Abstract
Reduced uptake of 123I-meta-iodobenzylguanidine (123I-MIBG) and orthostatic hypotension (OH) are independently associated with worse clinical outcomes of Parkinson’s disease (PD). However, their interactive influence on PD has not been studied. The role of 123I-MIBG myocardial uptake, as a biomarker of PD severity, was investigated, conditional on the mediating effects of OH. A total of 227 PD patients were enrolled. Their motor and nonmotor aspects were assessed with standardized tools. Global disease burden was estimated by averaging the scaled z-scores of the assessment tools. Every patient went through 123I-MIBG scan, and OH was evaluated with the head-up tilt-test. The mediating role of orthostatic blood pressure changes (ΔBP) on the association between cardiac sympathetic denervation and disease burden was investigated. Low heart-to-mediastinum (H/M) ratio with less than 1.78 was seen in 69.6% of the patient population, and 22.9% of patients had OH. Low H/M ratio was associated with OH, and these patients had worse disease burden than subjects with normal 123I-MIBG uptake (global composite z-score: normal 123I-MIBG vs. abnormal 123I-MIBG; −0.3 ± 0.5 vs. 0.1 ± 0.7; p < 0.001). The mediation models, controlled for age and disease duration, revealed that the delayed H/M ratio and global composite score were negatively associated, irrespective of orthostatic ΔBP. Adverse relationship between cardiac sympathetic denervation and disease burden was shown without any interference from orthostatic blood pressure fluctuations. This result suggested that extracranial cardiac markers might reflect disease burden, regardless of labile blood pressure influence.
Similar content being viewed by others
Introduction
Cardiovascular dysautonomia is increasingly accepted as a prodromal ‘window’ in which Parkinson’s disease (PD) can be detected1,2,3,4,5. These findings have been reported as clinical biomarkers for predicting PD clinical outcomes and consequences6,7,8,9,10.
Cardiac postganglionic sympathetic denervation, reflected more accurately in the decreased delayed heart-to-mediastinum (H/M) ratio upon 123I-meta-iodobenzylguanidine (123I-MIBG) myocardial scintigraphy scan11, contributes to orthostatic hypotension (OH)4. Reduced myocardial uptake of 123I-MIBG indicates norepinephrine transporter dysfunction in sympathetic neurons, and may reflect physiological consequences in residual functional cardiac sympathetic axons7,12. Reduced uptake of 123I-MIBG was utilized to discriminate PD from vascular Parkinsonism, drug-induced Parkinsonism, and atypical parkinsonian syndromes (APS)2.
Some PD patients have a normal 123I-MIBG uptake analogous to APS, and this imaging phenotype was previously given the term “scans without evidence of cardiac norepinephrine deficit (SWEND)”13. PD patients with SWEND had mild Hoehn and Yahr (H&Y) stage, short disease duration, slow progression of motor dysfunction, a lower incidence of the wearing-off phenomenon, and a lower prevalence of nonmotor symptoms7,10,11,12,14,15.
Most studies have investigated the association of cardiac sympathetic denervation with orthostatic hypotension and the impact on clinical outcomes separately, but the interacting effects of these two biomarkers on PD patient symptoms have seldom been explored4,16. As a pathophysiologic contributor, OH is a possible biomarker of pathologic burdens2,4,7,12,17, thus the confounding effects of OH need to be considered when assessing the role of 123I-MIBG myocardial scintigraphy results in PD patients.
In this study, we investigated whether a mediating role for OH could be disproved in the context of a significant association between cardiac sympathetic denervation and clinical disease burden. The refutation of this role would emphasize the sustained value of 123I-MIBG uptakes as a pathologic biomarker of disease severity despite its contribution to OH.
Orthostatic hypertension (OHT) is also a type of cardiovascular dysregulation which can be anticipated in PD patients18,19. This study also aimed to document the nature of OHT in early PD patients.
Results
Baseline characteristics
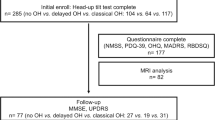
Baseline characteristics are summarized in Table 1. A total of 227 patients with mild PD stage were included. The mean age of the population was 69.6 ± 9.2 years old, and 105 (46.3%) were female. The disease duration was 1.1 ± 1.0 years. Total Unified Parkinson’s Disease Rating Scale (UPDRS) and H&Y were 23.4 ± 11.9 and 2.0 (Interquartile range, IQR, 0.0), respectively. The Mini-Mental Status Examination (MMSE) was 26.8 ± 3.0 and Clinical Dementia Rating (CDR) was 0.5 (IQR, 0.0). The mean uptake of early and delayed 123I-MIBG H/M ratio was 1.58 ± 0.31 and 1.55 ± 0.37, respectively. 69.6% (158/227) of patients were defined as having decreased H/M ratio on 123I-MIBG scintigraphy (normal 123I-MIBG group vs. abnormal 123I-MIBG group: 2.04 ± 0.16 vs. 1.34 ± 0.18, respectively). Twenty patients were found to be hypertensive when in the supine position; 40% (8/20) and 30% (6/20) of patients with supine hypertension (SH) had co-existing OH and OHT, respectively. Among the patients studied, 22.9% (52/227) and 26.4% (60/227) were classified into OH and OHT groups, respectively.
Comparisons between normal 123I-MIBG group and abnormal 123I-MIBG group
The group with low delayed H/M ratio had worse outcomes on motor and nonmotor measurements. Total and Part III UPDRS scores were higher (normal 123I-MIBG group vs. abnormal 123I-MIBG group: total UPDRS, 20.4 ± 11.1 vs. 24.8 ± 12.0, respectively, p = 0.010; and for Part III, 13.6 ± 8.0 vs. 16.7 ± 8.7, respectively, p = 0.013). H&Y stages did not differ between groups (normal 123I-MIBG group vs. abnormal 123I-MIBG group: 2.0 [1.0] vs. 2.0 [0.0], p = 0.112; Fisher’s exact test, p = 0.307). The scores of Nonmotor Symptoms Scale (NMSS), Montgomery-Asberg Depression Rating Scale (MADRS), Epworth Sleepiness Scale (ESS), Parkinson’s Disease Sleep Scale-2 (PDSS-2), REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Scale for Outcomes in Parkinson’s Disease-Autonomic (SCOPA-AUT), and the Orthostatic Hypotension Questionnaire (OHQ) Part II were compared between groups; the abnormal 123I-MIBG group was scored higher than the normal 123I-MIBG group. PD patients with decreased delayed H/M ratio had worse overall disease burden than PD patients with normal 123I-MIBG (normal 123I-MIBG group vs. abnormal 123I-MIBG group: global composite score, −0.3 ± 0.5 vs. 0.1 ± 0.7, respectively, p < 0.001).
Decreased 123I-MIBG uptake was associated with OH, while normal 123I-MIBG uptake was associated with OHT (Fisher’s exact test, p = 0.002, p = 0.014; respectively). The magnitude of blood pressure (BP) drop was higher in the abnormal 123I-MIBG group (normal 123I-MIBG vs. abnormal 123I-MIBG: ΔSBPmin, 5.3 ± 11.0 vs. 12.9 ± 13.6, respectively, p < 0.001; ΔDBPmin, 0.4 ± 6.8 vs. 3.8 ± 8.0, respectively, p = 0.002), and BP rise was greater in the normal 123I-MIBG group (normal 123I-MIBG vs. abnormal 123I-MIBG: ΔSBPmax, −3.3 ± 11.8 vs. 2.7 ± 13.8, respectively, p = 0.002; ΔDBPmax, −5.9 ± 6.9 vs. −3.5 ± 7.8, respectively, p = 0.030).
The mediating effects of orthostatic blood pressure changes on clinical outcomes
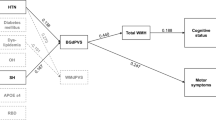
Table 2 and Fig. 1 present the mediating effects of BP changes (∆SBPmin, ∆DBPmin) on global composite score. When controlling for age and disease duration, delayed H/M ratio was negatively associated with global composite score (total effect [indirect + direct]: CI, [−0.701, −0.226]). Delayed H/M ratio was also negatively associated with orthostatic ∆BPmin (component a → b: ΔSBPmin vs. ΔDBPmin; CI, [−15.987, −7.355] vs. CI, [−7.966, −2.530]), but its inverse association was not maintained (component b → c: ΔSBPmin vs. ΔDBPmin; CI, [−0.004, 0.009] vs. CI, [−0.013, 0.009]). The overall indirect influence was not significant (a → b → c: ΔSBPmin vs. ΔDBPmin; CI, [−0.106, 0.051] vs. CI, [−0.049, 0.075]), but the direct negative associations between delayed H/M ratio and global composite score were maintained (component a → c: ΔSBPmin vs. ΔDBPmin; CI, [−0.687, −0.200] vs. CI, [−0.710, −0.237]). The results were similar when early H/M ratio (a’) and washout rate (a”) were set as predictors in the mediation analyses (Supplementary Table 1, Supplementary Fig. 1).
The subdomains of global composite score, partitioned by motor, sleep, and autonomic composite scores were analyzed (Table 3). A delayed H/M ratio negatively predicted each domain (total effect), and its inverse influence was not mediated by orthostatic ΔBP (indirect effect).
Bidirectionality of orthostatic blood pressure change
In an observational sub-analysis of orthostatic ∆BPmax, OHT group displayed a worsening trend for global composite score as the ∆BPmax decreased while the non-OHT group demonstrated a positive association as the ΔBPmax increased, especially ΔSBPmax (Supplementary Fig. 2). These associations lacked any statistical significance; however, orthostatic BP rise and drop was accompanied by trends toward adverse disease severity in both directions.
Comparison of washout rate between OHT and non-OHT
The washout rate (WR) was significantly opposite (normal 123I-MIBG vs. abnormal 123I-MIBG: WR, −4.87 ± 6.81 vs. 5.25 ± 7.03, respectively, p < 0.001). In a sub-analysis of sixty-nine normal 123I-MIBG patients, 37.7% (26/69) were orthostatic hypertensive. The WR was compared between those with OHT and without OHT. The comparison did not reveal any significant difference (non-OHT vs. OHT: −4.39 ± 7.29 vs. −5.65 ± 5.99, respectively; independent t-test, t = 0.743, df = 67.0, p = 0.460).
Discussion
Cardiac sympathetic denervation and orthostatic hypotension were encountered in early PD patients. OH was found more frequently in the abnormal 123I-MIBG group while orthostatic hypertension was more common in SWEND PD patients. PD patients with reduced 123I-MIBG uptake presented with worse clinical features. Cardiac denervation inversely predicted PD-related disease burden, irrespective of orthostatic blood pressure changes.
The population of this cohort was in the early mild PD disease stage. The prevalence of abnormal 123I-MIBG uptake was comparable to previous studies7,12,20. The frequency of OH in early PD patients was similar to another study21, and its association with impaired cardiac sympathetic integrity has been reported4,22. In this cohort, PD patients with decreased 123I-MIBG uptake showed more severe clinical presentations, and they were more susceptible to orthostatic BP drop7,23. PD patients with SWEND were associated with orthostatic BP rise, but had less disease burden.
Irrespective of age and disease duration, more severe cardiac sympathetic denervation was related to a larger drop in orthostatic BP (indirect component a → b) and worse disease severity (direct path a → c). Its negative influence was not carried through BP instability to affect PD patients (indirect path a → b → c). Thus, the total effect of sympathetic denervation negatively reflected the global disease burden, and was unaffected by BP lability; more severely impaired cardiac dysautonomia was directly associated with worse clinical outcomes (Table 2). Cardiac denervation inversely paralleled motor, sleep, and autonomic global severity domains in the subcomponent analyses (Table 3).
The inverse association between cardiac sympathetic denervation and worse disease severity could be explained by the degree of accumulated pathology. In a cross-sectional study, PD patients with cardiac sympathetic denervation were speculated to bear more pathologic burden that resulted in lack of compensatory reserve compared to patients with less cardiac sympathetic denervation, thus worse clinical manifestations7. This phenotype was further researched in a longitudinal study, and these patients were shown to be at a higher risk of developing motor complications12. This temporal association between PD with abnormal 123I-MIBG uptake and wearing-off phenomenon implied that pathologic burden at the periphery could mirror central pathophysiologic disease progression as cardiac sympathetic denervation progressed over time2,4,10,12. This centripetal degeneration of cardiac sympathetic response was suggested to represent common degenerative process in PD patients1, and suggests multiple origination sites for Lewy pathology spread24.
The lack of contribution of blood pressure to clinical burdens amid the paths could suggest myocardial 123I-MIBG scintigraphy is a potential biomarker that reflects Lewy body pathology2,17, in parallel with disease severity. The significant negative association (indirect component a → b), controlled by age and disease duration, confirmed the contributing action of cardiac sympathetic denervation on OH4.
Braak’s schema was disputed because its model failed to explain all the subtypes of PD, especially cardiac denervation in early PD24. Recent hypothesis of “body-first” subtype PD incorporates the cardiac pathobiology which may be the answer to the observed differences between normal and abnormal 123I-MIBG groups in this study25,26. This further enhances the extracranial biomarker role of cardiac denervation in PD.
Early H/M ratio and washout rate which reflect density of the presynaptic cardiac sympathetic nerve endings2, its tone27, and damaged or failing myocardium28, respectively, also manifested similar results that reinforced the hypothesis that 123I-MIBG scintigraphy is a mirror of disease burden (Supplementary Table 1, Supplementary Fig. 1).
OHT has seldom been investigated in PD patients, and its characteristics are largely unknown. In this study, despite a difference in estimation of ΔBP during head tilt, the prevalence of OH and OHT were comparable. Interestingly, although the analysis did not gain statistical significance, orthostatic BP incremental change was suggested to be related to worse clinical outcomes. Orthostatic BP drop also displayed a trend for worse outcomes, particularly with respect to ΔSBP. OHT is associated with other types of blood pressure lability, and with cardiovascular risk18. In accord with our observation, disrupted blood pressure control could be deleterious, regardless of the directionality of the BP changes19.
OHT was significantly associated with normal 123I-MIBG that included higher values of delayed H/M ratios. It also displayed lower and negative washout rate, which apparently signified that delayed H/M ratio was greater than early H/M ratio. In this regard, delayed H/M and WR could denote the same as WR contains the other in its equation. This was in correspondence to other studies indicating that delayed H/M and washout rate represented sympathetic tone2,27. Its greater sympathetic tone might have allowed normal 123I-MIBG group to culminate into higher orthostatic BP rise, particularly SBP, than abnormal 123I-MIBG group.
This result suggested that OHT could originate by a different cardiovascular dysregulation mechanism since it was related to normal 123I-MIBG. As OH in PD stems from cardiac, extra-cardiac denervation and arterial baroreflex failure4, orthostatic ∆BP rise could be the product of compensation of extant cardiac sympathetic tone amid other dysfunctions, which, when lost, could lead to OH. This may imply that OHT could be a prelude to OH in some PD. Our data did not depict any significant difference of washout rate between non-OHT and OHT among preserved 123I-MIBG group. Had this been manifested, the sick-but-not-dead phenomenon could have played a role in its pathophysiology;29 thus, further support the sequential conversion of OHT into OH as the disease progresses. The nature of OHT in PD needs to be further assessed.
There are several limitations in this study. First, because the enrolled PD patients were in the early phase of the disease, we could not completely exclude the possibility that some had atypical Parkinsonism. In particular, the PD with SWEND patient group might have inadvertently included some multiple system atrophy patients2. We attempted to reduce selection bias by using strict clinical diagnostic criteria for PD30,31 and structural neuroimaging. Nevertheless, it can be difficult to differentiate PD from multiple system atrophy from a nosological perspective. Second, we also could not exclude patients who had genetic Parkinsonism. Patients with the Parkin mutation might not present with nonmotor manifestations and abnormal 123I-MIBG uptake32,33. This study did not include familial PD or patients with young-onset PD (≤40 years). Third, direct biomarkers that provide objective measurements to assess PD disease severity are not available at the present; in addition, currently useful biomarkers do not always represent clinical symptoms. A clinical assessment-based approach, as in this study, is an alternative that evaluates disease severity, relevant to clinical practice. This study incorporated validated clinical tools that encompassed comprehensive motor and nonmotor disease domains. Fourth, there was not sufficient evidence that 123I-MIBG uptake represents myocardial synuclein deposition. Future studies that correlate 123I-MIBG myocardial scans with a direct synuclein accumulation biomarker are warranted. Moreover, cardiac imaging with 18F-fluorodopa, which may be a better substance to evaluate cardiac sympathetic innervation, was also investigated in PD16,34. Diverse studies with cross-validation between different imaging modalities will strongly enhance the results of this research. Finally, because early patients with mild PD stage were enrolled, the scores of questionnaires tended to center at the left of the scale with relatively large standard deviation. A larger recruitment of PD by engagement of multi-clinics to reduce the bias is warranted.
In this cohort, cardiac sympathetic denervation negatively predicted disease severity, independently of orthostatic blood pressure changes. Even though cardiac denervation is a contributor to orthostatic hypotension, the effect of cardiac denervation on disease burdens was not mediated by orthostatic blood pressure changes. Cardiac sympathetic denervation was suggested to represent the disease burden, in parallel with pathologic severity. Orthostatic hypertension was also observed, and it could potentially contribute to PD symptom clinical severity.
Methods
Patients
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, and all subjects provided written informed consent to participate. All experiments were conducted in accordance with relevant guidelines and regulations. The study was registered (Identification Number: KCT0005552) in the Clinical Research Information Service (CRIS; http://cris.nih.go.kr), which is an online clinical trial registration system established by the Korea Centers for Disease Control and Prevention (KCDC) with support from the Korea Ministry of Health and Welfare (KMOHW) and is affiliated with the Primary Registries in the World Health Organization (WHO) Registry Network.
Two hundreds twenty-seven de novo and drug-naïve PD patients between April 2014 and January 2020 were enrolled. PD was diagnosed based on the UK Parkinson’s Disease Society Brain Bank30, and its diagnosis was supported by positron emission tomography imaging studies using 18F-N-(3-fluoropropyl)-2beta-carbon ethoxy-3beta-(4-iodophenyl) nortropane31. All patients had decreased dopamine transporter uptake in the striatum, mainly in the posterior putamen. Patients underwent brain magnetic resonance imaging (MRI) to exclude secondary causes for this finding.
Demographics such as age, sex, body mass index, disease duration, smoking status and history of hypertension, diabetes mellitus, and dyslipidemia were investigated. Disease severity was investigated with the UPDRS and H&Y stage. Global cognition was assessed by the MMSE and CDR.
Patients were disqualified from the study if they had any of the following indications: (1) any symptoms or signs of atypical and/or secondary Parkinsonism, (2) positive family history of Parkinsonism by pedigree analysis, which included first degree relatives, (3) documentation of atrial fibrillation during the head up tilt test, (4) history of diabetic neuropathy, (5) history of symptomatic stroke that could affect general cognition and performance, and (6) history of medications such as tricyclic antidepressants or benzodiazepines that influence autonomic functions or patients who were taking medications at the time of diagnosis known to influence the central dopaminergic, noradrenergic, and/or serotonergic systems.
Patients were followed every 2–6 months for a minimum of 12 months from the time they began taking dopaminergic medication, and their diagnosis was reaffirmed after at least 12 months of follow-up by two neurologists (S.-W.Y., J.-S.K.).
Questionnaires
The patients were evaluated with the following questionnaires: (1) NMSS35, (2) MADRS36, (3) ESS37, (4) PDSS-238, (5) RBDSQ39, (6) OHQ40, and (7) the SCOPA-AUT41. Part I and II of the OHQ were summed separately. Because the SCOPA-AUT sexual dysfunction subsection had too many missing values, this subsection was omitted from that questionnaire’s summation. Missing values of sexual subsection was due to patients’ reluctance to reveal their sexual activities to the examiner. The sums of each questionnaire were analyzed.
Composite scores
Motor severity (motor composite score) was gauged by averaging the z-scores of the scaled UPDRS II and UPDRS III. The sums of each individual questionnaire for nonmotor features were standardized to z-scores. Nonmotor features were also divided into the sleep domain (sleep composite score: average z-scores for the ESS, PDSS-2, RBDSQ), and the autonomic domain (autonomic composite score: average z-scores for the OHQ and SCOPA-AUT), and were further analyzed separately. The affection domain was not analyzed because the MADRS data were not normally distributed, and the NMSS was excluded from subcomponent analysis due to its inclusive psychometric properties.
Parkinson’s disease overall burden (the global composite score) was estimated by averaging the scaled z-scores of all motor and nonmotor assessments. Higher z-scores indicated worse severity. All questionnaires were evaluated by investigators blind to patient clinical information.
Head-up tilt test
All patients were tested in the full resting state. Continuous electrocardiograph leads and non-invasive BP monitoring equipment were applied to the patients (YM6000, Mediana Tech, Redmond, WA, USA). A supine position was maintained for 20 min during recording of BPs and heart rates every 5 min, before tilting to 60 degrees (ENRAF NONIUS, Rotterdam, The Netherlands). At the tilted position, the same measurements were taken at 0, 3, 5, 10, 15, and 20 min.
After excluding the first supine BP at 0 min, average supine systolic and diastolic BPs were estimated from the measurements at 5, 10, 15, and 20 min. SH was defined if the average supine systolic and/or diastolic BPs (SBP/DBP) were ≥140/90 mmHg42.
The lowest SBP/DBP at 3 or 5 minutes during the tilted position were selected for the diagnosis of OH. The orthostatic BP changes in systole (ΔSBPmin) and diastole (ΔDBPmin) were also calculated (supine average BP minus lowest orthostatic BP). When patients were hypertensive with ≥140/90 mmHg in the supine position, ΔSBPmin and/or ΔDBPmin ≥30/15 mmHg within 5 min was applied to define OH; otherwise, ΔSBPmin and/or ΔDBPmin ≥20/10 mmHg were adopted43.
The highest SBP/DBP among tilted measurements at 3, 5, 10, 15, and 20 min were re-selected, and the orthostatic ∆BPs were calculated from the average BPs (supine average BP minus highest orthostatic BP). PD patients with SH were defined as having orthostatic hypertension (OHT) if ΔSBPmax and/or ΔDBPmax was ≤ −20/10 mmHg. PD patients without SH were categorized as OHT when their orthostatic BPmax was ≥140/90 mmHg or ΔBPmax was ≤ −20/10 mmHg19.
Positive orthostatic ΔBP indicated a drop in standing BP, and the negative ΔBP an increase in standing BP.
123I-metaiodobenzylguanidine (123I-MIBG) scintigraphy
123I-MIBG scintigraphy was performed using a dual-head camera equipped with a low-energy, high-resolution collimator (Siemens), and data were collected at 30-min (early) and 2-h (delayed) time points after the injection of 111 MBq of 123I-MIBG. A static image was obtained with a 128 × 128 matrix. Regions of interest were manually drawn around the heart and mediastinum. Tracer uptake was measured within each region of interest to calculate the H/M ratio. The lower limit of the reference value for delayed H/M ratio was calculated to be 1.7812,13. A delayed H/M ratio < 1.78 was defined as abnormal. Washout rate was calculated as the following: [(early H/M ratio − late H/M ratio)/early H/M ratio] x 10013.
Statistical analyses
All statistical analyses were conducted with Jamovi software (version 1.6; retrieved from https://www.jamovi.org) for the Mac, a graphical user interface for R, with the additional jAMM module. The jAMM module provides a GLM mediation model that utilizes the lavaan R package44. Descriptive statistics, and independent or Welch’s t-tests, the Mann–Whitney U test or Fisher’s exact test were performed as appropriate. Mediation models, partialized by the covariates of age and disease duration, were manipulated to assess whether there was a mediating role for orthostatic ∆BPmin (b) between cardiac sympathetic denervation (a) and disease burden (c). The direct association between delayed H/M ratio and composite scores (a → c) was established within the context of an indirect effect of orthostatic ∆BPmin (a → b → c). Their relative roles were estimated using the maximum likelihood method45 and statistical significance was defined as a two-tailed p value < 0.05.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Anonymized data generated during the current study are available from the corresponding author on reasonable request from individuals affiliated with research or health care institutions.
Code availability
Jamovi is a statistical spreadsheet and graphical user interface (GUI) for R. All the analyses were performed basically using jamovi software as mentioned in the method, statistical analysis section. The R package mentioned was utilized within jamovi GUI.
References
Orimo, S. et al. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131, 642–650 (2008).
Orimo, S., Yogo, M., Nakamura, T., Suzuki, M. & Watanabe, H. (123)I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in α-synucleinopathies. Ageing Res. Rev. 30, 122–133 (2016).
Goldstein, D. S. Orthostatic hypotension as an early finding in Parkinson’s disease. Clin. Auton. Res. 16, 46–54 (2006).
Jain, S. & Goldstein, D. S. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol. Dis. 46, 572–580 (2012).
Schrag, A., Horsfall, L., Walters, K., Noyce, A. & Petersen, I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 14, 57–64 (2015).
Choi, M. H., Yoon, J. H. & Yong, S. W. Cardiac sympathetic denervation and dementia in de novo Parkinson’s disease: a 7-year follow-up study. J. Neurol. Sci. 381, 291–295 (2017).
Kim, J. S. et al. Normal ‘heart’ in Parkinson’s disease: is this a distinct clinical phenotype? Eur. J. Neurol. 24, 349–356 (2017).
Kotagal, V., Lineback, C., Bohnen, N. I. & Albin, R. L. Orthostatic hypotension predicts motor decline in early Parkinson disease. Parkinsonism Relat. Disord. 32, 127–129 (2016).
Fereshtehnejad, S. M. et al. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. Jama. Neurol. 72, 863–873 (2015).
Tsujikawa, K. et al. Chronological changes of 123I-MIBG myocardial scintigraphy and clinical features of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 86, 945–951 (2015).
Sakakibara, R. et al. MIBG myocardial scintigraphy in pre-motor Parkinson’s disease: a review. Parkinsonism Relat. Disord. 20, 267–273 (2014).
Lee, J. E. et al. Cardiac sympathetic denervation can predict the wearing-off phenomenon in patients with Parkinson disease. J. Nucl. Med. 59, 1728–1733 (2018).
Ryu, D. W. et al. Initial versus follow-up sequential myocardial 123I-MIBG scintigraphy to discriminate Parkinson disease from atypical Parkinsonian syndromes. Clin. Nucl. Med. 44, 282–288 (2019).
Hamada, K. et al. Onset age and severity of motor impairment are associated with reduction of myocardial 123I-MIBG uptake in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 74, 423–426 (2003).
Spiegel, J. et al. Myocardial sympathetic degeneration correlates with clinical phenotype of Parkinson’s disease. Mov. Disord. 22, 1004–1008 (2007).
Goldstein, D. S. & Cheshire, W. P. Jr. Roles of cardiac sympathetic neuroimaging in autonomic medicine. Clin. Auton. Res. 28, 397–410 (2018).
Spiegel, J. Diagnostic and pathophysiological impact of myocardial MIBG scintigraphy in Parkinson’s disease. Parkinsons Dis. 2010, 295346 (2010).
Robertson, D. The pathophysiology and diagnosis of orthostatic hypotension. Clin. Auton. Res. 18, S2–S7 (2008).
Jordan, J., Ricci, F., Hoffmann, F., Hamrefors, V. & Fedorowski, A. Orthostatic hypertension: critical appraisal of an overlooked condition. Hypertension 75, 1151–1158 (2020).
Kawazoe, M. et al. Sensitivity and specificity of cardiac (123)I-MIBG scintigraphy for diagnosis of early-phase Parkinson’s disease. J. Neurol. Sci. 407, 116409 (2019).
Hiorth, Y. H., Pedersen, K. F., Dalen, I., Tysnes, O. B. & Alves, G. Orthostatic hypotension in Parkinson disease: a 7-year prospective population-based study. Neurology 93, e1526–e1534 (2019).
Nakamura, T. et al. Role of cardiac sympathetic nerves in preventing orthostatic hypotension in Parkinson’s disease. Parkinsonism Relat. Disord. 20, 409–414 (2014).
Kim, J. S. et al. Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J. Neurol. Sci. 362, 59–63 (2016).
Rietdijk, C. D., Perez-Pardo, P., Garssen, J., van Wezel, R. J. & Kraneveld, A. D. Exploring Braak’s hypothesis of Parkinson’s disease. Front. Neurol. 8, 37 (2017).
Borghammer, P. The α-synuclein origin and connectome model (SOC Model) of Parkinson’s disease: explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J. Parkinsons Dis. 11, 455–474 (2021).
Horsager, J. et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 143, 3077–3088 (2020).
Jeong, Y. J. et al. Relationship between the washout rate of I-123 MIBG scans and autonomic function in Parkinson’s disease. PLoS. One 15, e0229860 (2020).
Nakajima, K., Taki, J., Tonami, N. & Hisada, K. Decreased 123I-MIBG uptake and increased clearance in various cardiac diseases. Nucl. Med. Commun. 15, 317–323 (1994).
Goldstein, D. S. The “Sick-but-not-Dead” phenomenon applied to catecholamine deficiency in neurodegenerative diseases. Semin Neurol. 40, 502–514 (2020).
Gibb, W. R. & Lees, A. J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 51, 745–752 (1988).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Kim, Y. D. et al. Cardiac (123)I-metaiodobenzylguanidine scintigraphy in a patient with familial parkinsonism with Parkin gene mutation. J. Mov. Disord. 3, 42–44 (2010).
Kägi, G. et al. Nonmotor symptoms in Parkin gene-related parkinsonism. Mov. Disord. 25, 1279–1284 (2010).
Goldstein, D. S., Sewell, L. & Sharabi, Y. Autonomic dysfunction in PD: a window to early detection? J. Neurol. Sci. 310, 118–122 (2011).
Koh, S. B. et al. Validation of the Korean-version of the nonmotor symptoms scale for Parkinson’s disease. J. Clin. Neurol. 8, 276–283 (2012).
Ahn, Y. M. et al. A validation study of the Korean-version of the Montgomery-Asberg depression rating scale. J. Korean Neuropsychiatr. Assoc. 44, 466–476 (2005).
Cho, Y. W. et al. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep. Breath. 15, 377–384 (2011).
Yang, H. J. et al. Subtypes of sleep disturbance in Parkinson’s disease based on the cross-culturally validated Korean Version of Parkinson’s Disease Sleep Scale-2. J. Clin. Neurol. 16, 66–74 (2020).
Lee, S. A., Paek, J. H., Han, S. H. & Ryu, H. U. The utility of a Korean version of the REM sleep behavior disorder screening questionnaire in patients with obstructive sleep apnea. J. Neurol. Sci. 358, 328–332 (2015).
Kaufmann, H., Malamut, R., Norcliffe-Kaufmann, L., Rosa, K. & Freeman, R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin. Auton. Res. 22, 79–90 (2012).
Kim, J. Y. et al. Validation of the Korean version of the Scale for Outcomes in Parkinson’s Disease-Autonomic. J. Mov. Disord. 10, 29–34 (2017).
Fanciulli, A. et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin. Auton. Res. 28, 355–362 (2018).
Gibbons, C. H. et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J. Neurol. 264, 1567–1582 (2017).
Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Soft 48, 1–36 (2012).
Rijnhart, J. J. M., Twisk, J. W. R., Chinapaw, M. J. M., de Boer, M. R. & Heymans, M. W. Comparison of methods for the analysis of relatively simple mediation models. Contemp. Clin. Trials Commun. 7, 130–135 (2017).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2017R1D1A1B06028086). This study was also supported by Research Fund of Seoul St. Mary’s Hospital, The Catholic University of Korea.
Author information
Authors and Affiliations
Contributions
S-.W.Y., and J-.S.K. contributed the conception and design of the study; S-.W.Y., J-.S.K., Y-.,S.O., D-.W.R., S.H., J-.Y.Y., and K-.S.L. contributed to the acquisition and analysis of data; S-.W.Y., and J-.S.K. contributed to the interpretation of results drafting the text and preparing figure; S-.W.Y. drafted the manuscript; J-.S.K., Y-.,S.O., D-.W.R., S.H., J-.Y.Y., and K-.S.L. revised the manuscript. S-.W. Y., and J-.S.K. obtained funding. All authors read and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoo, SW., Kim, JS., Oh, YS. et al. Cardiac sympathetic burden reflects Parkinson disease burden, regardless of high or low orthostatic blood pressure changes. npj Parkinsons Dis. 7, 71 (2021). https://doi.org/10.1038/s41531-021-00217-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-021-00217-3
This article is cited by
-
Estimating motor progression trajectory pursuant to temporal dynamic status of cardiac denervation in Parkinson’s disease
Journal of Neurology (2024)
-
Cardiac sympathetic “morbidity” might reflect the neurobiology of early Parkinson’s disease
Journal of Neurology (2024)
-
A 3-year natural history of orthostatic blood pressure dysregulation in early Parkinson’s disease
npj Parkinson's Disease (2023)
-
Exploring the link between essential tremor and Parkinson’s disease
npj Parkinson's Disease (2023)