Abstract
With disease-modifying treatment for Parkinson’s disease (PD) associated with variants in the glucocerebrosidase gene (GBA1) under way, the challenge to design clinical trials with non-PD-manifest GBA mutation carriers (GBA1NMC) comes within close reach. To delineate trajectories of motor and non-motor markers as well as serum neurofilament light (sNfL) levels and to evaluate clinical endpoints as outcomes for clinical trials in GBA1NMC, longitudinal data of 56 GBA1NMC carriers and 112 age- and sex-matched GBA1 wildtype participants (GBA1wildtype) with up to 9 years of follow-up was analyzed using linear mixed-effects models (LMEM) and Kaplan–Meier survival analysis of clinical endpoints for motor and cognitive function. GBA1NMC showed worse performance in Pegboard, 20 m fast walking, global cognition as well as in executive and memory function at baseline. Longitudinally, LMEM revealed a higher annual increase of the MDS-UPDRS III bradykinesia subscore in GBA1NMC compared to GBA1wildtype, but comparable trajectories of all other motor and non-motor markers as well as sNfL. Kaplan–Meier survival analysis showed a significantly earlier progression to clinical endpoints of cognitive decline in GBA1NMC. Incidence of PD was significantly higher in GBA1NMC. In conclusion, our study extends data on GBA1NMC indicating early cognitive decline as a potentially characteristic feature. Comprehensive longitudinal assessments of cognitive function are crucial to delineate the evolution of early changes in GBA1NMC enabling a more accurate stratification and allow for a more precise definition of trial design and sample size.
Similar content being viewed by others
Introduction
It is well established that the classical motor manifestation of Parkinson’s disease (PD) is preceded by a phase which is characterized by the occurrence of several non-motor and early motor signs1. Non-motor symptoms include amongst others REM sleep behavioral disorder (RBD), hyposmia, autonomic dysfunction and neuropsychiatric symptoms such as depression and cognitive dysfunction whereas reduced arm swing and bradykinesia indicate early motor signs. However, kind and prevalence of these symptoms as well as time of occurrence and progression in relation to the onset of the classical motor manifestation is highly variable among individuals. With disease-modifying treatment options targeting different disease-specific pathways at hand, this poses challenges for designing clinical trials: Who should enter such clinical trials, when is the best time-point, how long should the intervention take, and what might be reasonable outcome measures2. Individuals with genetic mutations represent a valuable subgroup with a defined risk and known underlying pathophysiology for the development of PD. However, mutations in genes with high penetrance such as SNCA or bi-allelic PRKN and PINK1 are rare and thereby limiting the sample size whereas genes with more common mutations such as LRRK2 and GBA1 show reduced and age-dependent penetrance. Therefore, detailed longitudinal evaluation of clinical trajectories is needed in order to determine effect sizes of different assessments and biomarkers.
Heterozygous mutations in the GBA1 gene represent the most important genetic risk factor for Parkinson disease (PD) and dementia with Lewy Bodies (DLB)3 with reasonable prevalence, penetrance and occurrence across different populations. Clinically, people with PD carrying heterozygous GBA1 mutations (GBA1-PD) show more severe trajectories with faster progression of motor and non-motor impairment4,5, specifically more rapid and earlier development of cognitive decline6,7,8 compared to PD without GBA1 mutation. Importantly, this clinical phenotype is dependent on the GBA1 genotype with severe mutations predisposing to more prominent motor impairment and cognitive decline as compared to GBA1 risk variants and mild mutations4,6,9,10.
Given the prominent findings from the manifest disease phase, smaller studies have focused on non-PD-manifest GBA1 mutation carriers who did not meet diagnostic criteria for manifest PD or DLB at time of assessment (GBA1NMC)11. Longitudinal analysis over 2 and 6 years found greater deterioration in scales of depression, RBD, olfaction, global cognition as well as the Unified Parkinson’s Disease Rating Scale (UPDRS) part II and III scores in the GBA1NMC group compared to healthy controls without GBA1 mutation (GBA1wildtype)12,13,14. Focusing on a more detailed investigation of cognition, a cross-sectional study has recently shown that executive function assessed by the Stroop test was worse in GBA1NMC compared to GBA1wildtype and that reduced global cognitive function assessed with the Montréal Cognitive Assessment (MoCA) clustered with hyposmia. Verbal memory, overall motor score, presence of RBD or depression were similar between groups15. However, with clinical trials using disease-modifying compounds for PD at the horizon, there is still an urgent need for more sensitive and quantitative progression markers. Addressing this issue, we investigated trajectories of quantitative motor and non-motor parameters leveraged by a comprehensive assessment battery as well as serum levels of neurofilament light chain (sNfL) in GBA1 carriers compared to age- and sex-matched healthy controls in a prospective longitudinal study with up to 9 years of follow-up.
Results
Baseline characteristics
Details on demographic characteristics and frequencies of the different GBA1 variants are shown in Table 1. The total cohort (n = 168) included 762 assessments (n = 164 with 1–4 follow-up assessments) with a mean follow-up time of 6.3 ± 2.0 years in the GBA1NMC group versus 7.7 ± 1.2 years in the GBA1wildtype group. The 56 GBA1NMC accounted for a prevalence of 4.7% in the overall TREND study. GBA1wildtype and GBA1NMC were similar in sex (female 50.9% and 51.8%; p = 0.913) and mean age (63 years both groups, p = 0.982). There was a trend of a more frequent family history for PD in the GBA1NMC group (25.0% vs 13.4% p = 0.061), while a family history for dementia was more frequent in the GBA1wildtype group (55.4% vs 33.4%; p = 0.002). Years of education were higher in the GBA1wildtype group (mean years of education: 14.5 ± 2.3 years vs 13.5 ± 3.1 years; p = 0.041).
There were no significant differences with regard to severity of known non-motor symptoms (BDI II, RBDSQ, Sniffin Sticks, UMSARS: orthostatic, urinary, sexual, bowel dysfunction) between the GBA1wildtype and the GBA1NMC group (for details see Table 1).
In terms of motor function, GBA1NMC performed worse in the Purdue Pegboard test with the right hand (p = 0.033) and in fast walking of a 20 m distance starting with the right foot (p = 0.025) than the GBA1wildtype group, while there were trends in a similar direction for fast walking of the 20 m distance starting with the left foot (p = 0.067) and for fast walking while drawing crosses (p = 0.059) (Table 1). No differences were seen in mean MDS-UPDRS III total and sub-items scores (for details see Table 1).
GBA1NMC showed worse mean MMSE and MoCA scores compared to the GBA1wildtype group (both p < 0.001). The GBA1NMC group also showed significantly lower scores in the CERAD-Plus sum score (p = 0.036) as well as in the CERAD-Plus subtest scores for figure drawing (p = 0.004), figure recall (p = 0.031), and phonematic verbal fluency (p = 0.007). Similarly, they tended to perform worse in word list learning (p = 0.065) and in the TMT-A (p = 0.073).
Mean sNfL levels did not show significant differences between the GBA1wildtype and the GBA1NMC group at baseline (p = 0.373).
Longitudinal analyses
LMEM showed a significantly higher annual increase of the MDS-UPDRS III bradykinesia subscore of the GBA1NMC group compared to the GBAwildtype group (+1.13, 95% CI: −0.01–+2.26, p = 0.048; Table 2), but just missed significance level after adding age as a fixed factor remaining as a trend (+1.11, 95% CI: −0.06–+2.22, p = 0.051). All other non-motor and motor markers as well as serum NfL levels did not show significantly different slopes of GBA1NMC compared to GBAwildtype (for details see Table 2). However, adding age as a fixed factor in the model also revealed significant effects of age on several markers (Table 2).
Although a formal statistical analysis of groups stratified by GBA1 mutation severity was not possible due to small group sizes, exploratory descriptive analysis of trajectories showed steeper slopes of the MDS-UPDRS III total score as well as subscores for tremor, rigidity and bradykinesia in the GBA1mild and to a lesser extent in the GBA1risk group, while slopes of the MMSE, MoCA, CERAD-Plus and sNfL levels rather developed in parallel comparing GBA1risk, GBA1mild and GBA1severe with their respective age- and sex-matched GBA1wildtype groups.
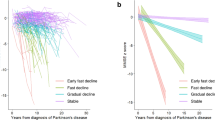
Kaplan–Meier survival analysis with log rank test and Cox regression analysis showed that GBA1NMC reached cognitive endpoints as defined by the MoCA (GBA1NMC: median 5 years, 95% CI: 4.1–5.9; vs GBA1wildtype: median 7 years, 95% CI: 6.5–7.5; p < 0.001) and the CERAD-Plus (GBA1NMC: median 7 years, 95% CI: 5.9–8.1; vs GBA1wildtype: median 8 years, 95% CI: 7.1–8.9 ; p = 0.001) significantly earlier compared to the GBA1wildtype group (Fig. 1 B, C). There was no difference in the clinical endpoint for motor function based on the MDS-UPDRS III total score (Fig. 1 A, p = 0.151).
Kaplan–Meier survival analysis with log rank test and Cox Regression analysis adjusted for age show that the asymptomatic GBAmutation group reach clinical endpoints for cognitive decline earlier than the GBAwildtype group (clinical endpoint of motor function based on the MDS-UPDRS III (a); clinical endpoints of cognitive function based on cut-offs for MCI established for the MoCA total score (b) and the CERAD-Plus battery (c)).
Incidence of PD and characteristics of PD converters
Five out of the 56 GBA1NMC (8.9%; 3 GBA1risk_and 2 GBA1mild) were diagnosed with PD according to clinical diagnostic criteria defined by classical PD motor symptoms in the course of the study whereas in the GBA1wildtype PD was diagnosed in 2 out of 112 participants (1.8%; p = 0.004). One GBA1-PD converter exhibited clinical characteristics of dementia with lewy bodies (DLB) already at baseline, consequently being excluded from the longitudinal analyses.
Descriptive characteristics of PD converters of the GBA1wildtype and GBA1NMC groups at baseline are included in Table 1 showing that GBA1-PD converters were slightly older than GBA1NMC and GBA1wildype. There was only one female GBA1-PD converter. Family history of PD, years of education and MDS-UPDRS total score as well as MDS-UPDRS tremor, bradykinesia and PIGD subscores were higher in GBA1NMC than in all other groups. Quantitative motor markers did not reveal any notable differences. MMSE, MoCa and CERAD-Plus sum scores as well as CERAD-Plus subtest showed comparable results compared to the other groups. NfL was remarkably higher than in the GBA1NMC group, but only slightly higher compared to the GBA1wildtype group.
Excluding all PD converters from the LMEM and Kaplan–Meier analyses did not relevantly influence the results.
Discussion
This study provides comprehensive longitudinal evaluation of GBA1NMC leveraging data of an assessment battery covering a broad panel of non-motor markers, motor and cognitive function as well as serum NfL levels of the largest cohort of GBA1NMC with the longest follow-up of up to 9 years to date.
Our findings indicate that GBA1NMC compared to age- and sex-matched GBA1wildtype show (i) worse performance in global cognitive function as well as in the subdomains of executive and memory function at baseline, (ii) faster longitudinal progression to clinical endpoints of cognitive performance defined by the MoCA and the CERAD-Plus battery, (iii) worse motor performance in Pegboard and 20 m fast walking at baseline, and a higher annual increase of the MDS-UPDRS III bradykinesia subscore, (iv) a higher prevalence of conversion to PD. However, performances in the MDS-UPDRS III total score at baseline as well as longitudinally were comparable, as were ratings of classical non-motor markers (except cognition) and sNfL levels. Surprisingly, a positive family history of dementia was more frequent in the GBA1wildtype group, which might be due to the high motivation of healthy individuals with a positive family history to participate in the TREND study as the study was explicitly designed and promoted to provide early detection of Parkinson’s disease and Alzheimer’s Dementia.
In summary, the faster progression to clinical endpoints of cognitive decline in the GBA1NMC group seems to characterize the profile of GBA1NMC.
In line with our findings, the two largest studies investigating GBA1NMC to date leveraging cross-sectional data from the Parkinson’s Progression Marker Initiative (PPMI) study16 and from a large Gaucher disease center17 showed higher MDS-UPDRS III and lower MoCA scores in GBA1NMC, but inconsistent differences in other non-motor features (e. g. RBD, mood and olfaction) compared to GBA1wildtype. Focusing on a more detailed investigation of cognitive function, a cross-sectional study has recently shown that executive function assessed by the Stroop test was worse in GBA1NMC compared to GBA1wildtype and that reduced global cognitive function based on the MoCA clustered with hyposmia. Contrary, overall motor score, presence of RBD or depression were similar between groups15. However, there is still only sparse longitudinal data available on the evolution of non-motor, motor, and fluid biomarkers in GBA1NMC. Three studies published by the same group with 2–6 years of follow-up data of a combined cohort of heterozygous GBA1NMC and biallelic Morbus Gaucher patients with subgroup analyses of the subgroup of heterozygous GBA1 cohort alone, found more deterioration in scales of depression, RBD, olfaction, global cognition (MoCA) as well as MDS-UPDRS part II and III scores in the GBA1NMC group compared to GBA1wildtype12,13,14.
All these studies consistently highlight cognitive performance of GBA1NMC as a key marker while motor and other non-motor signs have been shown to be affected in some but not in all investigations. This is of high relevance as clinical trials planned for GBA1NMC need to incorporate cognitive testing as a predictor and an outcome measure. While the MoCA as overall cognitive assessment seems sensitive to detect differences on a group level between GBA1NMC and GBA1wildtype, the field needs more data on comprehensive longitudinal cognitive test batteries of all relevant cognitive domains (attention, executive, memory, visuospatial) in order to estimate effect sizes of cognitive decline per year. Notably, subgroup analysis stratified by mutation severity as well as phenoconversion to PD and importantly also to DLB should be taken into account. These data will help to define cognitive outcome measures either per domain or as a composite score across domains and estimate sample sizes for a clinical trial.
In contrast to cognition, trajectories of the other assessed motor and non-motor markers as well as sNfL levels, rather developed in parallel and were primarily associated with time of follow-up and age. This seems to indicate that dynamics of these markers might be primarily associated with age. Also, the clinical tests used to assess these markers might not be sensitive enough and/or the analyzed cohort too small detect subtle early changes.
While there is increasing evidence for the utility of sNfL as a biomarker for disease progression in clinically established PD, sNfL seems not to be a sensitive marker in the non-manifest stage of PD. This is supported by evidence from a recent study of our group in a cohort of incident sporadic PD cases from the TREND study showing that sNfL levels are increased only shortly before the time point of conversion to clinically established PD18.
With the development of seed amplification assays (SAA) for the detection of disease-specific misfolded α-synuclein aggregates in various biospecimens, new options to identify subjects at risk on an individual levels have arisen enabling to establish biomarker-defined cohorts at-risk for PD19,20. It will be important to assess the evolution of motor, non-motor and fluid biomarkers in individuals who show a positive α-synuclein seeding answer in SAA.
Summarizing the results of our study, there is a great need to define and evaluate novel endpoints and outcomes for clinical trials of GBA1NMC. Single motor measures – even assessed with quantitative tools - and a variety of non-motor markers (except cognition) do not seem to be sensitive enough to consistently detect subtle changes in GBA1NMC. Therefore, in addition to the established endpoint of conversion to motor PD, it seems reasonable to seriously consider cognitive endpoints as additional outcomes for clinical trials and studies of GBA1NMC, in particular given that GBA1 mutations not only confer risk for motor PD but also for DLB as well as cognitive decline eventually resulting in dementia.
Notably, the risk of conversion might be different between mutation severity and age with those carrying severe mutations being younger whereas those with risk variants resemble idiopathic PD in term of age at onset.
Finally, our study with the – to date – longest longitudinal follow-up of GBA1NMC of up to 9 years demonstrates that even in genetically-defined at-risk populations larger, multicenter studies with higher numbers of carriers of severe GBA1 mutations and even longer follow-up periods are highly warranted and might be necessary to delineate trajectories of motor, non-motor and fluid biomarkers to predict conversion to PD and/or cognitive decline and to inform clinical trials that target GBA1.
We acknowledge the following limitations: (i) Our study is of exploratory nature and therefore, our findings need validation in prospective studies of even larger cohorts of GBA1NMC. In this context, stratification by mutation severity will be highly interesting. (ii) We had only a small number of PD converters defined by classical motor symptoms, which limits more sophisticated analysis such as principal component analysis of this specific subgroup. However, with ongoing follow-ups of the TREND study the number of PD converters might further increase yielding more valuable longitudinal data to delineate predictors of conversion to motor PD and cognitive decline. (iii) The group of GBA1NMC only included 3 individuals with severe GBA1 mutations so that a balanced and robust subgroup analysis by mutation severity was not possible. However, we argue that our findings would be even more pronounced with a higher number of individuals with severe GBA1 mutations. (v) As per the inclusion criteria of the TREND study that only recruited individuals older than 50 years of age, potential earlier changes of trajectories might not be detected. And (v) Linear mixed-effects models (LMEM) might be prone to a decrease of statistical power due to drop-out of participants with pronounced worsening of motor and cognitive function in the course of the study. Furthermore, while with LMEM continuous variables are compared over time, the Kaplan–Meier survival analysis is a time-to-event analysis using a defined endpoint as binary variable. This might explain the different results in our longitudinal analyses using these two statistical methods and further highlights the discussion the field has to make in order to design future studies and trials: which are the best outcome analyses to estimate effects but also that represent patient-related outcomes?
We conclude that our study extends data on the non-PD-manifest phase in GBA1NMC indicating early cognitive deterioration as a potentially characteristic feature. Consequently, comprehensive longitudinal assessments of cognitive function including evaluation of cognitive subdomains is crucial to delineate the evolution of early changes in GBA1NMC. This might enable a more accurate stratification of GBA1NMC and in turn allow for a more precise definition of trial design and sample size.
Methods
Participants
All participants were assessed as part of the TREND study (Tübingen Risk Evaluation for Neurodegenerative Diseases)21.
The TREND study is a prospective longitudinal study initiated in 2009 with biennial assessments of 1201 elderly participants aged between 50 and 80 years without neurodegenerative diseases. The study is performed at the Department of Neurology and the Department of Psychiatry of the University Hospital Tübingen, Germany comprising a large comprehensive assessment battery with mainly quantitative, unobtrusive measurements. For more details about the TREND study see https://www.trend-studie.de/. Study data are collected and managed using REDCap electronic data capture tools hosted at University of Tübingen22.
Genetic analysis
DNA was isolated from EDTA blood by salting out method and stored at 4 °C. Genetic screening for GBA1 variants was done by sanger sequencing of all exons of the GBA1 gene. Naming of GBA1 variants is based on the new nomenclature for GBA variants including the 39-aminoacid residue. In total, we identified 56 participants harboring a variant in the GBA1 gene (GBA1NMC). GBA1 variant severity was classified in risk variants (GBA1risk n = 48: 19 E365K, 26 T408M, 1 T336S, 1 N427K and 1 N427K + T408M), mild (GBA1mild n = 5: N409S) and severe mutations (GBA1severe n = 3: 1 H294Q, 1 L483P and 1 L483P + E365K) according to established genotype risks reported for PD23,24. To overcome age- and sex-related modifying effects within the total TREND cohort, we defined a nested case-control cohort out of the 1201 TREND participants in the relation of 1:2. We included the 56 GBA1NMC and randomly selected 112 age- and sex-matched healthy individuals without GBA1 mutation out of the TREND study cohort. All participants underwent genotyping and were also controlled for not carrying pathogenic mutations in the LRRK2 gene. Furthermore, all PD converters were also tested for not carrying pathogenic mutations in the recessive genes PRKN, PINK and DJ1.
Clinical investigations and assessments
Each participant underwent a standardized neurological examination by an experienced movement disorder specialist. Individuals with an incident diagnosis of PD at baseline according to the UK Brain Bank Criteria were excluded from the present analysis. Individuals who developed PD during the follow up period were excluded from the longitudinal analyses after the time point of their respective diagnosis.
Family history for PD and dementia, and years of education were assessed with standardized questionnaires. The German version of the Beck’s Depression Inventory II (BDI-II)25 was used to assess depressive symptoms. The RBD screening questionnaire (RBDSQ)26 was used to assesses sleep behavioral symptoms. Olfactory function was investigated with the 16 Sniffin’ Sticks test27. Autonomic symptoms, specifically orthostatic, urinary, and erectile dysfunction as well as constipation, were assessed using subitems 9 to 12 of the Unified Multiple Systems Atrophy Rating Scale (UMSARS)28.
Global cognitive function was assessed with the Mini Mental Status Examination (MMSE)29 and the Montreal Cognitive Assessment (MoCA)30. Since the MoCA was not available until 2009, MMSE scores from all visits of all patients were additionally converted into MoCA equivalent scores using a published algorithm31.
Detailed cognitive testing was performed using the extended German version of the Consortium to Establish a Registry for Alzheimer’s Disease-Plus (CERAD-Plus)32. The neuropsychological CERAD-Plus battery assesses 4 cognitive domains with the following respective subtests (in brackets): executive function (Trail Making Test [TMT] part B, semantic and phonemic verbal fluency), memory (word list learning, word list recall and figure recall), language (Boston Naming Test) and visuospatial function (Figure copy). Additionally, part A of the TMT was performed to assess psychomotor speed. Age, gender, and education adjusted z-scores were used.
Severity of motor symptoms was assessed by the MDS-UPDRS III. Additionally, subscores for tremor, rigidity, bradykinesia and postural instability-gait difficulty (PIGD) were calculated from the respective subitems of the MDS-UPDRS III as described before33,34. Purdue Pegboard was used for examination of hand dexterity and combined performance of fine motor speed and finger-eye coordination35. Gait speed was assessed quantitatively with the 3-meter Timed Up and Go Task (3 m TUG)36 and walking of a straight 20 m track with normal and fast speed as well as fast speed walking while making crosses and serial subtractions of 7 starting from 100 respectively.
Clinical endpoints were defined for motor and cognitive function according to established cut-offs. Motor deterioration reflecting subthreshold parkinsonism was assessed using the MDS-UPDRS III with a cut-off of >6 points excluding scores of the postural and action tremor items37. Cognitive endpoints for Mild Cognitive Impairment (MCI) were defined as (i) <26 points in the MoCA score as established38 and (ii) a decline of >0.03 based on the mean of the z-normalized CERAD-Plus total score as described recently39.
Serum Neurofilament light chain (sNfL)
Serum NfL levels were measured in duplicates by single-molecule array (SIMOA) technique on the Simoa HD-1 Analyzer (Quanterix, Lexington, Massachusetts), as established previously40.
Statistical analysis
Statistical analysis was performed using SPSS statistical software version 28.0 (IBM Corp., Armonk, NY) and RStudio software (release 2021.09.02 + 382) using R version 4.1.2 for data visualization. Analyses of cross-sectional data were performed using Student’s t test for continuous data and χ2 test for categorial variables. All statistical tests were two-sided and p values ≤ 0.05 were considered statistically significant. As all analyses were explorative, we did not correct for multiple testing.
Longitudinal analyses using linear mixed-effects models (LMEM) adjusting for age and years of education were performed to estimate the slopes of motor and non-motor parameters and NfL with the fixed factors group (GBA1wildtype, GBA1NMC) and time (time of follow-up in years from baseline), their interaction and the random variable subject, modeled by random intercepts. We analyzed the fixed effect of group, time and the interaction of group and time on the dependent variable, respectively. Kaplan-Meyer survival curves with log rank test and Cox regression analyses adjusted for age were used to estimate disease-free event of the defined motor and cognitive endpoints.
Ethical standards
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Ethical approval of the study was granted by the ethical committee of the University of Tübingen (Nr. 90/2009BO2) and written informed consent from all participants was obtained prior to study inclusion.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Anonymized data are available upon request to: benjamin.roeben@med.uni-tuebingen.de
References
Berg, D. et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 30, 1600–1611 (2015).
Berg, D. et al. Path to Parkinson Disease Prevention: Conclusion and Outlook. Neurology 99, 76–83 (2022).
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Maple-Grodem, J. et al. Association of GBA Genotype With Motor and Functional Decline in Patients With Newly Diagnosed Parkinson Disease. Neurology 96, e1036–e1044 (2021).
Brockmann, K. et al. GBA-associated PD presents with nonmotor characteristics. Neurology 77, 276–280 (2011).
Cilia, R. et al. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann. Neurol. 80, 662–673 (2016).
Brockmann, K. et al. GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov. Disord. 30, 407–411 (2015).
Mata, I. F. et al. GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson’s disease. Mov. Disord. 31, 95–102 (2016).
Gan-Or, Z. et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology 84, 880–887 (2015).
Jesus, S. et al. GBA Variants Influence Motor and Non-Motor Features of Parkinson’s Disease. PLoS One 11, e0167749 (2016).
Zimmermann, M. et al. Patient’s perception: shorter and more severe prodromal phase in GBA-associated PD. Eur. J. Neurol. 26, 694–698 (2019).
Beavan, M. et al. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 72, 201–208 (2015).
Avenali, M. et al. Evolution of prodromal parkinsonian features in a cohort of GBA mutation-positive individuals: a 6-year longitudinal study. J. Neurol. Neurosurg. Psychiatry 90, 1091–1097 (2019).
Mullin, S. et al. Evolution and clustering of prodromal parkinsonian features in GBA1 carriers. Mov. Disord. 34, 1365–1373 (2019).
Moran, E. E. et al. Cognitive Functioning of Glucocerebrosidase (GBA) Non-manifesting Carriers. Front. Neurol. 12, 635958 (2021).
Simuni, T. et al. Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson’s Progression Markers Initiative (PPMI): a cross-sectional study. Lancet Neurol. 19, 71–80 (2020).
Becker-Cohen, M. et al. A Comprehensive Assessment of Qualitative and Quantitative Prodromal Parkinsonian Features in Carriers of Gaucher Disease-Identifying Those at the Greatest Risk. Int J. Mol. Sci. 23, 12221 (2022).
Wilke, C. et al. Intraindividual Neurofilament Dynamics in Serum Mark the Conversion to Sporadic Parkinson’s Disease. Mov. Disord. 35, 1233–1238 (2020).
Berg, D. & Klein, C. alpha-synuclein seed amplification and its uses in Parkinson’s disease. Lancet Neurol. 22, 369–371 (2023).
Siderowf, A. et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using alpha-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 22, 407–417 (2023).
Gaenslen, A. et al. Prodromal features for Parkinson’s disease-baseline data from the TREND study. Eur. J. Neurol. 21, 766–772 (2014).
Harris, P. A. et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009).
Huh, Y. E. et al. beta-Glucocerebrosidase activity in GBA-linked Parkinson disease: The type of mutation matters. Neurology 95, e685–e696 (2020).
Lerche, S. et al. The Mutation Matters: CSF Profiles of GCase, Sphingolipids, alpha-Synuclein in PD(GBA). Mov. Disord. 36, 1216–1228 (2021).
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571 (1961).
Stiasny-Kolster, K. et al. Diagnostic value of the REM sleep behavior disorder screening questionnaire in Parkinson’s disease. Sleep. Med. 16, 186–189 (2015).
Hummel, T., Sekinger, B., Wolf, S. R., Pauli, E. & Kobal, G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 22, 39–52 (1997).
Wenning, G. K. et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov. Disord. 19, 1391–1402 (2004).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Bergeron, D. et al. Multicenter Validation of an MMSE-MoCA Conversion Table. J. Am. Geriatr. Soc. 65, 1067–1072 (2017).
Welsh-Bohmer, K. A. & Mohs, R. C. Neuropsychological assessment of Alzheimer’s disease. Neurology 49, S11–S13 (1997).
Muller, T. et al. 2-[123I]-iodolisuride SPET visualizes dopaminergic loss in de-novo parkinsonian patients: is it a marker of striatal pre-synaptic degeneration? Nucl. Med. Commun. 18, 1115–1121 (1997).
Stebbins, G. T. et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 28, 668–670 (2013).
Postuma, R. B., Lang, A. E., Massicotte-Marquez, J. & Montplaisir, J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology 66, 845–851 (2006).
Podsiadlo, D. & Richardson, S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 39, 142–148 (1991).
Heinzel, S. et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 34, 1464–1470 (2019).
Hoops, S. et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73, 1738–1745 (2009).
Lillig, R. et al. A new CERAD total score with equally weighted z-scores and additional executive and non-amnestic “CERAD-Plus” tests enhances cognitive diagnosis in patients with Parkinson’s disease: Evidence from the LANDSCAPE study. Parkinsonism Relat. Disord. 90, 90–97 (2021).
Kuhle, J. et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 54, 1655–1661 (2016).
Acknowledgements
We thank the participants for their continued participation in the TREND study and for providing biosamples. We acknowledge the work of numerous (doctoral) students and study nurses, who actively contributed to study organization, data collection, entry and monitoring. The TREND study is being conducted at Tübingen University Hospital and has been or is supported by the Hertie Institute for Clinical Brain Research, the German Center for Neurodegenerative Diseases, the Geriatric Center of Tübingen, the Center for Integrative Neuroscience, Teva Pharmaceutical Industries, Union Chimique Belge, Janssen Pharmaceuticals, and the International Parkinson Fund. The supporting institutions had no influence on the design, conduct, or analysis of the study. Biosamples were obtained from the Neuro-Biobank of the University of Tübingen, Germany, which is supported by the University of Tübingen, the Hertie Institute for Clinical Brain Research, and the German Center for Neurodegenerative Diseases.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Benjamin Roeben: Conception, design, execution of the clinical part of the study and statistical analysis, writing of the first draft of the manuscript. Inga Liepelt-Scarfone: Execution of the statistical analysis, critical review of the manuscript. Stefanie Lerche: Execution of the statistical analysis, critical review of the manuscript. Milan Zimmermann: Execution of the clinical part of the study, critical review of the manuscript. Isabel Wurster: Execution of the clinical part of the study, critical review of the manuscript. Claudia Schulte: Execution of the biochemical part study and GBA genotyping, critical review of the manuscript. Christian Deuschle: Execution of the biochemical part study, critical review of the manuscript. Gerhard W. Eschweiler: Execution of the clinical part of the study, critical review of the manuscript. Walter Maetzler: Execution of the clinical part of the study, critical review of the manuscript. Thomas Gasser: Execution of the study, critical review of the manuscript. Daniela Berg: Execution of the study, critical review of the manuscript. Kathrin Brockmann: Conception, organization and execution of the study, statistical analysis, manuscript preparation, critical review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.R. was supported by the Clinician Scientist program of the Medical Faculty of the University of Tübingen (grant #478-0-0). I.L.S. has received grants from Janssen Research and Development, a division of Janssen Pharmaceutica N.V. and grants from the Michael J. Fox Foundation; grants from European Commission, H2020-TWINN-2015, International Parkinson Fonds (Deutschland) GmbH (IPD), Novartis and German Ministry for Education and Research (BMBF). S.L. has nothing to disclose. M.Z. was supported by the Clinician Scientist program of the Medical Faculty of the University of Tübingen (grant #481-0-0). I.W. received funding from the Michael J. Fox Foundation in form of the Edmond J. Safra Fellowship in Movement Disorders. C.S. has nothing to report. C.D. has nothing to report. G.W.E. has received grants from the German Federal Ministry of Education of Research (BMBF), German Research Council (DFG), German Innovationsfonds but not from industrial partners. W.M. receives or received funding from the European Union, the German Federal Ministry of Education of Research, German Research Council, Michael J. Fox Foundation, Robert Bosch Foundation, Neuroalliance, Lundbeck, Sivantos and Janssen. He received speaker honoraria from Abbvie, Bayer, BIAL, GlaxoSmithKline, Heel, Licher MT, Pfizer, Takeda and UCB, was invited to Advisory Boards/Consultancies of Abbvie, Aptar Digital Health, Atheneum, Biogen, Kyowa Kirin, Lundbeck and Market Access & Pricing Strategy GmbH, is an advisory board member of the Critical Path for Parkinson’s Consortium, and an editorial board member of Geriatric Care. He serves as the co-chair of the MDS Technology Working Group. T.G. serves as associate editor at Journal of Parkinson’s disease; holds a patent re: KASPP (LRRK2) Gene, its Production and Use for the Detection and Treatment of Neurodegenerative Diseases; serves as a consultant for Cephalon, Inc. and Merck Serono; serves on speaker’s bureaus of Novartis, Merck Serono, SCHWARZ PHARMA, Boehringer Ingelheim, and Valeant Pharmaceuticals International; and receives research support from Novartis, the European Union, BMBF (the Federal Ministry of Education and Research), and Helmholtz Association. D.B. has served on scientific advisory boards for UCB/ SCHWARZ PHARMA, Lundbeck, Biogen and BIAL.; has received funding for travel or speaker honoraria from Lundbeck Inc., Novartis, UCB/ SCHWARZ PHARMA, Merck Serono, Biogen, Zambon, AbbVie, and BIALLtd.; and has received research support from Janssen, Teva Pharmaceutical Industries Ltd., Solvay Pharmaceuticals, Inc./Abbott, Boehringer, UCB, Michael J Fox Foundation, BMBF, dPV (German Parkinson’s disease association), Neuroallianz, DZNE,Center of Integrative Neurosciences and the Damp Foundation. K.B. has received research grants from the University of Tuebingen (Clinician Scientist), the German Society of Parkinson’s disease (dpv), the Michael J. Fox Foundation (MJFF), the German Centre for Neurodegenerative Diseases (DZNE, MIGAP) and the German Federal Ministry of Education and Research (BMBF) in the frame of ERACoSysMed2 (FKZ 031L0137B). She received Speaker honoraria from Abbvie, Lundbeck, UCB and Zambon. She serves as consultant for Roche.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roeben, B., Liepelt-Scarfone, I., Lerche, S. et al. Longitudinal cognitive decline characterizes the profile of non-PD-manifest GBA1 mutation carriers. npj Parkinsons Dis. 10, 88 (2024). https://doi.org/10.1038/s41531-024-00706-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00706-1