Abstract
People with schizophrenia show higher risk for abdominal obesity than the general population, which could contribute to excess mortality. However, it is unclear whether this is driven by alterations in abdominal fat partitioning. Here, we test the hypothesis that individuals with schizophrenia show a higher proportion of visceral to total body fat measured using magnetic resonance imaging (MRI). We recruited 38 participants with schizophrenia and 38 healthy controls matched on age, sex, ethnicity, and body mass index. We found no significant differences in body fat distribution between groups, suggesting that increased abdominal obesity in schizophrenia is not associated with altered fat distribution.
Similar content being viewed by others
People with schizophrenia show a 10–15 years shorter life expectancy1, and their mortality due to natural causes is up to 8-times higher than expected2. They have been shown to have an increased risk for abdominal obesity compared to the general population (odds ratio of 4.4 in a meta-analysis3). Abdominal obesity is the most prominent feature of the metabolic syndrome, and associates with blood lipid disorders, inflammation, insulin resistance or diabetes, and, downstream, increased risk of developing cardiovascular disease4. Obesity, therefore, might underpin many of the metabolic comorbidities that are seen in schizophrenia, including a higher prevalence of hypertension, high cholesterol and triglycerides, type 2 diabetes, insulin resistance, and the metabolic syndrome3. These, in turn, might be responsible for the increased cardiovascular mortality in this group5.
Few cross-sectional studies have investigated visceral adipose tissue mass or volume in treated schizophrenia6. Some have used proxies, such as bioelectrical impedance (BIA)7,8, while others have used gold standard magnetic resonance imaging (MRI)9,10 to visualise body fat distributions in cases and controls with conflicting results. MRI studies have reported no significant difference in visceral fat content between cases and controls9,10, whereas studies using BIA have shown both increases7 or decreases8 in visceral fat in schizophrenia. However, studies measuring visceral fat using MRI tend not to measure total body fat content, and it is unclear whether these studies are able to detect differences in distribution at the whole-body level in schizophrenia.
Our hypothesis was that participants with schizophrenia and controls, matched for body mass index (BMI), would show similar levels of total body fat, but an increased visceral fraction, as compared to controls.
Thirty-eight participants with schizophrenia, as well as the same number of matched healthy controls, underwent whole body fat MRI measures. As expected, due to the nature of case control matching there were no significant group differences for age, sex, ethnicity and BMI. A sample description is in Supplementary Table 1.
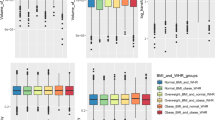
Table 1 describes MRI-derived fat measures according to diagnostic status. There were no significant differences between groups in total fat (d = 0.00, 95% CI: −0.46–0.46; p = 1.00), visceral fat (d = 0.06, 95% CI: −0.40–0.52; p = 0.79), or visceral to total ratio (d = 0.17, 95% CI: −0.29–0.62; p = 0.47).
In this work, we investigate the body fat distribution of people with chronic schizophrenia compared to healthy controls matched for age, sex, ethnicity, and BMI.
We show that subjects with schizophrenia do not show any differences in overall adipose tissue content or regional distribution, when compared to matched healthy controls. This compares to the presence of concentric cardiac remodelling and cardiac fibrosis in the same patient cohort11,12.
Our results are consistent with the majority of prior cross-sectional studies of visceral body fat in treated schizophrenia using a comparable methodology, and extend the previous literature on the topic by using whole-body MR imaging, to show that both body fat content and distribution were not different between cases vs. controls9,10,13,14,15. A recent meta-analysis6 compared levels of visceral adipose fat between people with schizophrenia and controls, showing significant increases in the treated sample (N = 53)—however the team could only include in the quantitative synthesis three13,14,15 out of the eight existing studies7,8,9,10,13,14,15,16. In Supplementary Table 2, we present our own summary of existing studies, showing that studies showing differences in visceral body fat mass between treated patients with schizophrenia and controls either did not match cases and controls by BMI, or used indirect measurement techniques, such as bioimpedance analysis.
Crucial to the interpretation of our work is that all participants with schizophrenia we included were chronic patients taking second-generation antipsychotics (SGAs), mostly clozapine and olanzapine. SGAs are known to cause increases in body weight17, and are potentially one of the causes of the increased prevalence of obesity in schizophrenia. Therefore, the finding of no difference in fat distribution in this sample, despite participants being exposed to these metabolically active compounds, is particularly meaningful. On the flip side, the findings in this cohort might not extend to untreated or minimally treated cohorts of patients with schizophrenia, so further research is needed in untreated patients. However, we note that previous studies looking at abdominal fat mass differences between drug-naïve people with a first episode of psychosis and matched healthy controls found no difference even before antipsychotic drugs were started—as evidenced in a recent meta-analysis6 after removing poor quality studies.
In this work, we find no differences in total or visceral fat volumes between treated people with schizophrenia and matched healthy controls. This does not imply no difference exists in terms of adipose tissue function in schizophrenia, as this cannot be captured by MRI. Indeed, previous research has shown that antipsychotics can cause a pro-inflammatory shift in adipose tissue in mice18; in humans, we have previously found that despite no differences in body-fat distribution, people with antipsychotic-treated chronic schizophrenia are characterised by alterations in systemic adipokine levels and pro-inflammatory changes19. Collectively, finding no differences in visceral body fat mass/distribution, while at the same time finding signs of adipocyte dysfunction and pro-inflammatory changes, might suggest that chronic, treated schizophrenia is characterised by the activation of specific molecular pathways19. These pathways might lead downstream to cardio-metabolic changes that might explain part of the additional cardiovascular morbidity and mortality seen in schizophrenia3.
Strengths of our study include excluding any history of cardiometabolic disease, including diabetes, hypertension, dyslipidaemia, and ischaemic heart disease, to make the case and control groups more comparable, given the different prevalence of these conditions in the two cohorts. Future larger studies could include and adjust the analyses for the presence of these conditions. In terms of limitations, it is possible that our negative findings might be down to a statistical type II error, however our sample size was among the largest of similar studies, and our effect size measures were very close to 0 for all outcomes. Further, we were able to match our samples for age, sex, ethnicity, and BMI. Data about further confounds, such as smoking, activity levels, and diet were not available for part of the cohort and could not be presented.
The lack of differences in visceral fat we observed in a closely matched control–case study add strength to similar findings from previous MRI studies. Differences in the prevalence of cardiometabolic disease in schizophrenia cannot be explained by specific alterations in body fat partitioning. Prospective studies will be useful to determine whether disease-related biological factors play a specific role in cardiometabolic disease in schizophrenia, or whether they simply reflect the same disease processes found in the wider population.
Methods
Participants
People with schizophrenia were recruited from community mental health services in London, UK. Healthy controls were recruited through the Hammersmith Hospital Healthy Volunteer Panel, London, UK, and through direct advertising, and were matched to patients for age (+/− 3 years), ethnicity, sex, and BMI (+/− 1).
Exclusion criteria for all participants were: age <18 or >65 years, pregnancy or breastfeeding, a history of cardiometabolic disease, including diabetes, hypertension, dyslipidaemia, ischaemic heart disease, any vascular disorder, other history of congenital/structural cardiac disease; or history of significant or continuing substance abuse. Inclusion criterion for patients was an ICD-10 diagnosis of schizophrenia. Exclusion criterion for healthy controls was a previous history or first-degree family history of schizophrenia or other psychotic disorder.
Written informed consent was obtained from all volunteers. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects were approved by the London—Camberwell St Giles Research Ethics Committee.
In addition to reviewing medical records, all subjects received a medical review and clinical examination to exclude medical co-morbidities. BMI was calculated as body mass (kg) divided by the square of body height (m2).
The patient sample in this study overlaps with our previously published MRI studies including cardiac and body fat measures by MRI11,12,19, while healthy controls show only partial overlap.
Magnetic resonance imaging
MR imaging was performed at a single site for all participants. All participants but 29 healthy controls were scanned on a 3 T Siemens Magnetom Prisma (Erlangen, Germany) using a combination of a 18-channel body coil and 12 elements of a 32-channel spine coil. An additional 29 whole-body fat imaging datasets were obtained at the same scanning site from healthy controls using a 1.5 T Phillips Achiva scanner (Phillips, Best, the Netherlands). Previous QA measures comparing whole-body fat imaging datasets from individuals scanned on both 1.5 and 3 T scanners demonstrate the data could be combined. A study of within-scanner variability (reproducibility) and across-scanner variability (including both 3 and 1.5 T scanners) found that the within-scanner repeatability (coefficient of variation = 2.9%) explained much of the overall reproducibility (CoV = 4.4%) for visceral fat volumes20.
Fat content and distribution were determined as follows: subjects were scanned using a rapid whole-body T1-weighted MRI protocol. Images were analysed by operators blind to diagnosis using SliceOmatic (Tomovision, Montreal, Quebec, Canada) and regional volumes were recorded in litres (L), including total adipose tissue and visceral fat21,22.
Statistical analysis
Differences between patients and controls were tested using χ2 tests for categorical variables, Kruskal–Wallis tests by ranks for non-normally distributed values, and analysis of variance (anova) for normally distributed measures. Effect sizes were calculated using Cohen’s d measure.
In all tests, a p value <0.05 (two-tailed) was taken as significant. Statistical analyses were performed in R.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available on request from the corresponding authors. The data are not publicly available due to ethics constraints and the potential for breaching participant privacy.
References
Plana-Ripoll, O. et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet 394, 1827–1835 (2019).
Harris, C. & Barraclough, B. Excess mortality of mental disorder. Br. J. Psychiatry 173, 11–53 (1998).
Vancampfort, D. et al. A meta‐analysis of cardio‐metabolic abnormalities in drug naïve, first‐episode and multi‐episode patients with schizophrenia versus general population controls. World Psychiatry 12, 240–250 (2013).
Després, J.-P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 444, 881–887 (2006).
Laursen, T. M., Munk-Olsen, T. & Vestergaard, M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr. Opin. Psychiatry 25, 83–88 (2012).
Smith, E. et al. Adiposity in schizophrenia: a systematic review and meta‐analysis. Acta Psychiatr. Scand. 144, 524–536 (2021).
Konarzewska, B. et al. Visceral obesity in normal-weight patients suffering from chronic schizophrenia. BMC Psychiatry 14, 1–9 (2014).
Kornetova, E. G. et al. Body fat parameters, glucose and lipid profiles, and thyroid hormone levels in schizophrenia patients with or without metabolic syndrome. Diagnostics 10, 683 (2020).
Kim, J.-H. et al. Body and liver fat content and adipokines in schizophrenia: a magnetic resonance imaging and spectroscopy study. Psychopharmacology 234, 1923–1932 (2017).
Ruppert, J. et al. Increased pericardial adipose tissue and cardiometabolic risk in patients with schizophrenia versus healthy controls. Eur. Arch. Psychiatry Clin. Neurosci. 268, 719–725 (2018).
Osimo, E. F. et al. Cardiac structure and function in schizophrenia: a cardiac MR imaging study. Br. J. Psychiatry 217, 450–457 (2020).
Pillinger, T. et al. Cardiac structure and function in patients with schizophrenia taking antipsychotic drugs: an MRI study. Transl. Psychiatry 9, 163 (2019).
Chouinard, V.-A. et al. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol. Psychiatry 24, 1513–1522 (2019).
Kozłowska, E. et al. The expression of toll-like receptors in peripheral blood mononuclear cells is altered in schizophrenia. Psychiatry Res. 272, 540–550 (2019).
Sapra, M., Lawson, D., Iranmanesh, A. & Varma, A. Adiposity-independent hypoadiponectinemia as a potential marker of insulin resistance and inflammation in schizophrenia patients treated with second generation antipsychotics. Schizophr. Res. 174, 132–136 (2016).
Blouin, M. et al. Adiposity and eating behaviors in patients under second generation antipsychotics. Obesity 16, 1780–1787 (2008).
Pillinger, T. et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7, 64–77 (2020).
Calevro, A. et al. Effects of chronic antipsychotic drug exposure on the expression of translocator protein and inflammatory markers in rat adipose tissue. Psychoneuroendocrinology 95, 28–33 (2018).
Osimo, E. F. et al. Adipose tissue dysfunction, inflammation, and insulin resistance: alternative pathways to cardiac remodelling in schizophrenia. A multimodal, case–control study. Transl. Psychiatry 11, 614 (2021).
Borga, M. et al. Reproducibility and repeatability of MRI‐based body composition analysis. Magn. Reson. Med. 84, 3146–3156 (2020).
O’donovan, G. et al. Fat distribution in men of different waist girth, fitness level and exercise habit. Int. J. Obes. 33, 1356–1362 (2009).
Thomas, E. L. et al. The missing risk: MRI and MRS phenotyping of abdominal adiposity and ectopic fat. Obesity 20, 76–87 (2012).
Acknowledgements
This work was funded by a Clinical PhD Fellowship to Dr Osimo jointly funded by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC) and the Medical Research Council (MRC) London Institute of Medical Sciences (LMS); by the BMA Foundation for Medical Research (Margaret Temple (2020) grant) to Dr Osimo and Prof Howes. This study was also funded by grants MC-A656–5QD30 from the Medical Research Council-UK, and the NIHR Biomedical Research Centre South London and Maudsley Foundation NHS Trust to Prof Howes. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. The authors would like to acknowledge the fantastic help provided by Ben Statton (Superintendent Research Radiographer), Alaine Berry (Senior Research Radiographer) and Marina Quinlan (Research Radiographer), who carried out the scans and provided great support by anonymising, storing and retrieving them as needed.
Author information
Authors and Affiliations
Contributions
E.F.O. and S.B. contributed to the design of the study, coordinated data collection, and recruited and scanned participants. E.F.O. performed data analyses, and drafted the manuscript. E.L.T. supervised data analyses and contributed to the manuscript. O.D.H. conceived and designed the study, supervised data analyses, and contributed to the manuscript. All authors have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Dr. Osimo, Dr. Brugger, and Professor Thomas report no conflicts of interest. Prof Howes is a part-time employee of H. Lundbeck A/S. He has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Angelini, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Jansenn, Lundbeck, Neurocrine, Otsuka, Sunovion, Recordati, Roche, and Viatris/Mylan. Prof Howes has a patent for the use of dopaminergic imaging.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osimo, E.F., Brugger, S.P., Thomas, E.L. et al. A cross-sectional MR study of body fat volumes and distribution in chronic schizophrenia. Schizophr 8, 24 (2022). https://doi.org/10.1038/s41537-022-00233-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00233-z