional composition and tender meat1. Unfortunately, oysters are a short-shelf-life product owing to their susceptibility to spoilage during storage and transportation, consequently incurring substantial economic losses2. Moreover, oysters are also known as important vectors of foodborne pathogens, including Salmonella enterica and Vibrio parahaemolyticus3,4. This is because oysters are active filter feeders that accumulate phytoplankton, zooplankton, and minute debris particles from ambient water, thereby facilitating the enrichment of potentially hazardous agents such as bacterial and viral pathogens5,6. Pathogen contamination in oysters represents a significant public health concern, especially given the growing market for raw oysters.
Biopreservation involves the utilization of microorganisms or their metabolites to prolong the shelf life of food7. The use of lactic acid bacteria (LAB) for food biopreservation has attracted increasing popularity in recent years because it causes fewer health concerns than chemical additives8. LAB are a group of gram-positive bacteria capable of utilizing carbohydrates to produce lactic acid as their primary metabolic product9. The bioactive soluble factors secreted by these microorganisms are referred to as postbiotics and include lactic acid, acetic acid, formic acid, propionic acid, hydrogen peroxide, diacetyl, peptide substances, etc. These compounds contribute to the flavour of fermented foods and exhibit inhibitory effects against spoilage and pathogenic microorganisms10. The application potential of LAB in food preservation is widely recognized. For instance, Hua et al.11 employed a postbiotic-fortified probiotic coating to inhibit Listeria monocytogenes and extend the shelf life of salmon fillets11. Khouadja et al. discovered that LAB could be used as a bioprotective culture in oyster depuration to prevent V. parahaemolyticus growth12. Importantly, bacteriocins are widely used in the food industry due to their biopreservation potential and antimicrobial properties13. Guerra et al.14 reported that a nisin-containing cellophane coating could reduce the percentage of viable total aerobic bacteria in fresh veal meat stored at 8 °C14. Mauriello et al.15 developed an active package obtained from nisin-treated film that reduced viable counts of Micrococcus luteus ATCC 10,240 cells in broth as well as in raw milk and pasteurized milk during storage15. Meira et al.16 developed a new antimicrobial package prepared by a casting method using nisin or pediocin for food preservation16.
To achieve the biopreservation described in the studies mentioned above, adequate LAB cells and their metabolites must be generated with the support of a nutritionally abundant medium, which is expected to account for a major proportion of the product cost. Soy whey is produced in significant quantities during tofu processing17. Research indicates that up to 8.98 kg of soy whey can be generated for every kg of soybeans utilized in tofu production18. The majority of soy whey is currently discarded as waste by tofu manufacturers due to the absence of economically viable technology. The disposal of untreated soy whey causes environmental pollution and wastes resources. In recent years, various endeavours have been undertaken to explore the possibility of reusing soy whey through nutrient recovery or biotransformation19. Based on previous studies, soy whey seems to supply sufficient support for LAB growth. For instance, in a study by Mitra et al.20, the growth of Lactococcus lactis in soy whey was comparable to that in De Man Rogosa Sharpe (MRS) broth. Zhou et al.18 reported that salted soy whey could ferment LAB to develop a sauce-like condiment18. In addition, soy whey contains multiple antimicrobial substances, including phenolic compounds, protein-derived peptides, saponins, and defensins21, which could enhance the biopreservation effectiveness of soy whey-fermented LAB suspensions.
This study describes the development of a biopreservation strategy for Pacific oysters (Magallana gigas) meat with the use of LAB (Lactiplantibacillus plantarum 299V)-fermented soy whey (FSW). In addition, the preservation effects of fermented soy whey—cell-free supernatant (FSW-CFS) and a cell mixture (FSW-cells) on oysters were compared (Fig. 1). Spoilage-related microbiological and chemical parameters were measured along with a storage of 12 days at 4 °C. The effectiveness of LAB-fermented soy whey on pathogenic bacteria S. enterica and V. parahaemolyticus introduced to the oyster by bioaccumulation was also evaluated. LAB-fermented soy whey is expected to change the organoleptic properties of raw oysters, and a sensory study was thus organized together with measurements of product texture and colour analysis to demonstrate the commercial potential of this technology. As L. plantarum 299V is a commercially available probiotic LAB with multiple beneficial effects for human health22, this product is also considered a novel vector for probiotic and postbiotic delivery.
Results and discussion
LAB fermentation in soy whey
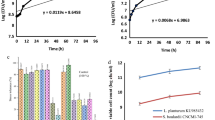
The growth kinetics of LAB in fermented soy whey with various initial spiking concentrations (6.6 ± 0.2 log CFU/mL, 7.5 ± 0.1 log CFU/mL, and 8.3 ± 0.1 log CFU/mL) are shown in Fig. 2A. All three groups exhibited evident growth during the first 24 h of fermentation (P < 0.05). The LAB levels detected in the three groups at 24 h were not significantly different (~8.5 log CFU/mL, P > 0.05), likely due to nutrient limitations imposed by the soy whey23. At 48 h, the number of viable LAB cells detected in the group with the lowest spike protein level was significantly greater than that in the other two groups (P < 0.05). When fermentation continued for 72 h, the LAB counts in all three groups decreased to levels below 8.0 log CFU/mL, likely due to nutrient limitations and the accumulation of metabolites. We thus selected the lowest LAB spiking level (~6.5 log CFU/mL soy whey) for the following tests, as it was the most economical solution.
Growth kinetics of LAB (A) and pH (B), with ~6.5 log CFU/mL as the starting concentration of LAB) during 72 h of fermentation in soy whey. A–C: The mean values with different letters at the same fermentation time are significantly different between different doses (P < 0.05). a–c: The mean values with different letters for the same dose are significantly different between different fermentation times (P < 0.05).
As shown in Fig. 2B, the pH of the fermentation system decreased drastically from 6.0 ± 0.0 to 3.8 ± 0.1 during the first 24 h and stabilized thereafter. L. plantarum 299V is a homofermentative LAB, and its major metabolite is lactic acid. However, this LAB strain can also produce various categories of metabolites, including organic acids, aldehydes, ketones, and fatty acids. Moreover, L. plantarum 299V can metabolize phenolic compounds in fermentation matrices (e.g., soy whey, coffee grounds, and green tea) and produce new phenolic metabolites18,24. Many of these metabolites, including dihydroferulic acid, dihydrocaffeic acid, and phenyllactic acid, have been reported to have antimicrobial effects25,26. These metabolites might continue to be generated at a later stage of fermentation. Therefore, a comparison study was conducted on the antimicrobial effect of 24 h and 48 h fermented soy whey on oysters and revealed no better antimicrobial effect of 48 h fermented soy whey than 24 h fermented soy whey on oysters during a storage period of 12 days (psychrophilic bacteria count, Pseudomonas, and Enterobacteriaceae counts, Table S1). These results suggested that the antimicrobial metabolites in the soy whey fermentation system in this study mostly accumulated during the first 24 h along with the generation of organic acids. The fermentation duration was thus set as 24 h for the following tests.
Effects of fermented soy whey on pathogenic bacteria that accumulate in oysters
In this study, V. parahaemolyticus and S. enterica were spiked into seawater, and the pathogenic bacteria were introduced to the oysters due to their filter-feeding activities. This approach was used to simulate the genuine contamination routes of pathogenic bacteria in oysters27,28. After being harvested, the oysters were shucked, immersed in different treatment solutions (there was no significant difference in the treatment dose between groups, P > 0.05, Fig. 3A, B), and stored at 4 °C. V. parahaemolyticus (Fig. 3A) and Salmonella (Fig. 3B) were enumerated from individual oysters on day 0 (30 min after treatment), day 1, day 2, and day 4.
V. parahaemolyticus A and Salmonella B on refrigerated oysters during a storage period of 4 days. The vertical bars represent the standard deviations (n = 3). The broken lines indicate the detection limit of 2 log CFU/g. *, 1 out of 3 replicates were below the detection limit; **, 2 out of 3 replicates were below the detection limit; ***, 3 out of 3 replicates were below the detection limit. The triangle symbols (Δ) indicate the ratios of the weight of each oyster to that of the 40 mL solution added.
Both V. parahaemolyticus and Salmonella counts remained stable in the control groups during the tested periods (P > 0.05, Fig. 3A, B). For V. parahaemolyticus, both FSW and FSW-CFS treatment reduced the V. parahaemolyticus counts to levels lower than the detection limit (<2.0 log CFU/g, >2.0 log CFU/g reductions, Fig. 3A) by Day 2. In comparison, Salmonella was slightly more resistant. The Salmonella counts decreased to levels lower than the detection limit (<2.0 log CFU/g, >1.0 log CFU/g reductions, Fig. 3B) by day 4.
L. plantarum 299V cells also demonstrated antimicrobial effects against V. parahaemolyticus and Salmonella, but the effects were much less pronounced (Fig. 3). By day 4, only one and two out of three samples were below the detection limit for V. parahaemolyticus and Salmonella, respectively, whereas the remaining samples still exhibited pathogenic bacteria levels comparable to those of the control groups (~4 log CFU/g of V. parahaemolyticus and ~3.5 log CFU/g of Salmonella). This was because L. plantarum 299V is mesophilic, and its metabolic activities at the chilled storage temperature are expected to be low29.
Indeed, the use of LAB for inhibiting pathogenic bacteria, including V. parahaemolyticus and Salmonella, has been previously reported30,31,32. In this study, as L. plantarum 299V actively fermented soybean whey and accumulated adequate amounts of antimicrobial metabolites, the resulting mixture demonstrated a remarkable antagonistic effect against V. parahaemolyticus and Salmonella. In addition, our results showed that the antimicrobial components were able to migrate to the inner structures of the oysters during the immersion treatment, as the pathogenic bacteria were introduced via bioaccumulation and, therefore, were expected to be mostly distributed in the intestine, gills, etc.33. Taken together, these results demonstrated that treatment with L. plantarum 299V fermented soy whey could reduce foodborne pathogens and thus enhance the safety of oyster consumption. Similarly, Chenet al.34 reported that using the bacteriocinogenic Weissella hellenica D1501 as a bioprotective culture of tofu could significantly suppress microbial spoilage34.
Effects of fermented soy whey on the microbial quality of oysters
As shown in Fig. 4A, the LAB counts plated on MRS agar remained constant during storage, and there was no significant difference between the FSW and FSW-cells groups (>8 log CFU/g, P > 0.05). Since the LAB counts in FSW and FSW-cells groups were more than 4 log greater than those in the control group, we could approximate the counts on MRS plates in the FSW and FSW-cells groups as the L. plantarum 299V counts. Therefore, these results suggested the excellent stability of the probiotic L. plantarum 299V in this product. On the other hand, naturally occurring LAB are considered spoilage-causing microorganisms in many food products35, which explains the gradual increase in LAB counts in the control group (P < 0.05). In comparison, the CFS of L. plantarum 299V inhibited the naturally occurring LAB in oysters, especially at the end of the tested storage period (P < 0.05, Fig. 4A).
LAB counts (A), psychrophile bacteria (B), Pseudomonas spp. (C), and Enterobacteriaceae (D) in refrigerated oysters during a storage period of 12 days. The vertical bars represent the standard deviations (n = 3). A–C: The mean values with different letters at the same time points are significantly different between different treatments (P < 0.05). a–c: The mean values with different letters for the same treatment are significantly different between different time points (P < 0.05). *: Below the detection limit (2.00 log CFU/g). The triangle symbols (Δ) indicate the ratios of the weight of each oyster to that of the 40 mL solution added.
The psychrophilic bacteria count is considered an important spoilage indicator of the oyster product developed in this study since the product should be stored under refrigeration conditions. The limit of acceptance for psychrophilic bacteria in seafood is 6 log CFU/g36. In the control group, drastic increases in the psychrophilic bacteria count were observed from 3.8 ± 0.6 log CFU/g on day 0 to 7.9 ± 0.1 log CFU/g on day 12 (P < 0.05, Fig. 4B). The L. plantarum 299V cells in the FSW-cells group exhibited significant inhibition of psychrophilic bacterial growth, as detected on day 4, day 8, and day 12 (P < 0.05, Fig. 4B). However, owing to the metabolites of L. plantarum 299V, the psychrophilic bacteria counts in the FSW and FSW-CFS groups were below the detection limit (<2 log CFU/g) on day 4, day 8, and day 12 (Fig. 4B).
Specifically, Pseudomonas spp. were identified as significant spoilage-causing microorganisms in aquatic products during refrigeration37, mainly due to the accumulation of unpleasant odours and harmful metabolites38. The Enterobacteriaceae family has been reported to be an important component of the natural microflora in seafood and is associated with spoilage39. In addition, many microorganisms within the Enterobacteriaceae family are human pathogens or opportunistic pathogens, including Salmonella and (pathogenic) Escherichia coli. L. plantarum 299V has been reported to have substantial antagonistic effects on Enterobacteriaceae30. In this study, very similar trends were observed for the Pseudomonas counts (Fig. 4C) and the Enterobacteriaceae counts (Fig. 4D) with respect to the psychrophilic bacteria counts (Fig. 4B). In the control group, the most drastic increases in Pseudomonas counts and Enterobacteriaceae counts were observed on day 8 compared to day 4 (P < 0.05, Fig. 4C, D). Compared with those in the control group, significant reductions in Pseudomonas and Enterobacteriaceae counts were detected in the FSW-cells group on day 4, day 8, and day 12 (P < 0.05, Fig. 4C, D). However, in the FSW and FSW-CFS groups, the Pseudomonas counts and Enterobacteriaceae counts were below the detection limits (<2 log CFU/g) on day 4, day 8, and day 12 (Fig. 4C, D).
Effects of fermented soy whey on the chemical quality of oysters
Total volatile basic nitrogen (TVB-N) constitutes a major freshness indicator for seafood products because it accounts for the accumulation of nitrogen-containing decomposition products40. The principal constituents of TVB-N, notably trimethylamine, dimethylamine, and ammonia, largely originate from the breakdown of nitrogen-containing compounds facilitated by microbial activity41. The changes in TVB-N measured in this study (Table 1) were highly consistent with the changes in the abundance of spoilage-causing microorganisms (Fig. 4B–D). The TVB-N levels in the control group increased continuously during storage (P < 0.05), exceeding 15 mg/100 g on day 12, which was recognized as the highest acceptable level in oysters, as reported previously42. Both the FSW and FSW-CFS treatments displayed excellent inhibition of TVB-N, resulting in TVB-N levels below 9 mg/100 g on day 12 (Table 1). Compared with those in the control group, treatment with FSW-cells also reduced TVB-N levels in the oysters but to a lesser extent than in the FSW and FSW-CFS groups (P < 0.05, Table 1).
Biogenic amines (BAs) are a group of non-volatile nitrogenous compounds derived from the decarboxylation of free amino acids or the amination of carbonyl-containing organic compounds facilitated by microbial metabolism43. Numerous studies have supported BAs as indicators of freshness in seafood43,44,45,46. In addition, BA is not only a good indicator of seafood spoilage but is also associated with serious health concerns due to its toxicity when consumed in sufficient quantities47. In this study, among the tested BAs, spermine (SPM) was detected at the highest level in the oysters on day 0, followed by spermidine (SPD) and phenethylamine (PHE) (P < 0.05, Table 1). Cadaverine (CAD), putrescin (PUT), histamine (HIS), tryptamine (TRY), and tyramine (TYR) were not detected (Table 1 for CAD and PUT, data not shown for the rest). SPM and SPD showed decreasing trends, whereas PHE, CAD, and PUT showed increasing trends during storage in the control group (Table 1). This observation was consistent with numerous previous reports48,49 and could be due to differences in the reaction rates of amine synthesis and amine deamination, as indicated by Halsz et al.50. Interestingly, despite these differences in the control group, the FSW treatment reduced all the detected BAs in the oysters. By day 12, the SPM, SPD, PHE, and CAD levels in the FSW and FSW-CFS groups were significantly lower than those in the control group (P < 0.05). PUT remained undetected in the FSW and FSW-CFS groups, whereas it was detected at 26.59 ± 0.18 mg/kg oyster tissue in the control group (Table 1).
Effects of fermented soy whey on the organoleptic properties of oysters
Texture attributes, including hardness, elasticity, stickiness, and chewiness, are related to the denaturation of muscle structure and are often considered a freshness indicator of oysters51. As shown in Table 2, in all the tested groups, the texture attributes inevitably decreased during storage, with the most drastic changes in hardness and chewiness detected on day 4 compared with day 0 (P < 0.05). However, FSW treatment was still observed to help maintain the texture properties of the oyster products. On day 12, significantly greater hardness, cohesiveness, and chewiness values were detected in the FSW group than in the control group (P < 0.05), and these values were comparable to those of the control group on day 4 (P > 0.05, Table 2).
Colour affects the overall appearance and acceptability of oysters and is also widely used to evaluate the freshness of products1. In the control group, the brightness, indicated by the L* values, and redness, indicated by the a* values, decreased significantly with increasing storage time (P < 0.05, Table 3). FSW treatment was very effective in maintaining the colour of the oyster products during storage. On day 12, the L* and a* values in the FSW group were significantly greater than those in the control group (P < 0.05) and were comparable to those in the control group on day 0 (P > 0.05). The same trend was observed for ΔE, indicating the total colour change, where FSW induced the least colour changes by day 12 compared to the other tested groups (P < 0.05, Table 3).
Finally, a sensory evaluation was organized using a 9-point hedonic scale. This method has been used to reflect consumers’ acceptance of food products52,53. The histograms in Fig. 5 show the proportions of consumers selected for each category on the scale. As a result, for all four parameters, including overall acceptance, appearance, smell, and taste, positive responses were received from a majority of the participants. More than 60% of the participants gave scores between 6 (like slightly) and 8 (like very much) for all four parameters. In addition, as described previously53, highly accepted food products typically have asymmetrical tension on positive responses, and products with low acceptance simply show negative responses. In this study, the FSW-treated oysters exhibited hedonic asymmetry in all four parameters, indicating high market acceptance and thus commercial potential of this product.
Category distribution of the 9-point hedonic scale showing positive hedonic asymmetry regarding the overall (A), appearances (B), smells (C), and tastes (D) of oysters. 1. Dislike extremely; 2. dislike very much; 3. dislike moderately; 4. dislike slightly; 5. neither like nor dislike; 6. like slightly; 7. like moderately; 8. like very much; 9. like extremely.
In summary, a productive and sustainable probiotic fermentation solution was formulated with the use of food waste and applied to fresh oyster preservation. Throughout the 12-day refrigeration storage period, the postbiotic fermentation solution demonstrated notable effectiveness in curbing bioaccumulated pathogenic bacteria, spoilage-causing microorganisms, TVB-N, and BAs in oysters. In comparison with the control group, this solution also showed an advantage in maintaining the texture and colour of the oyster product. The positive responses received from the sensory evaluation contributed to the commercial value of this solution. Finally, as probiotic bacteria together with their metabolites (also termed “postbiotics”) have been reported to have human health-promoting effects, the solution reported in this study could not only improve the shelf life and safety of fresh oysters but also indicate a new probiotic carrier for promoting human health.
Methods
Bacterial cultures
L. plantarum 299V (Jarrow Formulas, Inc., Canada) was cultured in MRS broth (Oxoid, Hampshire, England) under aerobic conditions11. Static cultivation was performed at 37 °C in test tubes, and one passage of growth lasted for 48 h. S. enterica serovar Typhimurium (ATCC 14028) and S. enterica serovar Enteritidis (ATCC 13076) were cultured in tryptic soy broth (TSB; Oxoid, Hampshire, UK) and incubated aerobically at 37 °C for 24 h as one passage30. V. parahaemolyticus strains (ATCC 17802 and ATCC 17803) were individually cultivated in TSB supplemented with 2 g/100 ml NaCl and incubated for 24 h at 37 °C as one passage. All frozen bacterial cultures were activated for two continuous passages before being used in the experiments.
Soy whey preparation
The preparation of soy whey was conducted following the methodology outlined by Zhou et al.18. Initially, the soybeans were immersed in deionized (DI) water for 16 h. Subsequently, the immersed soybeans were drained and homogenized with fresh DI water at a ratio of 1:6 (w[dry soybeans beans]/v[DI water]) to form a slurry. The slurry was subjected to pressing and filtration using cheesecloth, facilitating the separation of soybean pulp from the resulting soymilk. The soymilk was boiled for 5 min. Magnesium chloride (10 g/100 ml) as the coagulant was added to the soymilk at a ratio of 1:50 when it was cooled to a temperature range of 80–90 °C. The mixture was stirred briefly before allowing it to stand for 15 min to promote coagulation. The coagulated tofu was subsequently pressed to collect the soy whey. Finally, the soy whey underwent pasteurization at 105 °C for 30 min (Hirayama Manufacturing Corporation, Japan).
Soy whey fermentation and oyster treatment
Reactivated L. plantarum 299V was inoculated into pasteurized soy whey at different doses of ~6.5 log CFU/mL, ~7.5 log CFU/mL, and ~8.5 log CFU/mL. The incubation at 37 °C lasted for 72 h. Samples were intermittently collected to measure the viable cell counts and pH (Mettler Toledo, Giessen, Germany).
As shown in Fig. 1, for the “fermented soy whey (FSW)” group, the fermented soy whey was used directly. For the “fermented soy whey - cell-free supernatant (FSW-CFS)” group, the fermented soy whey was centrifuged at 8000 × g for 10 min, and the cell-free supernatant (CFS) was collected and filtered through 0.22 μm syringe filters (Hydrophobic PTFE) before being used. For the “FSW-cells” group, the bacteria pellets were collected after centrifuging the fermented soy whey, followed by being washed twice with 1 g/L peptone water and resuspended in sterile water to the same volume as the original fermented soy whey.
Live Pacific oysters (M. gigas) were collected from a local oyster farm in Singapore and transported to the laboratory on ice on the day of harvest. The oysters were rinsed with sterile water, shaken with a sterile knife, and immersed in the solutions described above. Each oyster weighing ~15–20 g was packaged individually with 40 mL of fermentation solution. In the control group, the oysters were immersed in sterile DI water. The oysters soaked in solution were stored at 4 ± 1 °C in low-temperature incubators (Gaia Science Pte Ltd., Singapore), and microbial and chemical analyses were conducted on days 0 (30 min after immersion), 4, 8, and 12.
Bioaccumulation of pathogenic bacteria in oysters
The live oysters were rinsed with sterile water and then acclimated in high-density polyethylene (HDPE) tanks with artificial seawater (every 50 oysters in 50 L of seawater; the salinity of the seawater was 26 g/L, prepared by dissolving instant ocean salts in tap water) for 4 h at room temperature (22 ± 1 °C). Subsequently, Salmonella (a cocktail of S. Typhimurium ATCC 14028 and S. Enteritidis ATCC 13076 mixed at a ratio of 1:1) or V. parahaemolyticus (a cocktail of ATCC 17802 and ATCC 17803 mixed at a ratio of 1:1) were spiked into the artificial seawater at a final concentration of 4.5–5.5 log CFU/mL. After 24 h, the oysters were shaken and immersed in the aforementioned solutions.
Microbial analysis of the oysters
The oysters were individually transferred to stomacher bags and homogenized with 0.1% peptone water at a ratio of 1:9 using an Interscience Bag Mixer 400 (France) equipped with a slapping stomacher. Subsequently, the LAB was enumerated on MRS agar after incubation at 37 °C for 48 h. Psychrophilic total counts (PTCs) were performed on plate count agar (PCA; OXOID, UK) plates at 15 °C for 7 days. Pseudomonas spp. counts were determined on Pseudomonas CFC selective agar (OXOID, UK) at 30 °C for 2 days. Enterobacteriaceae were enumerated on violet red bile glucose (VRBG, OXOID) agar after incubation at 37 °C for 24 h. Salmonella were enumerated on xylose lysine deoxycholate (XLD) agar (OXOID, UK) incubated at 37 °C for 24 h. V. parahaemolyticus was enumerated on thiosulfate-citrate-bile salt-sucrose (TCBS) agar (OXOID, UK) incubated at 37 °C for 24 h.
TVB-N measurement
TVB-N was measured using a modified semimicro steam distillation method described by Chung et al.54. Briefly, each oyster was first homogenized by a Tefal Optitouch Hand Blender, and 5 g of the homogenized oyster tissue was mixed with 50 mL of DI water and mixed with a BioRS-24 mini-rotator bioscan rotator at 30 rpm for 30 min. Subsequently, the mixture was centrifuged at 10,000 × g for 5 min at 4 °C, and the resulting supernatants were collected. For the distillation, 5 mL of the supernatant was transferred to a digestion tube, and 5 mL of MgO (10 g/L) was added immediately before the distillation. The distillation procedure was performed using an automated Kjeldahl apparatus (Vapodest 20, Gerhardt, Germany). The final product from the distillation was collected with boric acid (2 g/100 ml) and 0.1 mL of indicator solution (a mixture of methyl red and bromocresol green at a 1:5 ratio). The distillate was titrated using a 0.01 mol/L hydrochloric acid solution until blue-purple coloration was observed.
BAs measurement
As described above, each oyster was first homogenized in a Tefal Optitouch Hand Blender. Five grams of the homogenized oyster tissue was mixed with 15 mL of 0.6 mol/L perchloric acid and then subjected to centrifugation at 10,000 × g for 15 min at 4 °C. The extraction of the pellet was repeated, and the cumulative supernatant volume was adjusted to 50 mL using 0.6 mol/L perchloric acid. Next, 1 mL of the extract solution or a standard solution of BA was combined with 200 μL of 2 mol/L NaOH and 300 μL of saturated NaHCO3. This mixture was then subjected to derivatization by adding 1 mL of 1 g/100 ml dansyl chloride (Sigma, Germany). The resulting reactant was incubated at 40 °C for 45 min in darkness, and the residual dansyl chloride was removed by adding 100 μL of ammonia and then incubated for 30 min. The resulting solution was adjusted to 5 mL using acetonitrile, followed by centrifugation at 3,000 × g for 10 min at 4 °C, and the supernatant was then filtered through a 0.22 μm membrane filter. BA quantification was conducted using a high-performance liquid chromatography (HPLC) system (Alliance 2695, Waters, MA, USA) equipped with a C18 Diamondsil column (250 mm × 4.6 mm, 5 μm) and a photodiode array (PDA) detector. The column temperature was maintained at 30 °C, and the flow rate was set at 0.8 mL/min. The elution solvents consisted of ammonium acetate (0.1 mol/L) for washing and acetonitrile for elution. The gradient elution process followed the pattern of increasing the elution solvent concentration from 50% to 90% for 0–35 min and then decreasing it from 90% to 50% for 35–45 min. Identification and quantification of individual BAs were accomplished by comparing both the retention time and peak area of the BA standard compounds. Individual BAs were identified and quantified by comparing their retention times and peak areas with those of BA standard compounds.
Colour and texture analysis
The surface colour attributes (L*, a*, b*) were obtained by a colorimeter (CM-5, Konica Minolta, Tokyo, Japan) as described by Ma et al.55. Before experimentation, the colorimeter was calibrated for black and white references following the manufacturer’s guidelines. Ten repeated measurements were taken on both the ventral and dorsal sections of each oyster. The ΔE (total colour change) value was calculated using oysters on day 0 as a control, and the formula was as follows: ΔE=\(\root\of{{({L}^{* }-{L}_{0}^{* })}^{2}+{({a}^{* }-{a}_{0}^{* })}^{2}+{({b}^{* }-{b}_{0}^{* })}^{2}}\), where L0*, a0*, b0* represent the brightness, redness, and yellowness values of the oyster on day 0, respectively, while L*, a*, and b* denote the corresponding attributes on day N of storage.
The texture of the oyster’s body trunk was determined using a TA. An XT2i texture analyser (Stable Micro Systems, Ltd., Godalming, Surrey, UK) with a P/50 probe was used. The test speed was 1 m/s, incorporating pretest and posttest velocities of 1 m/s and 10 m/s, respectively. The sample deformation was 50%. Four texture parameters, namely, hardness, springiness, chewiness, and cohesiveness, were manually evaluated for each oyster sample through the instrument’s built-in software macro.
Sensory evaluation
The sensory evaluation was performed according to the methods described by Yang and Lee52. The participants (n = 37, aged 21–50 years) were recruited at the National University of Singapore. Participants with known allergies to shellfish or any other chronic disease were excluded from this study.
After storing the samples in L. plantarum 299V fermented soy whey (FSW) for 4 days, the oysters were drained briefly, steamed in boiled water for 10 min as described by Jiang et al.56, and then cooled at room temperature for <1 h before being served. The participants were required to observe, smell, chew, and spit out the oysters. The participants were asked to evaluate the samples on a 9-point hedonic scale for the overall acceptance, appearance, smell, and taste of the oysters: (1) dislike extremely; (2) dislike very much; (3) dislike moderately; (4) dislike slightly; (5) neither like nor dislike; (6) like slightly; (7) like moderately; (8) like very much; (9) like extremely.
Informed consent was obtained from the participants before sensory evaluation. The study was approved by the Ethical Committee of the National University of Singapore (approval no. FST-DERC-2023-002).
Statistical analysis
The data presented were derived from three independent replicates (n = 3) and are expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) with a significance threshold of P < 0.05 was conducted to analyse the significant differences by using SPSS software.
Data availability
Data will be made available on request.
References
Rodezno, L. A. E. et al. Modified atmosphere packaging (MAP) on nutritional stability and quality retention of oyster meat (Crassostrea virginica) during frozen storage. J. Packag. Technol. Res. 7, 11–22 (2022).
Chen, L. et al. Identification of potential peptide markers for the shelf-life of Pacific oysters (Crassostrea gigas) during anhydrous preservation via mass spectrometry-based peptidomics. LWT 134, 109922 (2020).
Caron, G. et al. Campylobacter jejuni outbreak linked to raw oysters in Rhode Island, 2021. J. Food Prot. 86, 100174 (2023).
Lo, Y., Wang, C., Chen, B., Hu, C. & Chou, C. Prevalence and antimicrobial resistance of Salmonella in market raw oysters in Taiwan. J. Food Prot. 80, 734–739 (2017).
Adams, C., Mayer, L. M., Rawson, P., Brady, D. C. & Newell, C. Detrital protein contributes to oyster nutrition and growth in the Damariscotta estuary, Maine, USA. Aquac. Environ. Interact. 11, 521–536 (2019).
Chen, L. et al. Molecular mechanism of protein dynamic change in Pacific oyster (Crassostrea gigas) during depuration at different salinities uncovered by mass spectrometry-based proteomics combined with bioinformatics. Food Chem. 394, 133454 (2022).
Kaveh, S. et al. Bio-preservation of meat and fermented meat products by lactic acid bacteria strains and their antibacterial metabolites. Sustainability 15, 10154 (2023).
Khubber, S., Marti-Quijal, F. J., Tomasevic, I., Remize, F. & Barba, F. J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds for food and by-products. Curr. Opin. Food Sci. 43, 189–198 (2022).
Anisah, Harun, H., Jannah, H., Amelia, R. & Purwati, E. Molecular identification and antimicrobial potency of probiotic lactic acid bacteria Pado (Fish Fermentation) Nagari Balingka IV Koto district-west Sumatra as a functional food. IOP Conf. Ser. Earth Environ. Sci. 1188, 012039 (2023).
Dejene, F., Regasa Dadi, B. & Tadesse, D. In Vitro antagonistic effect of Lactic acid bacteria isolated from fermented beverage and finfish on pathogenic and foodborne pathogenic microorganism in Ethiopia. Int. J. Microbiol. 2021, 1–10 (2021).
Hua, Q., Wong, C. H. & Li, D. Postbiotics enhance the functionality of a probiotic edible coating for salmon fillets and the probiotic stability during simulated digestion. Food Packag. Shelf Life 34, 100954 (2022).
Khouadja, S., Haddaji, N., Hanchi, M. & Bakhrouf, A. Selection of lactic acid bacteria as candidate probiotics for Vibrio parahaemolyticus depuration in pacific oysters (Crassostrea gigas). Aquac. Res. 48, 1885–1894 (2017).
Gálvez, A., Abriouel, H., López, R. L. & Omar, N. B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 120, 51–70 (2007).
Guerra, N. P., Macias, C. L., Agrasar, A. T. & Castro, L. P. Development of a bioactive packaging cellophane using Nisaplin® as bio preservative agent. Lett. Appl. Microbiol. 40, 106–110 (2005).
Mauriello, G., Luca, E. D., Storia, A. L., Villani, F. & Ercolini, D. Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett. Appl. Microbiol. 41, 464–469 (2010).
Meira, S. M. M., Zehetmeyer, G., Werner, J. O. & Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 63, 561–570 (2017).
Xu, D. et al. Soy whey as a promising substrate in the fermentation of soy sauce: a study of microbial community and volatile compounds. Int. J. Food Sci. Technol. 56, 5799–5811 (2021).
Zhou, R. Y., Huang, X., Liu, Z., Chua, J. & Liu, S. Evaluating the effect of lactic acid bacterial fermentation on salted soy whey for development of a potential novel soy sauce-like condiment. Curr. Res. Food Sci. 5, 1826–1836 (2022).
Chua, J. & Liu, S. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 91, 24–32 (2019).
Mitra, D. et al. Value-added production of Nisin from soy whey. Appl. Biochem. Biotechnol. 162, 1819–1833 (2010).
Sun, P. et al. Antimicrobial activity of tofu whey and steam blanching pea water for enhancement of shelf-life of 3D printed mashed potatoes. Food Biosci. 50, 102049 (2022).
Abdelazez, A., Abdelmotaal, H., Zhu, Z. T., Fang-Fang, J. & Meng, X. C. Potential benefits of Lactobacillus plantarum as probiotic and its advantages in human health and industrial applications: a review. Adv. Environ. Biol. 12, 16–27 (2018).
Barssoum, R. et al. Biochemical limitations of Bacillus thuringiensis based biopesticides production in a wheat bran culture medium. Res. Microbiol. 174, 104043 (2023).
Wang, R., Sun, J., Lassabliere, B., Yu, B. & Liu, S. Q. UPLC-Q-TOF-MS based metabolomics and chemometric analyses for green tea fermented with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. Curr. Res. Food Sci. 5, 471–478 (2022).
Koistinen, V. M. et al. Metabolite pattern derived from Lactiplantibacillus plantarum - Fermented rye foods and in Vitro gut fermentation synergistically inhibits bacterial growth. Mol. Nutr. Food Res. 66, 2101096 (2022).
Zhang, J. et al. Isolation, purification, identification, and discovery of the antibacterial mechanism of LD-phenyllactic acid produced by Lactiplantibacillus plantarum CXG9 isolated from a traditional Chinese fermented vegetable. Food Control 132, 108490 (2022).
Brillhart, C. D. & Joens, L. A. Prevalence and characterization of Salmonella Serovars isolated from oysters served raw in restaurants. J. Food Prot. 74, 1025–1029 (2011).
Xi, D., Liu, C. & Su, Y. Effects of green tea extract on reducing Vibrio parahaemolyticus and increasing shelf life of oyster meats. Food Control 25, 368–373 (2012).
Wong, C. H. & Li, D. Comparison of two strategies enhancing the antagonistic effect of lactic acid bacteria in edible coating against Listeria monocytogenes on fresh-cut apple slices. LWT 182, 114923 (2023).
Li, Y., Ten, M. M. Z., Zwe, Y. H. & Li, D. Lactiplantibacillus plantarum 299v as starter culture suppresses Enterobacteriaceae more efficiently than spontaneous fermentation of carrots. Food Microbiol. 103, 103952 (2022).
Sorée, M. et al. Screening of marine lactic acid bacteria for Vibrio parahaemolyticus inhibition and application to depuration in Pacific oysters (Crassostrea gigas). J. Appl. Microbiol. 134, lxac081 (2023).
Swieca, M. et al. Lactobacillus plantarum 299V improves the microbiological quality of legume sprouts and effectively survives in these carriers during cold storage and in vitro digestion. PLoS ONE 13, e0207793 (2018).
Lee, R., Lovatelli, A. & Ababouch, L. Bivalve Depuration: Fundamental and Practical aspects (FAO Fisheries Technical Paper, 2008).
Chen, C., Rui, X., Lu, Z., Li, W. & Dong, M. Enhanced shelf-life of tofu by using bacteriocinogenic Weissella hellenica D1501 as bioprotective cultures. Food Control 46, 203–209 (2014).
Borch, E., Kantmuermans, M. L. & Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 33, 103 (1996).
Mol, S., Erkan, N., Ücok, D. & Tosun, Y. Effect of psychrophilic bacteria to estimate fish quality. J. Muscle Foods 18, 120–128 (2007).
Odeyemi, O. A., Burke, C. M., Bolch, C. C. J. & Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 280, 87–99 (2018).
Andreani, N. A. & Fasolato, L. The Microbiological Quality of Food 25–59 (Woodhead Publishing, 2017).
Chen, H., Liu, Z., Shi, Y. & Ding, H. H. Microbiological analysis and microbiota in oyster: a review. Invertebr. Surviv. J. 13, 374–388 (2016).
Bekhit, A. E. A., Holman, B. W. B., Giteru, S. G. & Hopkins, D. L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 109, 280–302 (2021).
Wu, T. H. & Bechtel, P. J. Ammonia, dimethylamine, trimethylamine, and trimethylamine oxide from raw and processed fish by-products. J. Aquat. Food Prod. Technol. 17, 27–38 (2008).
Wang, S. et al. Applications of colorimetric sensors for non-destructive predicting total volatile basic nitrogen (TVB-N) content of Fujian oyster (Crassostrea angulata). Food Control 153, 109914 (2023).
Alvarez, M. A. & Moreno-Arribas, M. V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 39, 146–155 (2014).
Qu, Y., Wang, J., Liu, Z., Wang, X. & Zhou, H. Effect of storage temperature and time on biogenic amines in canned seafood. Foods 11, 2743 (2022).
Baixas Nogueras, S., Bover Cid, S., Veciana Nogues, M. T., Marine Font, A. & Vidal Carou, M. C. Biogenic amine index for freshness evaluation in iced Mediterranean hake (Merluccius merluccius). J. Food Prot. 68, 2433–2438 (2005).
Kim, M., Mah, J. & Hwang, H. Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chem. 116, 87–95 (2009).
Omer, A. K., Mohammed, R. R., Ameen, P. S. M., Abas, Z. A. & Ekici, K. Presence of biogenic amines in food and their public health implications: A review. J. Food Prot. 84, 1539–1548 (2021).
Balamatsia, C. C., Paleologos, E. K., Kontominas, M. G. & Savvaidis, I. N. Correlation between microbial flora, sensory changes and biogenic amines formation in fresh chicken meat stored aerobically or under modified atmosphere packaging at 4°C: possible role of biogenic amines as spoilage indicators. Antonie Van Leeuwenhoek 89, 9–17 (2006).
Kim, J. H. et al. Effects of gamma irradiation on the biogenic amines in pepperoni with different packaging conditions. Food Chem. 89, 199–205 (2005).
Halsz, A., Bar Th, G., Simon-Sarkadi, L. & Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 5, 42–49 (1994).
Lekjing, S. & Venkatachalam, K. Effects of modified atmospheric packaging conditions on the quality changes of pasteurized oyster (Crassostrea belcheri) meat during chilled storage. J. Aquat. Food Prod. Technol. 27, 1106–1119 (2018).
Yang, J. & Lee, J. Korean consumers’ acceptability of commercial food products and usage of the 9‐point hedonic scale. J. Sens. Stud. 33, e12467 (2018).
Feng, Y. & O’Mahony, M. Comparison between American and Chinese consumers in the use of verbal and numerical 9-point hedonic scales and R-Index ranking for food and personal products. Food Qual. Prefer. 60, 138–144 (2017).
Chung, W. H., Howieson, J. & Chaklader, M. R. The ameliorative effects of low-temperature pasteurization on physicochemical and microbiological quality of raw Akoya pearl oyster (Pinctada fucata). Food Control 129, 108241 (2021).
Ma, X., Mei, J. & Xie, J. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea). Ultrason. Sonochem. 76, 105657 (2021).
Jiang, S., Zeng, M. & Zhao, Y. Thermal processed Crassostrea gigas impact the mouse gut microbiota. J. Funct. Foods 75, 104254 (2020).
Acknowledgements
This work was supported by the China Scholarships Council (File No. 202206330052) and a Reimagine Research Scheme from the National University of Singapore.
Author information
Authors and Affiliations
Contributions
Lipin Chen: Conceptualization, investigation, data curation, writing—original draft preparation; Qian Hua and Ten Mei Zhen Michelle: Investigation, data curation; Zhaojie Li and Changhu Xue: Supervision, writing—reviewing and editing; Dan Li: Conceptualization, supervision, writing—reviewing and editing, funding acquisition, project administration. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, L., Hua, Q., Ten, M.Z.M. et al. Lactiplantibacillus plantarum 299V-fermented soy whey improved the safety and shelf life of Pacific oysters (Magallana gigas). npj Sci Food 8, 77 (2024). https://doi.org/10.1038/s41538-024-00317-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-024-00317-3